Abstract
Cystic fibrosis (CF) is characterized by airway inflammation and chronic bacterial lung infection, most commonly with Pseudomonas aeruginosa, an opportunistic human pathogen. Despite the persistent airway inflammation observed in patients with CF, although phagocyte inducible nitric oxide synthase (iNOS) production is upregulated, expression of iNOS in the respiratory epithelium is markedly reduced. Given the antimicrobial action of NO, this may contribute to the chronic airway infection of this disease. To define the role of epithelium-derived NO in airway defense against P. aeruginosa, we infected differentiated human bronchial epithelial cells derived from a patient with CF (CFBE41o- cells) with different strains of this pathogen at low multiplicities of infection. Using cells transfected with human iNOS cDNA, we studied the effect of NO on P. aeruginosa replication, adherence, and internalization. P. aeruginosa adherence to iNOS-expressing cells was reduced by 44 to 72% (P = 0.02) compared with control values. Absolute P. aeruginosa uptake into these cells was reduced by 44%, but uptake expressed as a percentage of adherent bacteria did not differ from the control uptake. Survival of P. aeruginosa within iNOS-expressing cells was reduced at late times postinfection (P = 0.034). NO production did not alter host cell viability. NO production reduced P. aeruginosa adherence to human bronchial epithelial cells and enhanced killing of internalized bacteria, suggesting that a lack of epithelial iNOS in patients with CF may contribute to P. aeruginosa infection and colonization.
Cystic fibrosis (CF) is the most common inherited lethal disorder of Caucasian populations. This disease arises from mutations in the CF transmembrane conductance regulator (CFTR) gene (39) that lead to electrolyte imbalance in epithelial cell secretions and mucous plugging in affected organs. CF is characterized by a chronic bacterial lung infection which causes death in over 90% of individuals with CF (7). In infancy and early childhood, airway infection is usually caused by Staphylococcus aureus and Haemophilus influenzae. Later, chronic infection with the opportunistic pathogen Pseudomonas aeruginosa predominates and is present in 80% of individuals with CF (25).
Nitric oxide (NO) is a free radical gas with important signaling and antimicrobial actions (2, 32). NO is synthesized from l-arginine by NO synthase (NOS), of which there are three isoforms. Two of these, endothelial NOS and neuronal NOS, are constitutively expressed and produce small, nanomolar quantities of NO. The third NOS isoform, inducible NOS (iNOS), is expressed maximally following an inflammatory stimulus and produces relatively large, micromolar, quantities of NO. Although all three NOS isoforms can be expressed in normal lung tissue (11, 47), it is NO produced by iNOS which has antimicrobial activity against a number of bacteria, fungi, protozoans, and helminths (12, 46). iNOS in the lung can be expressed by macrophages, neutrophils, and bronchial epithelial cells. iNOS expression in these cells is upregulated by lipopolysaccharide (LPS) and by proinflammatory cytokines, such as interleukin-1, tumor necrosis factor alpha, and gamma interferon (43). In contrast, inflammatory stimuli, such as LPS, markedly suppress or leave unchanged epithelial expression of the other two isoforms (11). Upregulation of iNOS expression is also seen in airway inflammation in the respiratory epithelium of patients with inflammatory lung conditions, such as asthma (22) and bronchiectasis (30). Increased iNOS expression correlates with elevated NO concentrations in exhaled air. In bronchiectasis, this has been related to the extent of disease (26). iNOS is also constitutively expressed in human nasal and tracheal epithelial cells (19, 24). It is possible that low-level basal iNOS expression is related to the continued exposure of airway epithelium to inhaled microbes.
Given the observed upregulation of epithelial iNOS in asthma and bronchiectasis, it is surprising that in the airways of patients with CF, which are characterized by chronic severe inflammation, the amount of exhaled NO is not increased (50) and the expression of epithelial iNOS is reduced. In bronchial explants derived from patients with CF, iNOS expression is reduced in bronchial epithelial cells, although it was normal in infiltrating inflammatory cells (24, 30). Aberrant iNOS expression in intestinal epithelial cells can be restored in mice with CF engineered to express CFTR in the intestinal epithelium (49). As NO has antimicrobial activity and augments mucociliary clearance (23), a lack of NO production in the bronchial epithelium in patients with CF may contribute to the chronic airway infection characteristic of this disease.
There have been limited studies on the role of NO in host defense against P. aeruginosa. In mice, iNOS inhibition with S-methyl-isothiourea increases P. aeruginosa growth in excised lungs (24) and lung homogenates from infected animals (45). In both of these studies, bacterial quantification postinfection was performed with whole lungs, and thus the relative contributions of phagocyte and epithelial NO production were not determined. Given the normal expression of iNOS in phagocytes from patients with CF (30), these studies did not address the role that a lack of epithelial NO production in individuals with CF might have in bacterial clearance. In one study the workers examined the effect of epithelial iNOS expression on P. aeruginosa infection of human nasal turbinate explants from individuals who did not have CF (9). Bacteria were added at a high multiplicity of infection (MOI), and although iNOS inhibition reduced P. aeruginosa binding, this may have been due to the reduction in epithelial damage due to inhibition of NO production.
In order to determine the role of epithelial iNOS production in airway defense against P. aeruginosa, we developed a novel in vitro model of respiratory infection that mimicked the in vivo setting as closely as possible. We infected human bronchial epithelial cells from a patient with CF with P. aeruginosa at a low MOI in a 2-μl droplet. We transfected these cells with human iNOS cDNA to examine the effect of NO on P. aeruginosa replication, adherence, and internalization into bronchial epithelial cells derived from a patient with CF and the effect of these processes and of NO on host cell viability. We found that the P. aeruginosa adherence to iNOS-expressing CF transfectants is significantly reduced compared to the adherence in control cells. In contrast to other reports (35), we found that there was internalization of different P. aeruginosa strains into these epithelial cells from a patient with CF (Darling and Evans, submitted for publication). iNOS expression did not alter the degree of internalization of adherent P. aeruginosa. However, the survival of internalized P. aeruginosa within the cytoplasm of iNOS-expressing cells at late times following infection was reduced. As bacterial binding to epithelial cells is the first step required to establish an infection, the fact that epithelial NO production reduced P. aeruginosa binding suggests that it is important in airway defense against this organism. The lack of epithelial iNOS in the airways of patients with CF may contribute to increased P. aeruginosa adherence and colonization.
MATERIALS AND METHODS
Bronchial epithelial cells.
A human respiratory epithelial cell line, CFBE41o- (17), derived from a CF patient homozygous for mutant CFTRΔF508, was a kind gift from Dieter Gruenert, University of Vermont. The cells were maintained at 37°C in a humidified atmosphere consisting of 95% air and 5% CO2 in RPMI 1640 medium supplemented with 10% fetal calf serum (Labtech International, Ringmer, United Kingdom) and 2 mM l-glutamine. Membranous CFTR, as determined by immunohistochemical staining, was absent in these cells.
Construct formation and epithelial cell transfection.
Human iNOS cDNA, cloned into the EcoRI site of the vector pBSIISK+, was provided by I. Charles, University College London, London, United Kingdom (4). The iNOS cDNA insert was cut from this vector with XbaI (removing the 5′-terminal 66 bp not involved in iNOS expression) and cloned into the XbaI site of pCI-neo (Promega, Southampton, United Kingdom), a mammalian expression vector which carries the human cytomegalovirus immediate-early enhancer-promoter region. A control construct was made by cloning a noncoding cDNA into the same site of the pCI-neo vector.
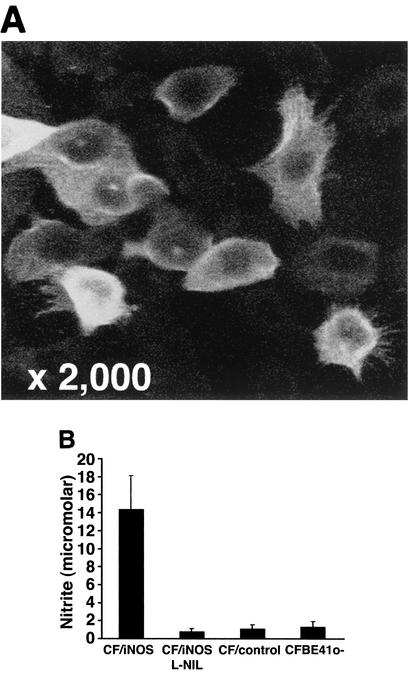
CFBE41o- cells were transfected with iNOS cDNA and with the noncoding plasmid to produce the CF/iNOS and CF/control cell populations, respectively, by using FuGENE 6 (Roche Diagnostics Ltd., Lewes, United Kingdom). Transfected cells were grown to confluence and cloned twice by limiting dilution. iNOS expression and NO production were measured following 48 h of incubation with sodium butyrate (5 mM), an inhibitor of histone deacetylation. iNOS expression was demonstrated by using immunohistochemistry and confocal microscopy (see below). NO production was measured by determining nitrite concentrations in 500 μl of culture supernatant based on the Griess reaction, as previously described (18). CF/iNOS clones were selected on the basis of iNOS expression in over 50% of the cells. Cells at passages 6 to 19 were used for infection experiments.
Culture conditions.
Untransfected CFBE41o- cells and stably transfected clones were differentiated by growth at an air interface as previously described (6). Briefly, cells from each population were seeded at a density of approximately 105 cells/cm2 into wells with porous membrane inserts (Anopore; pore size, 0.02 μm; diameter, 10 mm; Life Technologies, Paisley, United Kingdom). They were maintained in RPMI 1640 medium added above and below the porous insert. At confluence, the apical cell surface was exposed to air, leaving medium in only the basolateral chamber. At this point, the medium was changed to serum-free bronchial epithelial growth medium, made up of bronchial epithelial basal medium (Clonetics, Biowhittaker, Invitrogen, Workingham, United Kingdom) diluted 50:50 (vol/vol) with Dulbecco modified Eagle medium and supplemented according to the manufacturer's instructions. Intercellular tight junction integrity was assessed by measuring transepithelial resistance (TER) with an EVOM epithelial voltohmmeter (World Precision Instruments Incorporated, Sarasota, Fla.). TER readings were obtained across membranes with no cells, and the values were subtracted from the values obtained when cells were present. In addition, cells were examined by immunochemistry for the basolateral intercellular junction component E-cadherin (see below).
Confocal and fluorescence microscopy.
All steps were conducted at room temperature. Cells were washed three times with phosphate-buffered saline (PBS), fixed with 1% (vol/vol) paraformaldehyde in PBS for 30 min, and permeabilized with 0.2% Triton X-100 for 15 min. After blocking in 10% normal goat serum, iNOS monoclonal antibody (N39120; Transduction Laboratories, Lexington, Ky.) diluted 1:200 or E-cadherin monoclonal antibody (C20820; Transduction Laboratories) diluted 1:150 was applied for 4 h at room temperature or overnight at 4°C. Cells were counterstained without amplification for 1 h by using fluorescein isothiocyanate-goat anti-mouse immunoglobulin G (Molecular Probes, Leiden, The Netherlands) diluted 1:500 in PBS containing 0.1% bovine serum albumin. Cells were washed three times with PBS for 5 min between stages. After staining, the cells were mounted in Vectashield (Vector Laboratories Ltd., Peterborough, United Kingdom) for microscopy. Slides were examined by conventional fluorescence microscopy by using a Nikon Eclipse E600 (Nikon Ltd., Surrey, United Kingdom). Confocal microscopy was performed with a Bio-Rad MRC 1024 by using a Zeiss Axiovert microscope running with LaserSharp software.
Scanning electron microscopy was performed by washing cells twice with PBS and prefixing them at room temperature in 3% electron microscopy-grade glutaraldehyde in cacodylate buffer (pH 7.2) for 1 to 2 h. They were transferred to fresh cacodylate buffer and then postfixed in 1% osmium tetroxide, dehydrated through a graded series of alcohols, and critical point dried with liquid carbon dioxide. The cells were mounted on aluminum stubs, coated with gold, and examined by using a Cambridge Stereoscan S360 scanning electron microscope at an accelerating voltage of 1.5 kV.
Bacterial strains.
P. aeruginosa laboratory type strain PAO1 and a number of clinical isolates identified by standard tests were used. The clinical isolates were obtained from blood (PA1, PA3, and PA4), pus (PA5 and PA6), urine (PA2 and PA7), and sputum of a patient with CF (J1385). J1385 was a kind gift from J. Govan, University of Edinburgh, Edinburgh, United Kingdom. All strains had a mucoid phenotype.
Experimental design.
Infection studies were conducted in Anopore tissue culture wells by using untransfected CFBE41o- cells, two CF/iNOS clones, and two CF/control clones. Cell differentiation prior to infection was maximized by culture at an air interface for 10 to 14 days. At 48 h prior to infection, CF/iNOS and CF/control clones were incubated with 5 mM sodium butyrate to maximize protein expression. Such expression was present only in the basolateral chamber and was washed away immediately before the infection process was initiated. At 24 h prior to infection, a subset of CF/iNOS clones in wells were also incubated with the iNOS inhibitor l-N6-(1-iminoethyl)lysine hydrochloride (l-NIL) (Calbiochem-Novabiochem Limited, Nottingham, United Kingdom) at a concentration of 1 mM. Experiments were performed with two different CF/iNOS and CF/control clones.
Each strain was cultured in Luria-Bertani liquid medium at 37°C with agitation to the mid-logarithmic phase (optical density at 600 nm, 0.4). The cells were washed twice in sterile PBS and resuspended in tissue culture medium at a concentration of approximately 105 CFU/ml. A 2-μl droplet of this suspension, corresponding to approximately 200 CFU as measured by dilution plating, was added to the apical surface of the cells. The MOI with this inoculum was calculated by counting the number of epithelial cells in an uninfected well. The cell surface was washed with PBS, and adherent cells were detached by using 0.5 g of trypsin per liter along with 0.2 g of EDTA per liter in Hanks balanced salt solution for 20 min. Detached cells were pooled with cells obtained from washing and counted with a hemocytometer.
Replication of cell surface bacteria.
At 30 min and 3 and 6 h after infection with the laboratory type strain PAO1, the surface of each cell population was washed three times with sterile PBS. The wash suspensions were pooled and plated by using serial dilutions on Columbia blood agar, and viable CFU were counted. PAO1 replication was measured on three separate occasions by using two wells per cell type, with wash suspensions from each well additionally plated in duplicate.
Adherence and internalization assays.
After various times of cocultivation, the cell surface was washed three times with sterile PBS. Cell-associated bacteria were quantified by lysing the washed cells in sterile 0.5% Triton X-100 for 30 min at room temperature and counting viable CFU as described above.
Six hours following cocultivation, internalization was assessed by a gentamicin exclusion assay as previously described (14). Briefly, the cells were washed three times with sterile PBS and incubated with tissue culture medium containing 150 μg gentamicin per ml, which was more than 10 times the highest MIC for the different P. aeruginosa strains. At 7, 10, 15, and 24 h postinfection (1, 4, 9, and 18 h after the gentamicin treatment began, respectively), the gentamicin-containing medium was removed, and the cells were washed with sterile PBS. The cells were then lysed with Triton X-100, and serial dilutions of the cell lysates were plated as described above. Each association or internalization assay was performed in duplicate wells, and plating from each well was performed in duplicate. Each infection experiment was performed on three occasions. The percentage of uptake was determined as follows: (intracellular CFU/cell-associated CFU) × 100.
Cytotoxicity.
Cytotoxicity was determined by determining the release of lactate dehydrogenase (LDH) into culture supernatants by using a colorimetric assay (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions; 100% cytotoxicity was defined on the basis of the amount of LDH released in the presence of 2% Triton X-100.
Statistical analysis.
The mean and standard error of the mean were calculated for each cell population. The data were analyzed by Student's t test (two tailed), and a P value of <0.05 was considered significant.
RESULTS
Epithelial cell model.
In order to produce a model of human respiratory epithelial infection, we cultured the bronchial epithelial cell line CFBE41o- derived from a patient with CF on semipermeable supports and allowed differentiation to occur at an air interface for 10 to 14 days. Under these conditions, confluent cells produced numerous microvilli and small cilia (Fig. 1), as previously described (6). The cell layers formed a tight epithelial sheet with a TER value of 284.17 ± 23.74 Ω · cm2 (mean ± standard error of the mean; adjusted for background levels), a value similar to the value measured previously in a cell line derived from an individual who did not have CF (51).
FIG. 1.
Scanning electron micrograph of confluent differentiated CFBE41o- cells, showing microvilli and cilia (arrowheads).
To determine the effect of NO on P. aeruginosa infection of such monolayers, we established stable transfectants of this cell line expressing human iNOS. To maximize iNOS expression in these transfectants, we treated cells with sodium butyrate, an agent that inhibits histone deacetylation, increasing transcriptional activity. There was no significant difference in TER between untransfected and transfected cells, and measurements for transfectants were not changed by sodium butyrate treatment (Fig. 2A). The distribution of the intercellular adhesion molecule E-cadherin for the parent cell line and the distribution of E-cadherin for butyrate-treated transfectants were not different (Fig. 2B). CFBE41o- and CF/control cells did not express iNOS either constitutively or following treatment with proinflammatory cytokines, in keeping with previous studies with the CFBE41o- cell line (30). In addition, neither cell line expressed iNOS following infection with P. aeruginosa (data not shown). Following 48 h of incubation with 5 mM sodium butyrate, about 50% of the polarized CF/iNOS cells exhibited iNOS expression (Fig. 3A). This butyrate treatment had no effect on iNOS expression in CFBE41o- or CF/control cells, which remained undetectable by immunofluorescence and by NO output (Fig. 3B and data not shown). Importantly, the butyrate treatment had no effect on CFTR expression in either the parent cell line CFBE41o- or the transfected lines (CF/control or CF/iNOS) (data not shown). Confocal micrographs of these cells revealed that iNOS expression was apical, a feature of iNOS expression in a number of different epithelial cells that had both respiratory and renal origins (16). The implications of this subcellular distribution of iNOS for the effects of NO on bacterial entry are discussed below. Nitrite was detectable in CF/iNOS cell culture supernatants after 48 h of incubation with sodium butyrate but not in culture supernatants of CFBE41o- or CF/control cells similarly treated with butyrate (Fig. 3B). The nitrite levels produced by CF/iNOS cells were comparable to the levels previously described for A549 cells following stimulation with the proinflammatory cytokines tumor necrosis factor alpha, interleukin-1β, and gamma interferon (40). The NO output of CF/iNOS cells was reduced to control values following incubation with l-NIL (Fig. 3B). We were unable to detect the neuronal or endothelial isoforms of NOS in these cells in the presence or absence of butyrate (immunofluorescence assay) (data not shown).
FIG. 2.
Tight junction integrity of CFBE41o- cells and transfectants. (A) TER measured in cells cultured at an air interface for 12 days. TER was measured in transfectants both before (pre) and after (post) 48 h of incubation with sodium butyrate. The results are expressed as the means of triplicate measurements. The error bars indicate standard errors of the means. (B) Confocal photomicrographs of E-cadherin-specific immunostaining in the butyrate-treated cell populations.
FIG. 3.
Epithelial cell iNOS expression and NO production. (A) Confocal micrograph of iNOS-specific immunostaining in differentiated CF/iNOS transfectants. (B) Nitrite levels in supernatants from different differentiated cells. l-NIL was added where indicated. Transfected cells were treated for 48 h with 5 mM sodium butyrate. The results are expressed as means of triplicate measurements. The error bars indicate standard errors of the means.
Epithelial cell infection.
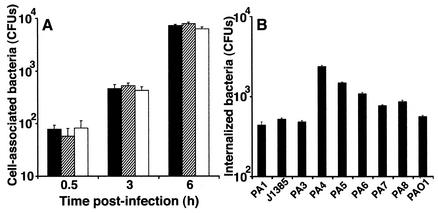
We developed an infection model in differentiated CFBE41o- cells using the laboratory type strain P. aeruginosa PAO1. We inoculated each 10-mm-diameter epithelial sheet with ∼200 CFU of P. aeruginosa, which corresponded to an MOI of 1 bacterium per 5 × 103 cells. Bacteria were added in 2 μl of growth medium to minimize the effects on the composition of the airway surface liquid. The numbers of cell-associated bacteria were initially measured at 30 min and 3 and 6 h postinfection. Over this period, the numbers of cell-associated bacteria steadily increased for all P. aeruginosa strains tested, including J1385 (Fig. 4A and data not shown). Surprisingly, at 6 h after infection, we found internalization of P. aeruginosa, which we quantified using a gentamicin protection assay. Reproducible internalization did not occur before 6 h, and the values were different for different P. aeruginosa strains (Fig. 4B). The fraction of internalized bacteria expressed as a percentage of the total cell-associated bacteria varied between 1.2 and 14%. This fraction was similar to that observed by Pier and coworkers for P. aeruginosa internalization into cells with wild-type CFTR (35).
FIG. 4.
Cell association and internalization of P. aeruginosa strains following infection of CFBE41o- cells. (A) Cell association expressed as means of three separate experiments, in which measurements at each time were taken from two wells per cell type and lysates from each well were also plated in duplicate. The error bars indicate standard errors of the means. Solid bars, strain PAO1; cross-hatched bars, strain PA3; open bars, strain PA4. (B) Internalization of different P. aeruginosa strains into CFBE41o- cells at 6 h postinfection. The percentages of internalization were markedly different for different strains. The results are expressed as the means of three separate experiments. The error bars indicate standard errors of the means.
Effect of NO production from iNOS on P. aeruginosa adherence and internalization.
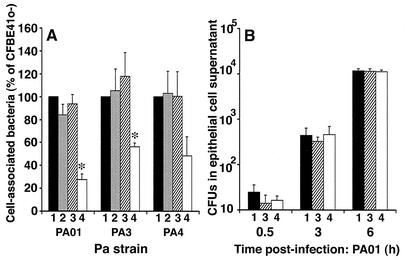
The association of three P. aeruginosa strains with the epithelial sheet was measured at 6 h postinfection, and internalization was measured 1 h later, following a 1-h incubation with gentamicin. We compared bacterial association and internalization in the parent line CFBE41o- and in the stably transfected CF/control and CF/iNOS cell lines; both of the transfected cell lines were treated with butyrate for maximal gene expression, as described in Materials and Methods. The cell association of all bacterial strains with two different CF/iNOS clones was reduced compared to the association with CFBE41o- cells and with two different CF/control clones; the reduction was significant for strains PAO1 (P = 0.009) and PA3 (P = 0.037) (Fig. 5A). To confirm that the observed changes in the iNOS-transfected cells were due to the production of NO, we added the NOS inhibitor l-NIL to the cells. Treatment of CF/iNOS cells with l-NIL eliminated the reduced cell association of P. aeruginosa (Fig. 5A). This suggested that NO either inhibited the growth of the different P. aeruginosa strains over the 6-h period or directly influenced bacterial adherence to the epithelial surface or the subsequent internalization. In order to distinguish between these possibilities, we measured the numbers of nonadherent bacteria recovered from the epithelial cells over the 6-h period. This bacterial population accumulated over time, but iNOS expression had no effect on the replication rate at any of the times studied (Fig. 5B). Thus, NO production did not influence the multiplication of bacteria at the cell surface but directly inhibited adherence or internalization.
FIG. 5.
(A) Effect of production of NO on adherence of P. aeruginosa (Pa) strains PAO1, PA3, and PA4. Cell-associated bacteria were measured at 6 h postinfection of CFBE41o- cells (bars 1), CF/control cells (bars 2), CF/iNOS cells in the presence of l-NIL (bars 3), and CF/iNOS cells alone (bars 4), and the data are expressed as percentages of the values observed with CFBE41o- cells. Association of P. aeruginosa with CF/iNOS transfectants in the absence of l-NIL was reduced for all three strains and was significant for PAO1 and PA3, as indicated by an asterisk (P < 0.05, as determined by Student's t test). Both the CF/control and CF/iNOS cells were treated with butyrate prior to infection as described in Materials and Methods. (B) Laboratory type strain PAO1 isolated from epithelial cell wash suspensions up to 6 h postinfection. The results are expressed as the means of three separate experiments, in which measurements at each time were taken from two wells per cell type and lysates were also plated in duplicate. The cell types were as described above for panel A. The error bars indicate standard errors of the means. The data for transfected cells are from one clone; similar results were obtained with an additional iNOS-expressing clone.
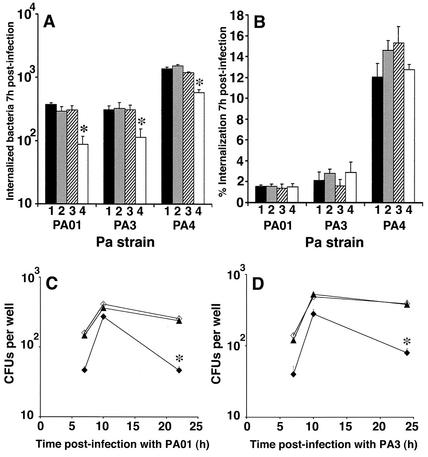
To determine which of these processes was altered by NO, we used a gentamicin protection assay to measure absolute internalization into the CF/iNOS clone and controls. There were significant reductions in absolute internalization for all three P. aeruginosa strains in the CF/iNOS cells compared to the controls (Fig. 6A); this effect was eliminated by treatment with l-NIL. However, the uptake expressed as a percentage of cell-associated bacteria did not differ from the control values (Fig. 6B). Thus, we concluded that NO affects the adherence of bacteria to the epithelial cell surface and does not influence the process of bacterial internalization.
FIG. 6.
(A) Effect of NO production on absolute P. aeruginosa (Pa) internalization into CFBE41o- cells (bars 1), CF/control cells (bars 2), CF/iNOS cells in the presence of l-NIL (bars 3), and CF/iNOS cells alone (bars 4). An asterisk indicates that there was a significant reduction compared to the values for CFBE41o- cells (P < 0.05, as determined by Student's t test). (B) P. aeruginosa internalization expressed as a percentage of the cell-associated bacteria measured 7 h postinfection (6 h following bacterial inoculation plus 1 h of incubation with 150 μg of gentamicin per ml). The results are expressed as the means of three separate experiments, in which measurements at each time were taken from two wells per cell type and lysates were also plated in duplicate. The error bars indicate standard errors of the means. (C and D) Intracellular survival of strains PAO1 (C) and PA3 (D) in CFBE41o- cells and in CF/iNOS cells in the presence and absence of l-NIL (triangles, open diamonds, and solid diamonds, respectively). The results are expressed as the means of three separate experiments. The error bars indicate standard errors of the means. An asterisk indicates that a value is significantly different from the control values (P < 0.04, as determined by Student's t test).
We also studied P. aeruginosa binding and uptake in the 16HBE14o- respiratory epithelial line, which expresses wild-type CFTR. When the same model of infection was used, no internalization of bacteria was found in these cells. In a subsequent analysis of the role of CFTR in mediating internalization of P. aeruginosa into the CFBE41o- cell line, using transfected wild-type CFTR, we found that CFTR did not affect bacterial adherence but inhibited internalization (Darling and Evans, submitted).
Effect of iNOS on bacterial and host cell fate.
We monitored the fate of the bacteria internalized 6 h after infection in the continued presence of gentamicin to remove extracellular organisms. In all cell populations, the number of internalized P. aeruginosa cells increased up to 10 h postinfection (Fig. 6C and D). At later times, the number of viable intracellular bacteria fell. The rate of intracellular killing was significantly higher in CF/iNOS cells than in controls for both the PA3 and PAO1 strains (Fig. 6C and D). Treatment with the NOS inhibitor l-NIL eliminated this effect (Fig. 6C and D).
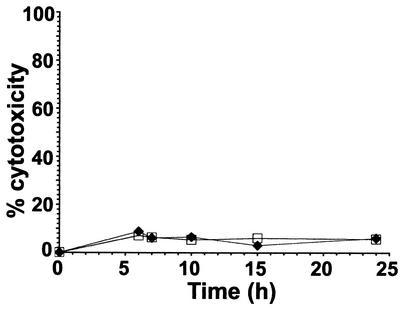
We attempted to measure host cell viability following infection using propidium iodide staining and flow cytometric analysis. However, production of a suspension of cells grown on semipermeable supports required prolonged digestion with trypsin, which led to extensive cell necrosis, even in uninfected cells. Thus, cell viability after infection was determined by measuring the LDH released into the growth medium (Fig. 7). This analysis showed that neither infection nor NO production significantly affected cell viability. The lack of an increase in the LDH level was in keeping with the macroscopic appearance and the results of E-cadherin-specific immunostaining, which remained unchanged up to 24 h postinfection (data not shown).
FIG. 7.
Viability of CF/iNOS cells in the presence (squares) and absence (diamonds) of l-NIL following infection with PA3, as assayed by LDH release from infected cells. The results are expressed as the percentages of cytotoxicity. No significant differences were observed either in the presence or in the absence of l-NIL.
DISCUSSION
Using a transfected human bronchial epithelial cell line that was derived from a patient with CF and constitutively expressed NO from iNOS, we demonstrated that the presence of NO results in a significant reduction in the binding of different P. aeruginosa strains to epithelial cells. NO did not affect the growth of extracellular bacteria. Absolute internalization was reduced, although P. aeruginosa uptake expressed as a percentage of cell-associated bacteria was not altered by iNOS. At later times following infection, iNOS transfectants exhibited enhanced killing of intracellular P. aeruginosa. The lack of epithelial iNOS in patients with CF that has been described (30) may thus have an important effect on colonization and the damage to the airways produced by P. aeruginosa.
Our novel in vitro model examines the initial contact of P. aeruginosa with respiratory epithelium from a patient with CF that might occur early in the course of this condition. Bacterial attachment to mucosal surfaces is the initial event in establishing infection and producing mucosal damage (21), and modification of this process is important in P. aeruginosa pathogenesis. NO did not influence the growth of nonadherent P. aeruginosa at the epithelial surface, and thus its effect on binding must be directed at P. aeruginosa adhesins or host cell receptors. A number of different pseudomonad proteins have been proposed as important adhesins for attachment to the surface of the respiratory epithelium. Pilin, the structural component of projecting surface pilus filaments, is believed to be an important surface protein of P. aeruginosa, mediating the initial contact with epithelial cells because of its extension from the cell surface (34). One study found that P. aeruginosa type 4 pilin accounts for about 90% of the adherence of P. aeruginosa to human lung pneumocyte A549 cells (5), and the binding to human endothelial cells by nonpiliated P. aeruginosa strains is significantly less than the binding by piliated P. aeruginosa strains (38). However, pili mediate twitching motility in P. aeruginosa, and removal of pilin reduces bacterial motility, possibly affecting the ability to reach the epithelial cell. This might account for the reduced binding seen in pilin-defective mutants. For example, in one study the workers found that loss of binding by pilin mutants to corneal epithelial cells could be overcome by centrifuging the bacteria onto the cells (15). The nonpilin P. aeruginosa adhesins include LPS (20) and alginate (8), although other workers have found that alginate can inhibit cell binding (29). In the early stage of infection, before chronic infection has been established, most clinical isolates from patients with CF are nonmucoid (34), and so the role of alginate in the initial P. aeruginosa infection is unclear. P. aeruginosa LPS has been observed to bind to membranous CFTR in cells from individuals who do not have CF (35) that are infected at a high MOI. In the model used in the work described here, we observed internalization of several P. aeruginosa strains into cells lacking surface CFTR. The percentages of adherent bacteria that were internalized were similar to the value obtained by Pier et al. for cells from individuals who did not have CF (35). There are clearly, therefore, additional mechanisms within respiratory epithelial cells for binding and internalization of P. aeruginosa that do not involve CFTR (Darling and Evans, submitted). AsialoGM1 may also play a role in P. aeruginosa adherence (42), although in the experiments described here asialoGM1 was not upregulated following exposure to P. aeruginosa supernatant and incubation with polyclonal anti-asialoGM1 antibody had no effect on bacterial binding (data not shown). Other proposed adhesins include Muc1 mucins (27), which are thought to bind to P. aeruginosa flagellin (28), the outer membrane protein OprF (1), and fibronectin and α5β1 integrin (41), as well as heparan sulfate (36). Which of these is predominant or important in in vivo infections is not clear.
We have recently demonstrated that epithelial iNOS is expressed in the apical compartment of polarized epithelial cells, in close association with the submembranous cortical actin cytoskeleton (16). In this location, it delivers vectorial NO output towards the apical face of the cell. This is clearly an ideal means of delivering highly reactive NO to invading pathogens while minimizing the potentially damaging effects of high intracellular NO concentrations. NO did not, however, have a detectable bactericidal effect on P. aeruginosa present on the apical surface of epithelial cells (Fig. 5B). Its specific effect on adherence must, therefore, result from an effect on bacterial adhesins or host cell receptors; this possibility is discussed further below.
The data in Fig. 5 and 6 show that NO reduces adherence of P. aeruginosa to human respiratory epithelial cells. It may be that NO, although necessary for this effect, is not on its own sufficient. NO can react with a variety of other molecules, including superoxide radical to yield peroxynitrite and thiols to give nitrosothiols. The use of butyrate to maximize iNOS gene expression should also increase transcription of a number of other genes, whose products might interact with NO. Further work will be required to identify other molecules, if any, that might interact with NO to inhibit pseudomonad adherence to respiratory epithelial cells and to determine under what conditions they are produced in human airway cells.
How might NO alter bacterial adhesins or host cell receptors? We propose that NO produces a chemical modification of either a key bacterial adhesin or a host cell receptor. Nitrosylation of protein-SH groups can alter function significantly (48). The S-nitrosylation status of hemoglobin, for example, confers the NO-responsive and -resistant conformations of this protein (48). Although the microbial targets of NO are still incompletely determined, the antimicrobial action of NO may result from modification of surface thiols or metal centers involved in critical enzymatic or regulatory functions. In Bacillus cereus, for example, nitrosylation of surface thiols has been implicated in the inhibition of spore outgrowth (31). In the parasite Leishmania infantum (44) and in human immunodeficiency virus type 1 (33), S-nitrosylation of a cysteine residue has been shown to inhibit proteases, suggesting that inactivation of cysteine proteases is a general mechanism of NO-related antimicrobial activity. In addition, NO can react with superoxide to generate peroxynitrite, which can mediate protein tyrosine nitration (10). Posttranslational nitrotyrosination of α-tubulin, for example, by reactive nitrogen species generated under pathophysiological conditions invokes conformational changes resulting in microtubule dysfunction and loss of epithelial barrier integrity (10). Nitrotyrosination may also have effects on microbial protein function and hence on pathogenesis. Further work should determine whether either or both of these reactions alter P. aeruginosa adhesins or cell surface receptors.
Following adhesion of P. aeruginosa to the surface of respiratory epithelial cells, bacteria can be internalized in a strain-dependent fashion (37; this study). It has been proposed that internalization is dependent on apical expression of CFTR and produces clearance of adherent P. aeruginosa, enhancing host defense (35). However, some patients with CF express a nonfunctional apical CFTR (e.g., G551D) that has the necessary domain to bind and internalize P. aeruginosa, and yet these patients have chronic colonization of the airways with P. aeruginosa as severe as that found in patients with no surface CFTR (e.g., ΔF508) (52). Moreover, we found internalization of P. aeruginosa in cells from a patient with CF (Fig. 4B) and prolonged survival of the bacteria within the cells (Fig. 6C and D) without host cell cytotoxicity (Fig. 7). Thus, P. aeruginosa internalization may be of benefit to the microbe; reduction of absolute numbers of internalized bacteria (Fig. 6A) may be an additional protective mechanism provided by epithelial NO production.
Following uptake of P. aeruginosa by bronchial epithelial cells, NO production was associated with a delayed increase in intracellular killing, evident at 24 h after infection (Fig. 6C and D). NO on its own may have a microbicidal effect within the cell, but this effect is considerably enhanced by the reaction of NO with superoxide to produce peroxynitrite (53). The reason for the delayed effect of NO production on intracellular bacterial growth is not clear. It may reflect a time lag in microbial killing following contact with NO or movement of bacteria from a protected intracellular site, such as a particular vesicle population. Microbial manipulation of intracellular NO delivery has been demonstrated following infection of macrophages with Salmonella enterica serovar Typhimurium. Following entry into the host cell, a type III secretion system encoded by this pathogen interferes with intracellular localization of iNOS and excludes peroxynitrite from bacterium-containing vacuoles (3).
Our finding that iNOS expression reduces P. aeruginosa adherence is contrary to the findings reported by Dowling and colleagues, who demonstrated that there was reduced P. aeruginosa binding with iNOS inhibition (9). This group infected nonpolarized primary human nasal cells from an individual who did not have CF with 9.5 × 106 ± 0.5 × 106 bacteria per well in 20 μl of broth. Epithelial damage was reduced with iNOS inhibition. NO autotoxicity has been described in infection models and airway inflammation (13), and damaged epithelium has been associated with increased P. aeruginosa binding to cells from patients with CF (37). Reduced P. aeruginosa binding associated with iNOS inhibition may have occurred secondary to the protection against epithelial damage. The high bacterial counts may also have overwhelmed the effect of NO to cause substantial modification of pathogen adhesins or host cell receptors and may have produced additional toxic effects that were not seen at a lower MOI. In our study, epithelial toxicity, as demonstrated by the supernatant LDH concentration (Fig. 7) and immunostaining for E-cadherin (data not shown), did not increase over time. Our findings are therefore not confounded by NO autotoxicity.
Our results suggest that infecting epithelial cells at a very low MOI allows study of NO activity without confounding toxicity effects that may occur with higher numbers of bacteria. We demonstrated that there was a reduction in P. aeruginosa adherence and intracellular survival in iNOS-expressing cells from a patient with CF. A lack of iNOS expression in respiratory epithelium from patients with CF (24, 30, 50) may favor P. aeruginosa binding and transfer of toxins secreted via the type III system. As bacterial adherence is an important initial step in establishing P. aeruginosa infection and as P. aeruginosa plays such a major role in the etiology of lung damage during CF, replacing epithelial NO in patients with CF might be of therapeutic benefit early in this disease.
Acknowledgments
We thank Dieter Gruenert, University of Vermont, for providing the cell line derived from a patient with CF, Ian Charles, University College London, for providing the human iNOS cDNA plasmid, John Govan, University of Edinburgh, for providing P. aeruginosa strain J1385, and Tim Ryder, Imperial College, for performing the scanning electron microscopy.
This work was supported by a research training fellowship from the Wellcome Trust to K.E.A.D., by the CF Trust, and by a Lister Institute Jenner fellowship to T.J.E.
Editor: V. J. DiRita
REFERENCES
- 1.Azghani, A. O., S. Idell, M. Bains, and R. E. Hancock. 2002. Pseudomonas aeruginosa outer membrane protein F is an adhesin in bacterial binding to lung epithelial cells in culture. Microb. Pathog. 33:109-114. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 3.Chakravortty, D., I. Hansen-Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles, I. G., R. M. Palmer, M. S. Hickery, M. T. Bayliss, A. P. Chubb, V. S. Hall, D. W. Moss, and S. Moncada. 1993. Cloning, characterization, and expression of a cDNA encoding an inducible nitric oxide synthase from the human chondrocyte. Proc. Natl. Acad. Sci. USA 90:11419-11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi, E., T. Mehl, D. Nunn, and S. Lory. 1991. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect. Immun. 59:822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cozens, A. L., M. J. Yezzi, K. Kunzelmann, T. Ohrui, L. Chin, K. Eng, W. E. Finkbeiner, J. H. Widdicombe, and D. C. Gruenert. 1994. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 10:38-47. [DOI] [PubMed] [Google Scholar]
- 7.Davies, J., A. Dewar, A. Bush, T. Pitt, D. Gruenert, D. M. Geddes, and E. W. Alton. 1999. Reduction in the adherence of Pseudomonas aeruginosa to native cystic fibrosis epithelium with anti-asialoGM1 antibody and neuraminidase inhibition. Eur. Respir. J. 13:565-570. [DOI] [PubMed] [Google Scholar]
- 8.Doig, P., N. R. Smith, T. Todd, and R. T. Irvin. 1987. Characterization of the binding of Pseudomonas aeruginosa alginate to human epithelial cells. Infect. Immun. 55:1517-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowling, R. B., R. Newton, A. Robichaud, P. J. Cole, P. J. Barnes, and R. Wilson. 1998. Effect of inhibition of nitric oxide synthase on Pseudomonas aeruginosa infection of respiratory mucosa in vitro. Am. J. Respir. Cell. Mol. Biol. 19:950-958. [DOI] [PubMed] [Google Scholar]
- 10.Eiserich, J. P., A. G. Estevez, T. V. Bamberg, Y. Z. Ye, P. H. Chumley, J. S. Beckman, and B. A. Freeman. 1999. Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc. Natl. Acad. Sci. USA 96:6365-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ermert, M., C. Ruppert, A. Gunther, H. R. Duncker, W. Seeger, and L. Ermert. 2002. Cell-specific nitric oxide synthase-isoenzyme expression and regulation in response to endotoxin in intact rat lungs. Lab. Investig. 82:425-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang, F. C. 1997. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flak, T. A., and W. E. Goldman. 1996. Autotoxicity of nitric oxide in airway disease. Am. J. Respir. Crit. Care Med. 154:S202-S206. [DOI] [PubMed] [Google Scholar]
- 14.Fleiszig, S. M., D. J. Evans, N. Do, V. Vallas, S. Shin, and K. E. Mostov. 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65:2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleiszig, S. M., T. S. Zaidi, and G. B. Pier. 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63:4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glynne, P. A., K. E. Darling, J. Picot, and T. J. Evans. 2002. Epithelial inducible nitric-oxide synthase is an apical EBP50-binding protein that directs vectorial nitric oxide output. J. Biol. Chem. 277:33132-33138. [DOI] [PubMed] [Google Scholar]
- 17.Goncz, K. K., L. Feeney, and D. C. Gruenert. 1999. Differential sensitivity of normal and cystic fibrosis airway epithelial cells to epinephrine. Br. J. Pharmacol. 128:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 19.Guo, F. H., H. R. De Raeve, T. W. Rice, D. J. Stuehr, F. B. Thunnissen, and S. C. Erzurum. 1995. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc. Natl. Acad. Sci. USA 92:7809-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta, S. K., R. S. Berk, S. Masinick, and L. D. Hazlett. 1994. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect. Immun. 62:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn, H. P. 1997. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene 192:99-108. [DOI] [PubMed] [Google Scholar]
- 22.Hamid, Q., D. R. Springall, V. Riveros-Moreno, P. Chanez, P. Howarth, A. Redington, J. Bousquet, P. Godard, S. Holgate, and J. M. Polak. 1993. Induction of nitric oxide synthase in asthma. Lancet 342:1510-1513. [DOI] [PubMed] [Google Scholar]
- 23.Jain, B., I. Rubinstein, R. A. Robbins, K. L. Leise, and J. H. Sisson. 1993. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem. Biophys. Res. Commun. 191:83-88. [DOI] [PubMed] [Google Scholar]
- 24.Kelley, T. J., and M. L. Drumm. 1998. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J. Clin. Investig. 102:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerem, E., M. Corey, R. Gold, and H. Levison. 1990. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J. Pediatr. 116:714-719. [DOI] [PubMed] [Google Scholar]
- 26.Kharitonov, S. A., A. U. Wells, B. J. O'Connor, P. J. Cole, D. M. Hansell, R. B. Logan-Sinclair, and P. J. Barnes. 1995. Elevated levels of exhaled nitric oxide in bronchiectasis. Am. J. Respir. Crit. Care Med. 151:1889-1893. [DOI] [PubMed] [Google Scholar]
- 27.Lillehoj, E. P., S. W. Hyun, B. T. Kim, X. G. Zhang, D. I. Lee, S. Rowland, and K. C. Kim. 2001. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L181-L187. [DOI] [PubMed] [Google Scholar]
- 28.Lillehoj, E. P., B. T. Kim, and K. C. Kim. 2002. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am. J. Physiol. Lung Cell. Mol. Physiol. 282:L751-L756. [DOI] [PubMed] [Google Scholar]
- 29.Massengale, A. R., F. J. Quinn, A. Williams, S. Gallagher, and S. C. Aronoff. 2000. The effect of alginate on the invasion of cystic fibrosis respiratory epithelial cells by clinical isolates of Pseudomonas aeruginosa. Exp. Lung Res. 26:163-178. [DOI] [PubMed] [Google Scholar]
- 30.Meng, Q. H., D. R. Springall, A. E. Bishop, K. Morgan, T. J. Evans, S. Habib, D. C. Gruenert, K. M. Gyi, M. E. Hodson, M. H. Yacoub, and J. M. Polak. 1998. Lack of inducible nitric oxide synthase in bronchial epithelium: a possible mechanism of susceptibility to infection in cystic fibrosis. J. Pathol. 184:323-331. [DOI] [PubMed] [Google Scholar]
- 31.Morris, S. L., R. C. Walsh, and J. N. Hansen. 1984. Identification and characterization of some bacterial membrane sulfhydryl groups which are targets of bacteriostatic and antibiotic action. J. Biol. Chem. 259:13590-13594. [PubMed] [Google Scholar]
- 32.Murad, F. 1998. Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone? Recent Prog. Horm. Res. 53:43-59. (Discussion, 53:59-60.) [PubMed]
- 33.Persichini, T., M. Colasanti, G. M. Lauro, and P. Ascenzi. 1998. Cysteine nitrosylation inactivates the HIV-1 protease. Biochem. Biophys. Res. Commun. 250:575-576. [DOI] [PubMed] [Google Scholar]
- 34.Pier, G. B. 1985. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J. Infect. Dis. 151:575-580. [DOI] [PubMed] [Google Scholar]
- 35.Pier, G. B., M. Grout, T. S. Zaidi, J. C. Olsen, L. G. Johnson, J. R. Yankaskas, and J. B. Goldberg. 1996. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 271:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plotkowski, M. C., A. O. Costa, V. Morandi, H. S. Barbosa, H. B. Nader, S. de Bentzmann, and E. Puchelle. 2001. Role of heparan sulphate proteoglycans as potential receptors for non-piliated Pseudomonas aeruginosa adherence to non-polarised airway epithelial cells. J. Med. Microbiol. 50:183-190. [DOI] [PubMed] [Google Scholar]
- 37.Plotkowski, M. C., S. de Bentzmann, S. H. Pereira, J. M. Zahm, O. Bajolet-Laudinat, P. Roger, and E. Puchelle. 1999. Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am. J. Respir. Cell. Mol. Biol. 20:880-890. [DOI] [PubMed] [Google Scholar]
- 38.Plotkowski, M. C., A. M. Saliba, S. H. Pereira, M. P. Cervante, and O. Bajolet-Laudinat. 1994. Pseudomonas aeruginosa selective adherence to and entry into human endothelial cells. Infect. Immun. 62:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riordan, J. R., J. M. Rommens, B. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. L. Chou, et al. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066-1073. [DOI] [PubMed] [Google Scholar]
- 40.Robbins, R. A., P. J. Barnes, D. R. Springall, J. B. Warren, O. J. Kwon, L. D. Buttery, A. J. Wilson, D. A. Geller, and J. M. Polak. 1994. Expression of inducible nitric oxide in human lung epithelial cells. Biochem. Biophys. Res. Commun. 203:209-218. [DOI] [PubMed] [Google Scholar]
- 41.Roger, P., E. Puchelle, O. Bajolet-Laudinat, J. M. Tournier, C. Debordeaux, M. C. Plotkowski, J. H. Cohen, D. Sheppard, and S. de Bentzmann. 1999. Fibronectin and alpha5beta1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur. Respir. J. 13:1301-1309. [PubMed] [Google Scholar]
- 42.Saiman, L., and A. Prince. 1993. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Investig. 92:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salkowski, C. A., G. Detore, R. McNally, N. van Rooijen, and S. N. Vogel. 1997. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo: the roles of macrophages, endogenous IFN-gamma, and TNF receptor-1-mediated signaling. J. Immunol. 158:905-912. [PubMed] [Google Scholar]
- 44.Salvati, L., M. Mattu, M. Colasanti, A. Scalone, G. Venturini, L. Gradoni, and P. Ascenzi. 2001. NO donors inhibit Leishmania infantum cysteine proteinase activity. Biochim. Biophys. Acta 1545:357-366. [DOI] [PubMed] [Google Scholar]
- 45.Satoh, S., K. Oishi, A. Iwagaki, M. Senba, T. Akaike, M. Akiyama, N. Mukaida, K. M. Atsushima, and T. Nagatake. 2001. Dexamethasone impairs pulmonary defence against Pseudomonas aeruginosa through suppressing iNOS gene expression and peroxynitrite production in mice. Clin. Exp. Immunol. 126:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt, H. H., and U. Walter. 1994. NO at work. Cell 78:919-925. [DOI] [PubMed] [Google Scholar]
- 47.Shaul, P. W. 2002. Regulation of endothelial nitric oxide synthase: location, location, location. Annu. Rev. Physiol. 64:749-774. [DOI] [PubMed] [Google Scholar]
- 48.Stamler, J. S., S. Lamas, and F. C. Fang. 2001. Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106:675-683. [DOI] [PubMed] [Google Scholar]
- 49.Steagall, W. K., H. L. Elmer, K. G. Brady, and T. J. Kelley. 2000. Cystic fibrosis transmembrane conductance regulator-dependent regulation of epithelial inducible nitric oxide synthase expression. Am. J. Respir. Cell. Mol. Biol. 22:45-50. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, S. R., S. A. Kharitonov, S. F. Scott, M. E. Hodson, and P. J. Barnes. 2000. Nasal and exhaled nitric oxide is reduced in adult patients with cystic fibrosis and does not correlate with cystic fibrosis genotype. Chest 117:1085-1089. [DOI] [PubMed] [Google Scholar]
- 51.Wan, H., H. L. Winton, C. Soeller, G. A. Stewart, P. J. Thompson, D. C. Gruenert, M. B. Cannell, D. R. Garrod, and C. Robinson. 2000. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o. Eur. Respir. J. 15:1058-1068. [DOI] [PubMed] [Google Scholar]
- 52.Welsh, M. J., and A. E. Smith. 1993. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 73:1251-1254. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, L., C. Gunn, and J. S. Beckman. 1992. Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys. 298:452-457. [DOI] [PubMed] [Google Scholar]