Abstract
MtsABC is a Streptococcus pyogenes ABC transporter which was previously shown to be involved in iron and zinc accumulation. In this study, we showed that an mtsABC mutant has impaired growth, particularly in a metal-depleted medium and an aerobic environment. In metal-depleted medium, growth was restored by the addition of 10 μM MnCl2, whereas other metals had modest or no effect. A characterization of metal radioisotope accumulation showed that manganese competes with iron accumulation in a dose-dependent manner. Conversely, iron competes with manganese accumulation but to a lesser extent. The mutant showed a pronounced reduction (>90%) of 54Mn accumulation, showing that MtsABC is also involved in Mn transport. Using paraquat and hydrogen peroxide to induce oxidative stress, we show that the mutant has an increased susceptibility to reactive oxygen species. Moreover, activity of the manganese-cofactored superoxide dismutase in the mutant is reduced, probably as a consequence of reduced intracellular availability of manganese. The enzyme functionality was restored by manganese supplementation during growth. The mutant was also attenuated in virulence, as shown in animal experiments. These results emphasize the role of MtsABC and trace metals, especially manganese, for S. pyogenes growth, susceptibility to oxidative stress, and virulence.
Streptococci, pneumococci, and enterococci form a group of important pathogens with the ability to cause both superficial and invasive disease. During the establishment of an infection, bacteria must acquire many essential nutrients, some of which are scarcely available and/or safeguarded. A very important group of proteins in this process is the ATP binding cassette (ABC) transporters (21). In gram-positive bacteria, the prototypical ABC transporter consists of a lipoprotein, a hydrophobic membrane protein, and an ATPase, with the two latter present as homo- or heterodimers. The lipoprotein is tethered to the outer side of the cell membrane by means of a lipid modification of the N-terminal cysteine residue (52) and functions as a ligand binding protein. Metal ions such as manganese, copper, iron, cobalt, and zinc are essential trace elements but are also potentially harmful, which necessitates careful regulation of metal homeostasis (39). In recent years, a family of streptococcal ABC metal transporters has attracted considerable interest. Initially, before it was understood that the proteins were involved in metal transport, a lipoprotein family designated LraI was described (26). The family was later extended and renamed (cluster 9) and now includes members from at least 10 species of streptococci and enterococci (10, 12). Studies of Streptococcus pneumoniae and Streptococcus gordonii have suggested that manganese ion is transported by PsaBCA and ScaCBA, respectively (12, 31). PsaA (the lipoprotein component of the transporter) is also a virulence factor (3) and an interesting vaccine candidate (53). In Streptococcus mutans, the orthologous transporter Slo (also called Fim) was suggested to be involved in iron accumulation (29, 50). Streptococcus parasanguis contains another member of the family, FimA, which may be involved in the development of endocarditis (6). Immunization with the lipoprotein conferred protective immunity in an endocarditis model (54) and also offered protection against endocarditis caused by other viridans streptococci (30). The orthologous EfaA from Enterococcus faecalis has also been suggested to affect virulence (35, 48).
The Streptococcus pyogenes member of this family, MtsABC, has been described previously (25). In that study, the authors focused on the metal-binding properties of recombinantly expressed MtsA (lipoprotein). Support was found for iron and zinc as ligands of MtsA. The transporter was consequently deemed to have multiple specificities for metals. In addition, it was demonstrated that an MtsABC-deficient mutant had reduced accumulations of 55Fe and 65Zn. The mtsABC operon is polycistronic, but attenuation of the transcription by a stem-loop structure results in an abundance of monocistronic mtsA transcript. Recently, a review of the phylogeny of 47 metal-binding receptors was presented, including MtsA (10). These proteins can be divided into two paralogous subclusters, with a specificity for either zinc or manganese (12). For example, the Adc permease in S. pneumoniae transports Zn and is paralogous to the Psa permease, which transports Mn (12). A related publication stresses the importance of manganese homeostasis in bacteria and provides an interesting discussion of the metal specificity of transporters that may be involved (23). Relatively little is known about metal transport and the metal requirements of the important human pathogen S. pyogenes, although a few studies have described the effects of iron starvation on the growth and production of known or putative virulence factors (13, 19, 34, 37, 49). A recent review of iron uptake systems in gram-positive bacteria lists (at least) three separate systems in S. pyogenes (4). In this work, we investigated the role of metal transport in S. pyogenes by radioactive metal ion incorporation assays and the growth characteristics of an mtsABC mutant under different environmental conditions. We also describe an interesting connection between defective manganese transport and reduced functionality of superoxide dismutase (SOD), a crucial enzyme for defense against reactive oxygen species (ROS) in S. pyogenes (17). Details on the mechanisms of aerotolerance have begun to emerge, with the identification of two peroxidases, an NADH oxidase, and the peroxide stress response regulator PerR (18, 28, 46). Also, an early report suggested that the hyaluronic acid capsule may be important for oxygen resistance (11) in S. pyogenes. Our results provide evidence for a complex interplay between metal homeostasis, response to oxidative stress, and virulence.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. pyogenes strain AP1 is of serotype M1 and originates from the World Health Organization Collaborating Center for references and research on streptococci, Institute of Hygiene and Epidemiology, Prague, Czech Republic. The RJ1 strain is a previously described isogenic mutant constructed by insertion-duplication mutagenesis (25). In brief, the RJ1 chromosome contains a truncated mtsA gene followed by plasmid pFW13 (42) containing a Kmr cassette and several transcriptional terminators. A promoterless mtsABC operon is located immediately downstream of the plasmid. S. pyogenes was grown in Todd-Hewitt broth (Difco) supplemented with 0.25% yeast extract (THY) in 5% CO2 at 37°C or in metal ion-depleted THY medium (Cx-THY). Cx-THY was obtained by overnight (ON) treatment of THY medium with 5% Chelex-100 resin (Bio-Rad), sterile filtration, and addition of 100 μM CaCl2 and 2 mM MgCl2. Aerobic growth was performed at 37°C in rotating Erlenmeyer flasks with ambient air. The medium was preequilibrated under these conditions for at least 1 h prior to the inoculation of bacteria. For growth curves, precultures were grown ON in THY medium, washed once in fresh THY or Cx-THY medium, resuspended at a concentration of 2 × 108 CFU ml−1, and inoculated 1:100 in fresh medium in duplicate samples. RJ1 was grown in the presence of 150 μg of kanamycin (Sigma) ml−1. Sterile filtered metal salt solutions were used for metal supplementation. FeSO4 was always prepared fresh in order to minimize the effects of oxidation.
Metal accumulation.
Precultures were prepared as described above and inoculated 1:100 in 1 ml of fresh Cx-THY medium containing 120 pmol (0.25 μCi) of 55FeCl3 (Amersham Pharmacia Biotech) or 150 pmol (1 μCi) of 54MnCl2 (NEN). When the cultures reached an A620 of 0.6, bacteria were pelleted by centrifugation at 10,000 × g for 3 min. Supernatants were collected, and bacteria were resuspended in 1 ml of fresh Cx-THY. Pilot experiments were performed in which viable counts were made on sample tubes to ensure that the procedure resulted in comparable amounts of bacteria. Cells were recentrifuged and resuspended as described above for a total of three washes. Bacteria were finally resuspended in 200 μl of Cx-THY and mixed with 5 ml of Ready Safe scintillation cocktail (Beckman). The culture supernatants collected initially were likewise mixed with scintillation cocktail. Radioactivity was then measured in a β-counter by using the 32P window for 54Mn and a calibrated window for 55Fe. The fraction of radioisotope associated with the bacterial pellet was calculated by dividing the counts per minute of the pellet by the sum of the counts per minute of the pellet and the counts per minute of the supernatant. Experiments were performed at least three times in duplicate or triplicate samples.
Oxidative stress.
Bacteria were grown ON in THY medium and reinoculated 1:200 in fresh THY. When cells reached an A620 of 0.5, an aliquot of 100 μl was removed (time zero), and 5 mM H2O2 was added to the tubes. Samples were collected at various time points (5, 10, 15, 30, and 60 min), 5 mg of catalase (Sigma)/ml was added, and tubes were put on ice. Appropriate bacterial dilutions were performed and plated onto THY agar plates. Cells were counted, and the results were expressed as the percentage of survival by dividing the number of CFU at different time points with the initial number of CFU before the H2O2 challenge (time zero). For growth in the presence of paraquat (Sigma), ON cultures of wild-type (WT) and mutant strains were inoculated in fresh THY at a concentration of 3 × 105 CFU ml−1. Paraquat was added at concentrations of 2 and 10 mM, and MnCl2 and ferric citrate were used at concentrations of 30 μM. After 15 h of incubation at 37°C with 5% CO2, the A620 was recorded.
SOD activity assay.
The preparation of streptococcal cell extracts was performed essentially as described previously (7). Bacteria were grown on THY plates in ambient air. Colonies were scraped off the plates into extraction buffer (0.1 M Tris-HCl [pH 7.6]) and pelleted by centrifugation, and the supernatant was removed. Bacterial pellets were frozen for 10 min at −80°C and thawed at 37°C for 5 min. The freeze-thaw cycle was repeated three times. Pellets were resuspended in 500 μl of extraction buffer and sonicated 8 times for 15 s, with 30 s on ice between each round of sonication. Samples were centrifuged (14,000 × g) at 4°C for 15 min, and the supernatants were transferred into fresh tubes, aliquoted, and immediately frozen. The protein concentration was determined according to the Bradford method with the Coomassie protein assay reagent (Pierce). Twenty micrograms of protein per sample was separated by 8% native polyacrylamide gel electrophoresis (PAGE). The SOD activity assay was performed as previously described (2). In brief, the gel was immersed in 2.5 mM nitroblue tetrazolium (Sigma) for 20 min and in activation solution for 15 min (36 mM KH2PO4 [pH 7.8], 28 μM riboflavin [ Sigma], 28 mM N,N,N′,N′-tetramethylethylenediamine [Bio-Rad]). After rapid rinsing in distilled H2O, the gel was exposed to visible light on a transilluminator until adequate contrast was achieved (5 to 10 min). SOD activity inhibits the photoinduced coloration of the gel and results in clear, uncolored zones.
RNA methods.
To prepare total RNA from S. pyogenes, AP1 and RJ1 bacteria were cultured in 20 ml of THY and harvested in the exponential phase of growth (A620 ≅ 0.5). Cells were centrifuged (5,000 × g, 10 min, 4°C); resuspended in 125 μl of 100 mM Tris-HCl (pH 8) containing 15 mg of lysozyme (Sigma) ml−1, 100 U of mutanolysin (Sigma), and 20 mM ribonucleoside vanadyl complexes (Sigma); and incubated for 30 min at 37°C. Then 2.5 ml of Tri reagent (Sigma) was added to each sample, and samples were incubated for 5 min at room temperature before the addition of 0.5 ml of chloroform. Following extraction and precipitation, RNA was resuspended in 50 μl of 0.1% (vol/vol) diethylpyrocarbonate-treated H2O, spectrophotometrically quantified, and immediately frozen at −70°C.
For Northern blot experiments, 10 μg of total RNA was separated on a 1% agarose gel in HEPES buffer (20 mM Na-HEPES [pH 7], 5 mM sodium acetate, 1 mM EDTA) and 17% (vol/vol) formaldehyde. RNA was transferred onto Hybond-N filters (Amersham Pharmacia Biotech), cross-linked, and hybridized with different probes produced by PCR with chromosomal DNA from strain AP1 as the template. A 487-bp mtsA-specific probe was obtained with the primers mtsA-forward (5′-TAC GAA CCA TTA CCA GAA GAT-3′) and mtsA-reverse (5′-CTT CTT CTT CGG TGT TAA TTT CCC AG-3′). The 470-bp spy0385-specific probe was generated with the primers spy0385-forward (5′-ATA GCG GGC TGA AAG ATT GAG GTC-3′) and spy0385-reverse (5′-CAA GAT TAT TAC CAC CAA AAC AGG G-3′), and primers sodA-forward (5′-TTT ACC AGA ACT TCC ATA CGC G-3′) and sodA-reverse (5′-TCT TTT TGA GAT TGG AAA CCC-3′) were used to generate a 620-bp fragment specific for sodA. PCR products were purified on MicroSpin S-200 HR columns (Amersham Pharmacia Biotech) and labeled with [α-32P]dATP by using Megaprime (Amersham Pharmacia Biotech). Hybridization was performed at 46 to 50°C ON in a rotating hybridization oven. Membranes were washed extensively with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer and 0.1% sodium dodecyl sulfate and dried. Membranes were then exposed on a BAS-III Imaging plate and scanned with a Bio-Imaging Analyzer BAS-2000 (Fuji Photo Films, Tokyo, Japan).
Animal experiments.
Bacteria were grown in THY medium until the A620 was 0.4, washed three times in sterile phosphate-buffered saline, and diluted in phosphate-buffered saline to final concentrations of 108, 107, and 106 CFU ml−1. Viable counts were performed to verify the accuracy of the dilutions. Seven- to nine-week-old inbred BALB/c mice were used. Mice received a subcutaneous injection of 900 μl of air together with 100 μl of bacterial solution. A total of three experiments were performed. In the first (n = 3 per group) and second (n = 5 per group) experiments, 4 groups were inoculated with 106 and 105 CFU of the WT or mutant ml−1. The third experiment comprised 6 groups (n = 5) of mice infected with 107, 106, and 105 CFU of AP1 and RJ1 bacteria ml−1. Animals were checked at regular intervals three times per day. Moribund mice (ruffled fur, inactive, and unresponsive to painful stimuli) were euthanized and recorded as dead (a total of 5 animals distributed among the groups were euthanized). Animals were observed for at least 1 week after the last casualty. Statistical analysis (Fisher's exact test) was performed with InStat (Graphpad software).
Homology modeling.
The mature MtsA polypeptide sequence from S. pyogenes strain SF370 was submitted to the internet-based server SWISS-MODEL, which uses ProMODII for modeling (47). Automated selection of templates resulted in the use of PsaA (1PSZ) and TroA (1TOA). The model built was visualized and analyzed with SwissPdbViewer for Macintosh (20).
RESULTS
The mtsABC mutant shows impaired growth in metal-depleted and aerobic environments.
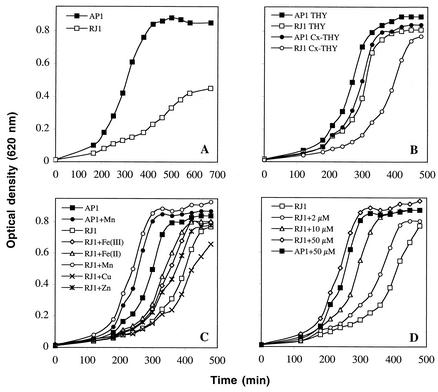
We observed that the mtsABC mutant exhibited abnormal colony morphology when grown on plates, especially in ambient air. The RJ1 mutant strain colonies were consistently smaller than those of the WT (data not shown). Closer scrutiny of RJ1 growth (at 5% CO2 atmosphere) in liquid medium indicated a discrete difference in growth between the WT and mutant, unlike what was initially reported (25). We therefore examined growth also under aerobic conditions in THY medium, and the mutant strain showed a pronounced growth defect (Fig. 1A), with a doubling time of 142 min compared to 73 min for the WT. To investigate a possible metal-dependent growth defect, THY medium was depleted of metals by treatment with Chelex-100 resin and then supplemented with CaCl2 (100 μM) and MgSO4 (2 mM), resulting in Cx-THY. When grown in Cx-THY in a 5% CO2 atmosphere, the mutant strain showed a clear impairment of growth (Fig. 1B) compared to the WT strain, which had a modest or no growth deficit (doubling times of 67 and 43 min, respectively). Cx-THY was also used under aerobic conditions, but there was no growth of bacteria during the time span used (data not shown). Cx-THY medium was then supplemented with 50 μM concentrations of various metal cations (Fig. 1C). The only metal ion giving a pronounced and reproducible improvement of mutant growth was manganese. Ferric and ferrous iron also enhanced mutant growth, but the finding was not always reproducible. When grown in the presence of MnCl2, the RJ1 growth rate was similar to or higher than that of the WT. The WT was also grown with metal supplementation, and manganese showed some stimulatory effect in metal ion-depleted THY (Fig. 1C). The impact of manganese on RJ1 growth was further investigated by decreasing the concentration (50 to 2 μM) of supplementary Mn (Fig. 1D). In these experiments, a certain reconstitution of growth was seen even with 2 μM MnCl2. Full reconstitution was seen with 10 μM of MnCl2. Supplementation with 50 μM manganese ion also increased the growth of the WT strain, but similar levels of growth were seen for RJ1 with 50 μM MnCl2.
FIG. 1.
Growth of WT (AP1) and mutant (RJ1) mtsABC strains under different environmental conditions. Experiments were performed at least three times with duplicate samples, and representative growth curves are shown. (A) Growth in THY under aerobic conditions. (B) Comparison of WT and mutant growth in THY or metal-depleted THY (Cx-THY) in a 5% CO2 atmosphere. (C) Growth in a 5% CO2 atmosphere in Cx-THY with 50 μM supplements of the following metal salts: ferric citrate, FeSO4, MnCl2, CuCl2, ZnCl2. (D) Growth in a 5% CO2 atmosphere in Cx-THY supplemented with increasing concentrations (0 to 50 μM) of MnCl2.
MtsABC transports both iron and manganese.
A previous study of the mtsABC system showed that an mtsABC mutant had a 50% reduced accumulation of 55Fe and 65Zn compared to the WT, in ON cultures. In similar experiments, we arrested growth towards the end of the exponential phase (A620 = 0.6), and under those conditions, accumulation of 55Fe in the mutant was reduced to less than 10% of WT levels (data not shown). Two additional ABC transporters have been suggested to contribute to iron uptake in S. pyogenes. The polycistronically transcribed and iron-regulated genes spy0383 to spy0386 (gene numbers are according to the S. pyogenes genome project [14]) encode putative proteins with similarity to siderophore-dependent iron transporters (49). All three principal components of an ABC transporter are present in the operon. A recent publication describes Shp, a heme-associated surface protein (34). The shp gene is cotranscribed with spy1795 to spy1793, encoding a putative ABC transporter which may be involved in heme uptake. The transcription of shp and adjacent genes mainly occurs during the stationary phase. We investigated the expression of spy0385 (encoding a putative iron-binding lipoprotein) by total RNA extraction from log-phase bacteria and subsequent Northern blotting experiments. However, we failed to detect any transcript hybridizing with the probe, whereas strong hybridization occurred with the same membranes by using an mtsA probe (data not shown). We also searched for additional iron transporters in the streptococcal genome by using known or suggested binding proteins of ABC transporters as queries (Escherichia coli, FhuD, FepB, FeoB, and FecB [loci b0152, b0592, b3409, and b4290 in the E. coli K-12 MG1655 genome]; Staphylococcus aureus, FhuD1 and FhuD2 [accession numbers AF325854 and AF3258555]; S. pneumoniae, PitA, PiuA, and PiaA [SP0243, SP1032, and SP1872 in The Institute for Genomic Research serotype 4 S. pneumoniae genome]) and examining chromosomal regions where hits occurred for the presence of genes encoding putative ABC transporters. No putative iron transporters other than the three mentioned above were identified.
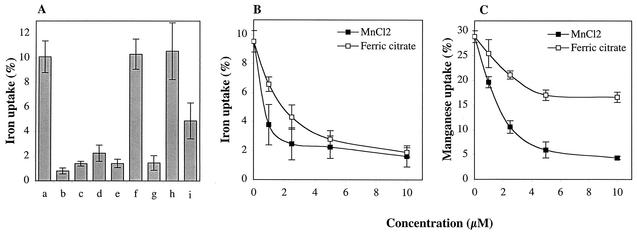
A problem with studies of metal accumulation in a complex medium such as THY is that only a very minor fraction (<1%) of the added radioisotope is taken up, which makes competition experiments difficult. In order to reduce the effects of competition from nonradioactive metals in the medium, we repeated the 55Fe accumulation experiments by using exponential-phase bacteria grown in Cx-THY. Under these conditions, the ratio between WT and mutant uptake was similar to that in experiments performed with normal THY medium (90% reduction of 55Fe accumulation in the mutant), but the accumulation of 55Fe was more than 10 times higher than in THY medium, rising to 10% of the added radioisotope for the WT strain. In control experiments, ferric citrate or 2,2-dipyrridyl (an iron chelator) was added during washes, at a concentration (1 mM) which was in large excess compared to the radioisotope. This did not significantly affect the amount of radioactivity found in the bacterial pellet, making it unlikely that nonspecific binding to the bacterial surface affected the results (data not shown). Competing metal salts were included in the experiments at a concentration of 10 μM (Fig. 2A). Ferric and ferrous ions reduced accumulation by 75 to 90%, and manganese was equally efficient. Zinc competed with 55Fe accumulation to a lesser extent, whereas copper did not compete. Dose-dependent manganese competition was also examined (Fig. 2B), indicating that Mn is equally or more effective than ferric iron in competing with 55Fe accumulation of the WT strain. The accumulation of manganese was then directly examined by using 54Mn. During log-phase growth in Cx-THY, WT bacteria incorporated 29.3% ± 1.6% of the added 54Mn while the mtsABC mutant incorporated only 3% ± 1.2%, which represents a 90% reduction of 54Mn accumulation (data not shown). Dose-dependent competition experiments with WT bacteria showed that ferric iron competes with 54Mn accumulation but to a lesser extent than manganese (Fig. 2C). Interestingly, a significant proportion of the total Mn accumulation remained at the highest dose (10 μM) of ferric citrate competition. In conclusion, MtsABC is capable of transporting not only iron and zinc but also manganese. Taken together with the metal supplementation results above, it is reasonable to assume that the impaired growth of the mtsABC mutant is caused by reduced intracellular availability of manganese, as a consequence of reduced transport.
FIG. 2.
Bacterial incorporation of radioactive metal ion isotopes during the exponential phase of growth in Cx-THY. (A) Accumulation of 55Fe in the presence or absence of 10 μM concentrations of competing metal salts. AP1 accumulation (a) was competed with ferric citrate (c), FeCl3 (d), FeSO4 (e), Na citrate (f), MnCl2 (g), CuCl2 (h), and ZnCl2 (i). Results for the mtsABC mutant RJ1 are seen in bar b. In this context, accumulation is defined as the radioactivity found in the bacterial pellet as a fraction of the total radioactivity added. The bars represent the means of three experiments performed with duplicate samples. Standard deviations are represented by the error bars. (B) Dose-dependent competition of 55Fe accumulation by MnCl2 and ferric citrate. Data points are means of two experiments performed with triplicate samples. Standard deviations are represented by the error bars. (C) Dose-dependent competition of 54Mn accumulation by MnCl2 and ferric citrate. Data points are means of two experiments performed with triplicate samples. Standard deviations are represented by the error bars.
The mtsABC mutant has increased susceptibility to ROS.
The reduced growth rate of the mtsABC mutant in an aerobic environment suggested that this strain has increased sensitivity to oxidative stress. The susceptibility to lethal hydrogen peroxide challenge was investigated in a time course study. Bacteria were grown to mid-exponential phase, and then 5 mM hydrogen peroxide was added. Samples were collected at various time points, and a viable count was performed (Fig. 3A). The mutant strain was killed more rapidly than the WT. At 5 min after the challenge, 9% of the mutant bacteria were still alive compared to 34% of the WT bacteria. The difference was statistically significant (P < 0.05) and reproducible. In a different assay, methyl viologen (paraquat) was used to induce oxidative stress (Fig. 3B). Cultures were grown ON in the presence of 0, 2, or 10 mM paraquat. The WT strain was moderately affected by the presence of paraquat, whereas the mtsABC mutant was unable to grow even at the lower concentration. To investigate whether this pronounced difference between the WT and mutant was due to decreased availability of manganese in the mutant, bacterial cultures were supplemented with 30 μM MnCl2 and grown in the presence of paraquat. Interestingly, this completely restored the capacity to grow under oxidative stress conditions. Ferric citrate was also used for supplementation but did not restore growth of the mutant. Some authors have reported that Mn has antioxidant properties per se (8), but under these conditions, such an effect seems unlikely since the WT strain did not benefit from the presence of additional manganese. It thus seems that the sensitivity to ROS in the mutant is at least partly dependent upon the deficiency in manganese accumulation. A recent paper described a thiol-specific antioxidant in S. parasanguis (51), carried immediately downstream of the fimA locus, which encodes proteins orthologous to MtsABC. A BLAST search of the entire S. pyogenes genome and scrutiny of open reading frames in the vicinity of the mtsABC locus showed that no putative protein similar to the S. parasanguis antioxidant is encoded by S. pyogenes.
FIG. 3.
Comparison of WT and mutant sensitivity to oxidative stress conditions. (A) WT (AP1) and mutant (RJ1) bacteria were grown in a 5% CO2 atmosphere until the A620 was 0.5, and 5 mM hydrogen peroxide was added. Samples were collected at various time points, and viable counts were performed. Results are presented as the percentages of viable bacteria at a certain time point compared with the number of bacteria prior to challenge (time zero). Data points represent means of five experiments, and error bars indicate the standard deviations. (B) Growth of WT (AP1) and mutant (RJ1) in the presence or absence of paraquat. ON cultures were reinoculated in fresh THY containing 0, 2, or 10 mM paraquat. MnCl2 or ferric citrate was added to some samples at a concentration of 30 μM. The A620 was recorded after 15 h of incubation. Results represent the means of four experiments performed with duplicate samples. Standard deviations are indicated by the error bars.
Reduced activity of SOD.
SODs are crucial in the defense against oxidative stress (15). The S. pyogenes genome encodes one SOD, which is Mn dependent (14, 17). The increased sensitivity to ROS in the mtsABC mutant was reversed by adding Mn, and it therefore seemed possible that SOD activity might be affected as a consequence of reduced intracellular availability of the cofactor. We prepared whole-cell lysates of bacteria grown aerobically. Total protein preparations were separated by native PAGE, and a SOD activity assay was performed (Fig. 4A). Equal amounts of total protein were applied by utilizing protein concentration measurements according to the Bradford method. Also, as a control, a second native PAGE was always performed in parallel and stained with Coomassie brilliant blue. As previously reported, only one zone of clearance was seen per sample, confirming the presence of a single SOD (17). The mutant consistently showed fainter, sometimes barely detectable, bands than did the WT. When bacteria were grown on THY plates supplemented with 50 μM MnCl2, the subsequent SOD activity assays no longer showed any difference between the WT and mutant. It was not possible to increase SOD activity by simply adding MnCl2 to the total protein extracts prior to loading the PAGE (data not shown). To investigate a possible downregulation of sodA transcription, we performed Northern blot analysis of WT and mutant bacteria by using a sodA probe (Fig. 4B). The probe hybridized with a single transcript, and there was no apparent difference in intensity between the WT and mutant. Therefore, it seems likely that the reduced SOD activity in the mtsABC mutant is a consequence of decreased cofactor availability within the bacteria.
FIG. 4.
Analysis of SOD activity and sodA transcription. (A) Cell extracts were prepared from WT (AP1) and mutant (RJ1) bacteria grown on THY plates in ambient air, with or without 50 μM MnCl2 supplementation. Twenty micrograms of protein per sample was separated by native PAGE. Gels were then subjected to an assay where SOD activity is visualized as clear zones. The experiment was performed three times, and the image shown represents the highest observed activity in RJ1 (without manganese supplementation). (B) Northern blot analysis of total RNA from WT and mutant strains. A 32P-labeled 620-bp probe corresponding to sodA was hybridized with the membrane. The experiment was performed twice with identical results.
MtsABC is required for full virulence.
There are several reports indicating that the homologues of MtsABC in S. pneumoniae (PsaBCA), S. parasanguis (FimCBA), and E. faecalis (EfaA) are relevant for virulence (3, 6, 48). Therefore, in order to examine whether the mtsABC mutant was attenuated in virulence, we infected BALB/c mice with WT and mutant bacteria by using the established air sac model (44). Three sets of animal experiments were performed, with three different bacterial doses: 107, 106, and 105 CFU. With the highest inoculum (107), none of the animals infected with the WT strain survived while 40% of the mice injected with the mutant strain survived. Using 106 and 105 CFU, all animals inoculated with the mutant strain survived while the groups injected with WT bacteria contained 38 and 62% survivors, respectively. The results (Table 1) showed a significantly reduced virulence of the mtsABC mutant. Fisher's exact test was used for the larger groups (106- and 105-CFU inocula) while the remaining group (107-CFU inoculum) was too small for reliable statistical evaluation. The statistical significance was a P value of <0.001 with 106 bacteria and a P value of <0.05 with 105 bacteria. The calculated dose at which 50% of animals die (or become moribund) was 2.4 × 105 CFU for the WT and 6.8 × 106 CFU for the mutant. There is thus an approximate 30-fold reduction in virulence of the mutant.
TABLE 1.
Virulence of WT and mtsABC mutant strains in mice
| Strain | No. of dead mice/total no. of micea for inoculum (CFU/mouse):
|
||
|---|---|---|---|
| 107 | 106 | 105 | |
| WT | 5/5 | 8/13 | 5/13 |
| Mutant | 3/5 | 0/13b | 0/13b |
Included among the dead mice are moribund mice (n = 5, distributed among different groups) that were euthanized.
These results are statistically significant from those of the WT strain, assessed by Fisher's exact test.
Homology modeling of MtsA.
The crystal structure of PsaA, the S. pneumoniae equivalent of MtsA, has been solved (32). We performed homology modeling of MtsA by using PsaA and TroA as templates (33). The mature MtsA polypeptide has 79% sequence identity with the corresponding PsaA sequence. TroA, a zinc-binding protein, shares a similar overall structure with PsaA and has some sequence similarity to MtsA (29% identity). A model encompassing most of the mature MtsA polypeptide (Asp32 to Lys308) was created. The root mean square deviation from the PsaA reference was 0.43 Å. The metal-binding site described for PsaA was completely conserved and consisted of His68, His140, Glu206, and Asp282. The overall fold of MtsA is a pair of β/α sandwich domains against a backbone helix (Fig. 5). The topology is a 2-1-3-4-linked parallel (β/α)4. MtsA thus represents another example of the atypical ligand binding protein fold recently described (9).
FIG. 5.
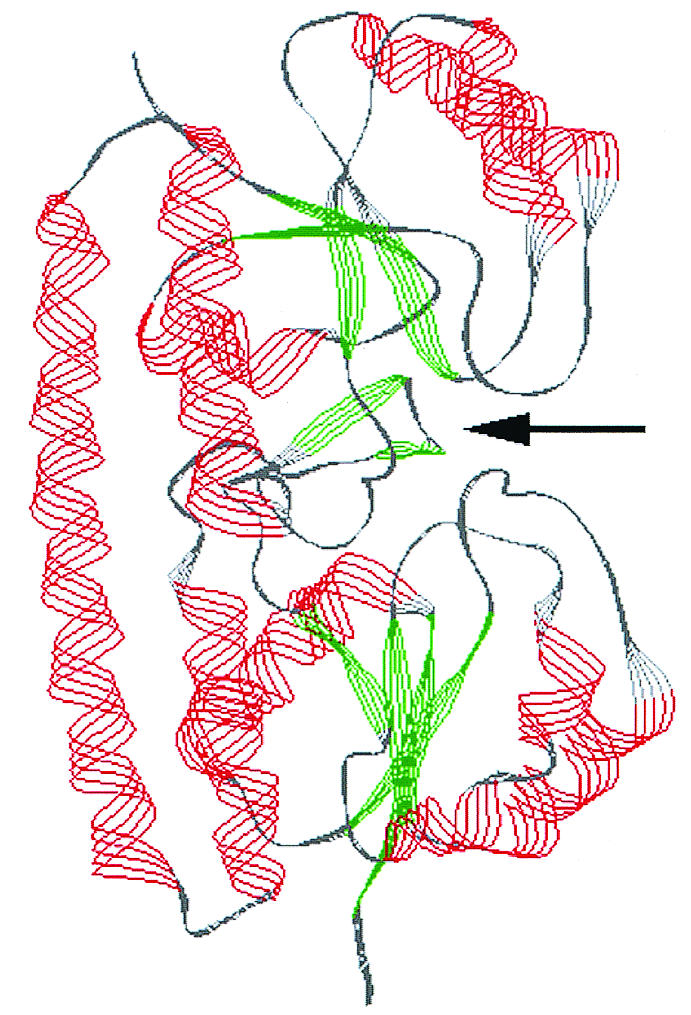
Homology modeling of MtsA. Overall fold of MtsA seen from a view orthogonal to the pseudosymmetry axis, with the N-terminal (membrane associated) region facing down. The β strands are shown in green, and the α helices are shown in red. The arrow indicates the approximate location of the putative metal-binding pocket.
DISCUSSION
Recent contributions to the understanding of bacterial metal transport have included the extension and subdivision of a family of ABC metal permeases (10, 12) and an overview of the role of manganese in bacterial physiology (23). The subdivision is based upon phylogenetic analysis of a large number of putative metal-binding receptors and proposes the existence of two subclusters dedicated to either manganese or zinc transport.
In a previous work, the identification and partial characterization of S. pyogenes MtsABC, an ABC metal permease now included in the Mn subcluster, was described. The observation that an mtsABC mutant had a growth deficiency prompted us to further investigate the role of MtsABC in S. pyogenes. Metal deprivation of THY medium accentuated the growth deficiency of the mutant, whereas the WT was modestly affected. In comparison, a previous report of S. pneumoniae showed that this bacterium has a growth deficiency in identical metal-depleted medium even without inactivation of genes encoding metal transporters (5). This suggests that the metal requirements of S. pyogenes are different and possibly less demanding. Growth of the mtsABC mutant was completely restored by manganese supplementation, and partial restoration was achieved with as little as 2 μM manganese. Iron also alleviated the growth deficiency somewhat, but these results were less convincing. Analogous studies of metal transport-deficient mutants in S. gordonii (scaCBA, Mn) and S. pneumoniae (psaBCA, Mn, and the paralogue adcCBA, Zn) (12, 31) showed that (i) Mn restored growth of scaCBA and psaBCA mutants and (ii) Zn restored growth of the adcCBA mutant. Interestingly, the addition of both Mn and Zn abolished rescue of growth in the adcCBA mutant, and 54Mn accumulation in WT S. gordonii was inhibited by Zn. The modeling of PsaA from crystallographic data was successful only when using Zn in the metal-binding site (32). However, another report discusses the geometry of the metal-binding site in PsaA, TroA, and related proteins (33). The authors implicate a correlation between the presence of a Glu residue in the metal-binding site (as in PsaA and MtsA) and coordination of Fe2+ or Mn2+ rather than Zn2+, which coordinates with His instead of Glu. In our initial report, we showed that recombinant MtsA had the capacity to bind both iron and zinc. We failed to show a direct interaction between Mn and MtsA, although there was some inhibition of zinc binding when competing with Mn. In light of the data presented here, it would seem that MtsABC is indeed involved in Mn transport. The inability to show a direct interaction may have been a result of nonoptimal conditions (i.e., buffer composition or pH), whereas the metal content analysis of recombinant MtsA is prone to be influenced by the intracellular availability of metal ions in E. coli. The homology between MtsA and PsaA is striking, and our attempt to model the tertiary structure of MtsA suggests that the overall fold is very similar and preserves the metal-binding site. If MtsABC and homologues have similar specificities, one interpretation is that several ligands can be bound and transported. PsaBCA and ScaCBA, for instance, may transport both Mn and Zn. In that case, the proposed subclusters (although phylogenetically correct) might not directly correspond to a devoted metal specificity.
Iron acquisition in bacteria has been widely studied and is of pronounced significance for microbial pathogenesis (45). We therefore initially focused on a characterization of the iron accumulation of S. pyogenes. When iron accumulation assays were performed during the exponential phase of growth, the difference between the WT and mutant was larger (90% reduction in the mutant) than in ON cultures (50% reduction). It is thus possible that MtsABC has a prominent role in iron accumulation during this phase but is later partly supplanted by other uptake systems, such as the iron-regulated proteins Spy0383 to Spy386 (49). Competition for iron accumulation was seen with iron (ferric and ferrous), manganese, and zinc. Manganese was equally or more effective than iron itself in competing with 55Fe accumulation. The accumulation of 54Mn was also examined, and the mtsABC mutant showed a 90% reduction of accumulation. It thus seems clear that MtsABC is also a manganese transporter. Competition for 54Mn accumulation was achieved with both ferric citrate and ferrous sulfate. It is difficult to distinguish between the effects of divalent and trivalent iron in the systems used. Ferrous iron is unstable and prone to oxidation. On the other hand, the S. pyogenes genome encodes a ferredoxin (14), which may push the equilibrium towards divalent iron. Ferric iron is essentially insoluble at neutral pH, and the role of putative siderophores remains to be investigated.
Under the presumption that MtsABC dominates both iron and manganese accumulation under the conditions used, it may be difficult to reconcile the fact that Mn competition of 55Fe accumulation is very efficient (Fig. 2B), whereas Fe competition of 54Mn accumulation reaches a plateau where approximately half of the accumulation remains (Fig. 2C). A speculation would be that the specificity of the metal-binding receptor may be different from that of the permease. The permease is likely the rate-limiting step in transport. For instance, Mn may have a low affinity for the lipoprotein but a high affinity for the permease. If receptor-independent accumulation may occur to some extent, complete competition at the level of the receptor may still allow significant Mn accumulation through the permease. Alternatively, there might be a second transporter contributing to Mn uptake, as suggested by the fact that growth of the mtsABC mutant was rescued by Mn supplementation. For example, the paralogous AdcCBA transporter discussed above is encoded by the genome of S. pyogenes (spy0093, spy0094, and spy0714) but differs from its S. pneumoniae counterpart in that the gene encoding the lipoprotein (adcA) is not found in the same locus as the other components. In addition to ABC transporters, the NRAMP family transporters are also known to mediate Mn translocation across cell membranes. We searched the S. pyogenes genome for putative proteins homologous to MntH (41) of E. coli and a Lactococcus lactis protein (L91569), both of which are members of an NRAMP cluster of proteins (cluster of orthologous groups 1914). However, no putative NRAMP proteins were found.
The role of oxidants in microbial pathophysiology is well documented (38). As a facultative anaerobe, S. pyogenes has the capacity to withstand growth under aerobic conditions. The mtsABC mutant showed a pronounced growth deficit under aerobic conditions, suggesting that the lack of certain metals increased the susceptibility to ROS. Experiments with paraquat as an inducer of oxidative stress showed that the mutant was unable to grow unless supplemented with 30 μM Mn. In comparison, aeration and paraquat stress had no inhibitory effect on the scaCBA mutant in S. gordonii (31). Several species of streptococci can grow in the absence of iron (24, 40), and it has been proposed that Mn can replace iron (1). A connection between Mn homeostasis and sensitivity to oxidative stress has been reported (27, 36). S. pyogenes lacks catalase but produces an Mn-dependent SOD (16, 17). In contrast to many other species which have several SODs, with different cofactors (Mn, Fe, and Cu/Zn), it has been suggested that Bacillus subtilis and most streptococci and enterococci mainly utilize the Mn SOD (22, 40). The present study shows that the activity of SOD in the mutant was reduced compared to that of the WT but could be restored by growth under Mn-supplemented conditions. The reduced SOD activity is unlikely the result of general downregulation of sodA transcription, as shown by Northern blot analysis. Formally, however, it cannot be excluded that there is a downregulation of sodA transcription at some point during growth. We interpret the reduced SOD activity as a consequence of low intracellular Mn availability, resulting in the degradation of misfolded SOD and/or disturbed function of noncofactored SOD.
ROS play an important role in host defense against bacterial infection (38), and there are examples of streptococci showing attenuated virulence as a consequence of disturbed SOD activity (43, 55). The ≈30-fold reduction in virulence seen in the mtsABC mutant may be partly attributed to such an effect. At the same time, the growth deficiency of the mutant may also contribute to virulence attenuation, depending on Mn availability in vivo. The metal accumulation studies and growth experiments suggest that Mn may be a crucial metal for S. pyogenes. The drastic reduction in iron accumulation of the mutant does not seem to have profound effects on growth, whereas Mn does. In conclusion, our study further details the contribution of MtsABC to iron transport and provides evidence for manganese as an additional ligand. Our data also point to an interesting connection between Mn transport, oxidative stress responses, and virulence in S. pyogenes. MtsABC is likely to have an important role in this complex interplay.
Acknowledgments
This work was supported by the Swedish Research Council (project 7480) and the Foundations of Kock and Österlund.
We thank Ingbritt Gustavsson and Andrea Stuart for expert technical assistance.
Editor: V. J. DiRita
REFERENCES
- 1.Archibald, F. 1986. Manganese: its acquisition by and function in the lactic acid bacteria. Crit. Rev. Microbiol. 13:63-109. [DOI] [PubMed] [Google Scholar]
- 2.Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276-287. [DOI] [PubMed] [Google Scholar]
- 3.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, J., and D. Holden. 2002. Iron acquisition by Gram-positive bacterial pathogens. Microbes Infect. 4:1149-1156. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J. S., S. M. Gilliland, and D. W. Holden. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40:572-585. [DOI] [PubMed] [Google Scholar]
- 6.Burnette-Curley, D., V. Wells, H. Viscount, C. L. Munro, J. C. Fenno, P. Fives-Taylor, and F. L. Macrina. 1995. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect. Immun. 63:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caparon, M. G., R. T. Geist, J. Perez-Casal, and J. R. Scott. 1992. Environmental regulation of virulence in group A streptococci: transcription of the gene encoding M protein is stimulated by carbon dioxide. J. Bacteriol. 174:5693-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheton, P. L., and F. S. Archibald. 1988. Manganese complexes and the generation and scavenging of hydroxyl free radicals. Free Radic. Biol. Med. 5:325-333. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, T. E., S. Y. Ku, D. R. Dougan, H. J. Vogel, and L. W. Tari. 2000. The structure of the ferric siderophore binding protein FhuD complexed with gallichrome. Nat. Struct. Biol. 7:287-291. [DOI] [PubMed] [Google Scholar]
- 10.Claverys, J. P. 2001. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 152:231-243. [DOI] [PubMed] [Google Scholar]
- 11.Cleary, P. P., and A. Larkin. 1979. Hyaluronic acid capsule: strategy for oxygen resistance in group A streptococci. J. Bacteriol. 140:1090-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dintilhac, A., G. Alloing, C. Granadel, and J.-P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-739. [DOI] [PubMed] [Google Scholar]
- 13.Eichenbaum, Z., E. Muller, S. A. Morse, and J. R. Scott. 1996. Acquisition of iron from host proteins by the group A streptococcus. Infect. Immun 64:5428-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridovich, I. 1997. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J. Biol. Chem. 272:18515-18517. [DOI] [PubMed] [Google Scholar]
- 16.Gerlach, D., W. Reichardt, and S. Vettermann. 1998. Extracellular superoxide dismutase from Streptococcus pyogenes type 12 strain is manganese-dependent. FEMS Microbiol. Lett. 160:217-224. [DOI] [PubMed] [Google Scholar]
- 17.Gibson, C. M., and M. G. Caparon. 1996. Insertional inactivation of Streptococcus pyogenes sod suggests that prtF is regulated in response to a superoxide signal. J. Bacteriol. 178:4688-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson, C. M., T. C. Mallett, A. Claiborne, and M. G. Caparon. 2000. Contribution of NADH oxidase to aerobic metabolism of Streptococcus pyogenes. J. Bacteriol. 182:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths, B. B., and O. McClain. 1988. The role of iron in the growth and hemolysin (streptolysin S) production in Streptococcus pyogenes. J. Basic Microbiol. 28:427-436. [DOI] [PubMed] [Google Scholar]
- 20.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, C. F. 2001. ABC transporters: physiology, structure and mechanism—an overview. Res. Microbiol. 152:205-210. [DOI] [PubMed] [Google Scholar]
- 22.Inaoka, T., Y. Matsumura, and T. Tsuchido. 1999. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 181:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubovics, N. S., and H. F. Jenkinson. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709-1718. [DOI] [PubMed] [Google Scholar]
- 24.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2000. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38:140-153. [DOI] [PubMed] [Google Scholar]
- 25.Janulczyk, R., J. Pallon, and L. Björck. 1999. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol. Microbiol. 34:596-606. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson, H. F. 1994. Cell surface protein receptors in oral streptococci. FEMS Microbiol. Lett. 121:133-140. [DOI] [PubMed] [Google Scholar]
- 27.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 28.King, K. Y., J. A. Horenstein, and M. G. Caparon. 2000. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J. Bacteriol. 182:5290-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 68:4441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitten, T., C. L. Munro, A. Wang, and F. L. Macrina. 2002. Vaccination with FimA from Streptococcus parasanguis protects rats from endocarditis caused by other viridans streptococci. Infect. Immun. 70:422-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolenbrander, P. E., R. N. Andersen, R. A. Baker, and H. F. Jenkinson. 1998. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J. Bacteriol. 180:290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The structure of pneumococcal PsaA. Structure 6:1553-1561. [DOI] [PubMed] [Google Scholar]
- 33.Lee, Y. H., R. K. Deka, M. V. Norgard, J. D. Radolf, and C. A. Hasemann. 1999. Treponema pallidum TroA is a periplasmic zinc-binding protein with a helical backbone. Nat. Struct. Biol. 6:628-633. [DOI] [PubMed] [Google Scholar]
- 34.Lei, B., L. M. Smoot, H. M. Menning, J. M. Voyich, S. V. Kala, F. R. Deleo, S. D. Reid, and J. M. Musser. 2002. Identification and characterization of a novel heme-associated cell surface protein made by Streptococcus pyogenes. Infect. Immun. 70:4494-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe, A. M., P. A. Lambert, and A. W. Smith. 1995. Cloning of an Enterococcus faecalis endocarditis antigen: homology with adhesins from some oral streptococci. Infect. Immun. 63:703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin, M. E., R. C. Strachan, H. Aranha, S. L. Evans, M. L. Salin, B. Welch, J. E. Arceneaux, and B. R. Byers. 1984. Oxygen toxicity in Streptococcus mutans: manganese, iron, and superoxide dismutase. J. Bacteriol. 159:745-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIver, K. S., A. S. Heath, and J. R. Scott. 1995. Regulation of virulence by environmental signals in group A streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect. Immun. 63:4540-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson, N. 1999. Metal ion transporters and homeostasis. EMBO J. 18:4361-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niven, D. F., A. Ekins, and A. A. al-Samaurai. 1999. Effects of iron and manganese availability on growth and production of superoxide dismutase by Streptococcus suis. Can. J. Microbiol. 45:1027-1032. [DOI] [PubMed] [Google Scholar]
- 41.Patzer, S. I., and K. Hantke. 2001. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J. Bacteriol. 183:4806-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podbielski, A., B. Spellerberg, M. Woischnik, B. Pohl, and R. Lütticken. 1996. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS). Gene 177:137-147. [DOI] [PubMed] [Google Scholar]
- 43.Poyart, C., E. Pellegrini, O. Gaillot, C. Boumaila, M. Baptista, and P. Trieu-Cuot. 2001. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect. Immun. 69:5098-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raeder, R., and M. D. Boyle. 1993. Association between expression of immunoglobulin G-binding proteins by group A streptococci and virulence in a mouse skin infection model. Infect. Immun. 61:1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 46.Ricci, S., R. Janulczyk, and L. Björck. 2002. The regulator PerR is involved in oxidative stress response and iron homeostasis and is necessary for full virulence of Streptococcus pyogenes. Infect. Immun. 70:4968-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwede, T., A. Diemand, N. Guex, and M. C. Peitsch. 2000. Protein structure computing in the genomic era. Res. Microbiol. 151:107-112. [DOI] [PubMed] [Google Scholar]
- 48.Singh, K. V., T. M. Coque, G. M. Weinstock, and B. E. Murray. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323-331. [DOI] [PubMed] [Google Scholar]
- 49.Smoot, L. M., J. C. Smoot, M. R. Graham, G. A. Somerville, D. E. Sturdevant, C. A. Migliaccio, G. L. Sylva, and J. M. Musser. 2001. Global differential gene expression in response to growth temperature alteration in group A streptococcus. Proc. Natl. Acad. Sci. USA 98:10416-10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spatafora, G., M. Moore, S. Landgren, E. Stonehouse, and S. Michalek. 2001. Expression of Streptococcus mutans fimA is iron-responsive and regulated by a DtxR homologue. Microbiology 147:1599-1610. [DOI] [PubMed] [Google Scholar]
- 51.Spatafora, G., N. Van Hoeven, K. Wagner, and P. Fives-Taylor. 2002. Evidence that ORF3 at the Streptococcus parasanguis fimA locus encodes a thiol-specific antioxidant. Microbiology 148:755-762. [DOI] [PubMed] [Google Scholar]
- 52.Sutcliffe, I. C., and R. R. B. Russell. 1995. Lipoproteins of gram-positive bacteria. J. Bacteriol. 177:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talkington, D. T., B. G. Brown, J. A. Tharpe, A. Koenig, and H. Russell. 1996. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesins A (psaA). Microb. Pathog. 21:17-22. [DOI] [PubMed] [Google Scholar]
- 54.Viscount, H. B., C. L. Munro, D. Burnette-Curley, D. L. Peterson, and F. L. Macrina. 1997. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect. Immun. 65:994-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yesilkaya, H., A. Kadioglu, N. Gingles, J. E. Alexander, T. J. Mitchell, and P. W. Andrew. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]