Abstract
The role of Helicobacter pylori lipopolysaccharide (LPS) Lewis antigens in infection is still not well known. We investigated the influence of Lewis antigen expression by H. pylori on its internalization by AGS cells and the epithelium of human gastric xenografts in nude mice using isogenic mutants in LPS biosynthetic genes. In vivo, colonization rates were unaffected by the change in H. pylori Lewis antigen expression, whereas the number of viable intracellular bacteria was significantly higher with wild-type H. pylori strains expressing Lewis antigens when compared to the isogenic mutants in both models. H. pylori strains expressing more Lewis X antigens (Lex) were internalized at a higher rate than those expressing less Lex, type II Lewis antigens (Lea or Leb) alone, or no Lewis antigens. Thus, Lewis antigens appear to be involved in the internalization of H. pylori by the gastric epithelium.
Helicobacter pylori is considered an extracellular pathogen but may reside inside gastric epithelial cells (2, 4, 11, 27). Adherence of H. pylori to epithelial cells may represent the first step in the process of colonization and endocytosis of this bacterium (3, 18, 24). H. pylori express Lewis antigens on their surface as part of their lipopolysaccharide (14). In Western populations most H. pylori strains express the type II glycoconjugate antigens, Lewis X (Lex) and Ley, whereas a small proportion express the type I glycoconjugates, Lea and Leb (15). Although no gastric Lex-binding lectin has been identified, the detection of pedestal formation and the association of Lex at this location suggests that Lex-Lex homotypic interaction between the eukaryotic cell Lex and the H. pylori Lex may be responsible for a Lex-mediated adherence (26). It has been shown that the adherence of H. pylori strains expressing Lex to cultured gastric epithelial cells was significantly inhibited by a monoclonal anti-Lex (1, 17). Edwards et al. showed that, in contrast to a Lex-positive parent strain, two strains that were unable to produce Lex did not adhere to the epithelial cells of the gastric pits of human antral tissue sections (7). In patients with chronic gastritis, Heneghan et al. found a significant relationship between bacterial Lex/y expression and H. pylori colonization density (10). These data suggest that H. pylori Lex antigens may play a role in colonization via a Lewis antigen-mediated adherence process. However, it has also been shown that H. pylori cells that do not express Lewis antigens but do express other complex carbohydrates may still colonize patients (23). Thus, the role of these antigens in colonization and internalization is unclear and requires further study. The goal of the present work was to investigate the internalization of H. pylori in AGS cells and to determine if the internalization is affected by Lewis antigen expression. Additional studies of bacterial colonization and internalization were undertaken in vivo using a recently developed model of H. pylori infection using human gastric xenografts in nude mice (13) to complement the in vitro studies.
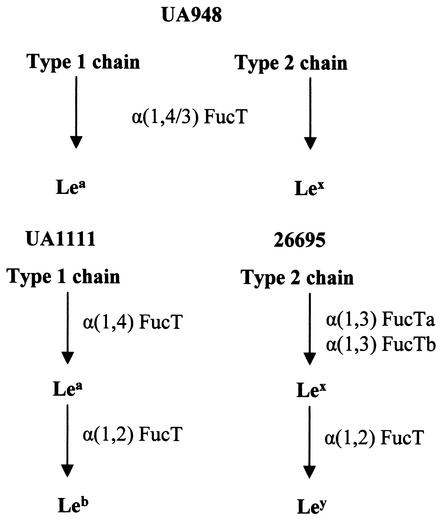
Strains used in this work and the corresponding genotypes and phenotypes are listed in Table 1. H. pylori strains UA948, 26695, and UA1111 express Lea and Lex, Lex and Ley, or Leb and only trace amounts of Lea, respectively (15, 21, 22). The difference in Lewis antigen expression in these wild-type strains reflects the interstrain variability of the fucosyltransferases (FucT) that are required in the synthesis of Lewis antigens (Fig. 1.). Isogenic mutants previously generated from the above-mentioned strains by insertional mutagenesis (chloramphenicol resistance gene cassette) of FucT genes were also studied: H. pylori UA948fucTa− (no detectable Lewis antigen), H. pylori 26695fucTa− (decreased amounts of both Lex and Ley), and H. pylori UA1111fucT2− (Lea) (21, 22; D. A. Rasko and D. E. Taylor, unpublished data). Strains were grown on Columbia agar supplemented with 10% horse blood or in brucella broth containing 5% fetal bovine serum under microaerobic conditions as previously described (13). For the mutants, the media were supplemented with chloramphenicol (20 μg/ml).
TABLE 1.
H. pylori strains used in this study
| Strain | Genotype | Lewis phenotype | Reference |
|---|---|---|---|
| UA948 | Wild type | Lea, Lex | 22 |
| UA948fucTa−a | α(1,3/4)fucTa::cat | 22 | |
| 26695 | Wild type | Lex, Ley | 15 |
| 26695fucTa−b | α(1,4)fucTa::cat | (Lex), (Ley) | Rasko et al., unpublished |
| UA1111 | Wild-type | Leb, (Lea) | 21 |
| UA1111fucT2−c | fucT2::cat | Lea | 21 |
In this strain the inactivation of fucTa is responsible for the total loss of Lewis antigen expression as this gene encodes the only functional FucT enzyme which contains both α1,3 and α1,4 FucT activities.
In this strain the decrease of Lex/y expression is due to the fact that only one (α[1,3]fucTa) of the two genes encoding α(1,3)FucT is inactivated. Thus, the second enzyme (α[1,3]FucTb) is still functional but does not fucosylate the LPS to the same degree leading to a decrease in Lex/y expression.
In this strain the absence of Leb production is due to the inactivation of fucT2 that encodes the only functional α(1,2)FucT, involved in the conversion of Lea to Leb. Thus, a significant amount of Lea is produced.
FIG. 1.
Synthesis of Lewis antigens in H. pylori strains UA948, 26695, and UA1111 (15, 21, 22).
Internalization of H. pylori into cultured AGS cells (ATCC CRL 1739) was quantitated using a standard gentamicin internalization assay. AGS cells were grown in F-12 K medium (American Type Culture Collection, Manassas, Va.) containing 2 mM l-glutamine, sodium bicarbonate (1.5 g/liter), and 10% fetal bovine serum. Bacteria grown in broth were washed with F-12 K before inoculation. AGS cells (106 cells/well) were then incubated at 37°C with H. pylori suspensions in F-12 K for 6 h at a multiplicity of infection of 100. Monolayers were washed three times, incubated for 1.5 h in the presence of gentamicin (50 μg/ml) to kill extracellular bacteria, and lysed using distilled water. The count of viable intracellular bacteria was determined by quantitative plating of serial dilutions of the lysates. The experiments performed in triplicate (106 cells/well) were repeated eight times for each strain, and the results were expressed as the mean percentage (± standard deviation) of the inoculum that was internalized in AGS cells.
Xenografts exhibiting human mature gastric epithelium and acidic secretion (pH range, 1.5 to 2) were obtained in nude mice as previously described (13). Prior to inoculation, one biopsy specimen was taken in each graft to determine the Lewis antigen status of the gastric mucosa. Immunostaining of the biopsy samples was performed by a standard avidin-biotin-peroxidase technique using mouse monoclonal anti-Lea, -Leb, -Lex, and -Ley antibodies (Signet Pathology Systems Inc., Dedham, Mass.) (26). All of the biopsy specimens were positive for all four Lewis antigens with the exception of three grafts in which only Leb, Lex, and Ley were detected. Bacterial inoculation was then performed as previously described (13). At 2, 4, and 8 weeks after inoculation, each graft was surgically opened, gastric juice was taken for pH determination, and mucus was sampled for qualitative culture onto blood agar. Three biopsy samples were then taken from adjacent sites in the antrum for quantitative culture and histology. At 8 weeks after inoculation, an additional biopsy sample was taken for electron microscopy. Biopsy specimens for culture were immediately placed in a semisolid agar transport medium (Portagerm pylori; bioMérieux) and weighed. One of these specimens was directly homogenized with an Ultra Turrax grinder (Labo-Moderne, Paris, France), and serial dilutions of the homogenate in sterile 0.9% NaCl were transferred onto blood agar plates. After 5 days of microaerobic incubation, bacterial counts were performed and expressed as CFU per gram of tissue. The second sample was used to determine the concentration of intracellular bacteria. This specimen was vigorously washed six times in broth, incubated for 2 h in the presence of gentamicin (100 μg/ml) to kill extracellular bacteria, washed six times, and then processed (29).
The expression of bacterial Lewis antigens was evaluated before inoculation and after 8 weeks of infection, testing isolates obtained from biopsy specimens treated with gentamicin (intracellular bacteria) and from mucus (extracellular bacteria). Lewis antigen expression was determined on bacteria grown in broth by an enzyme-linked immunosorbent assay previously described (26). For histological studies, specimens were processed by standard methods and stained with hematoxylin-eosin to assess the intensity of gastritis (13). For transmission electron microscopy, biopsy specimens were processed and examined as previously described (13).
Differences between means of internalization in AGS cells of wild-type strains and isogenic mutants were assessed by unpaired t test with Welch correction. Statistical analysis of the differences in colonization and internalization in gastric xenografts were performed using the Mann-Whitney test. A P of <0.05 was considered significant.
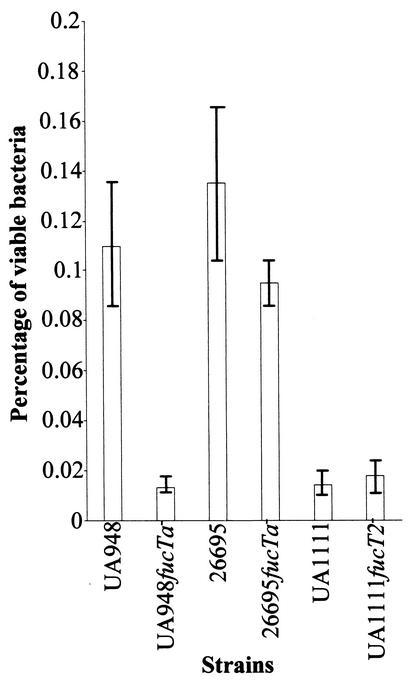
In vitro assays demonstrated that all six tested strains were able to penetrate in AGS cells. The level of internalization of H. pylori 26695 was comparable to that of H. pylori UA948 but significantly higher than that of each of the corresponding isogenic mutants (Fig. 2). Penetration rates of H. pylori UA948 and H. pylori 26695fucTa− were both significantly higher than those of H. pylori UA948fucTa−, H. pylori UA1111, and H. pylori UA1111fucT2−, for which no significant difference in intracellular uptake was observed.
FIG. 2.
Influence of Lewis antigen expression by H. pylori on its internalization by AGS cells. Viable bacterial uptake of wild-type strains UA948, 26695, and UA1111 was compared to that of isogenic mutants UA948fucTa−, 26695fucTa−, and UA1111fucT2− using a gentamicin assay. Results are expressed as the percentage of the inoculum that was internalized (mean ± standard deviation [error bars]).
All strains colonized all inoculated grafts for the 2-month period of the study. At any time period tested, culture of mucus and mucosa samples were positive for H. pylori only. The pH of the gastric juice measured at each time was increased to the range of 5 to 6.5. The levels of colonization determined by quantitative culture varied between 106 and 1.6 × 108 CFU/g of tissue. At any time, mutants colonized at levels comparable to those of wild type. In the three grafts with the Leb Lex Ley expression patterns, the colonization rates observed after inoculation of H. pylori strains UA948 (n = 1), UA1111 (n = 1), or UA1111fucT2− (n = 1) were similar to those observed in the other grafts (Lea Leb Lex Ley) infected with the same strains at all time points. Intracellular H. pylori were detected in all infected xenografts at all times after challenge.
At all time points, H. pylori 26695 was significantly more internalized than all the other strains, including H. pylori 26695fucTa− (Table 2). The internalization rate of H. pylori UA948 was higher than that of H. pylori UA948fucTa− and higher than those of all strains except H. pylori 26695 at 4 weeks. No difference in internalization was seen between the other strains tested. It is interesting that H. pylori UA948fucTa− was found significantly less internalized than all the other strains at 8 weeks and does not express any of the complete Lewis antigens.
TABLE 2.
Invasion of antral mucosa of human gastric xenografts after inoculation of H. pylori UA 948, UA 948fucTa−; UA 1111, UA 1111fucT2−; 26695 and 26695fucTa−
| H. pylori strain (no. of xenografts) | Ratio of the no. of gentamicin-resistant CFU/g of tissue to the total number of CFU/g of tissue × 100a at:
|
||
|---|---|---|---|
| 2 wk | 4 wk | 8 wk | |
| UA1111 (6) | 0.50 (0.04-2.00) | 0.10 (0.04-0.14) | 0.50 (0.08-2.00) |
| UA1111fucT2− (6) | 0.40 (0.03-1.20) | 0.10 (0.07-0.16) | 0.45 (0.05-2.00) |
| UA948 (5) | 0.48 (0.20-0.70) | 0.70 (0.30-1.00) | 0.66 (0.15-1.50) |
| UA948fucTa− (5) | 0.10 (0.04-0.50) | 0.06 (0.03-0.09) | 0.05 (0.01-0.10) |
| 26695 (6) | 3.14 (1.70-4.50) | 1.30 (0.40-2.00) | 1.90 (1.00-2.70) |
| 26695fucTa− (6) | 0.20 (0.01-0.80) | 0.09 (0.01-0.25) | 0.21 (0.08-0.40) |
Values are shown as mean (range).
To determine whether the expression of Lewis antigens of H. pylori strains had changed during in vivo experiments, Lewis antigen typing was performed on each isolate obtained, after 8 weeks of infection, from the mucus and the mucosa (after incubation with gentamicin and cell lysis). For all strains tested and for all xenografts, no detectable change in the expression of Lewis antigens by H. pylori between inoculation and 2 months of infection was observed.
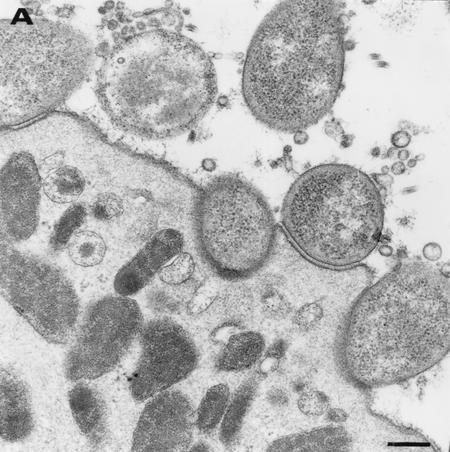
In inoculated grafts, erythematous areas were visible at the surface of the antrum at 2 weeks. These lesions were widespread from week 4 onwards. Histological examination of antral biopsy samples showed at any time a mild inflammation associated from week 4 with mild activity. These macroscopic and microscopic features were the same for all H. pylori-infected grafts. Electron microscopy studies confirmed the presence of both extracellular and intracellular bacteria in all biopsy specimens studied (Fig. 3). In all cases, numerous extracellular bacteria were seen within the mucous layer and often in close proximity to the surface of gastric epithelial cells. For each strain tested, bacteria were observed adhering to epithelial cells either through fibril-like strands or more intimate contact often associated with pedestal formations (Fig. 3A). In some cases, the outer membrane layer of the bacteria was apparently fused with the apical cell membrane associated with a nearly complete engulfment of the microorganism (Fig. 3B).
FIG. 3.
Transmission electron micrographs of the gastric antral epithelium from a xenograft infected by H. pylori UA948. (A) H. pylori organisms in proximity of or adherent to a mucous cell. (B) H. pylori bacterium nearly completely engulfed in a mucous cell. (C) H. pylori bacterium internalized in a mucous cell. Bar = 0.25 μm.
In this study, we have demonstrated for the first time that H. pylori internalization in human gastric epithelial cells may be influenced by Lewis antigen expression. H. pylori strains expressing Lex were internalized to greater levels both in vitro and in vivo than isogenic mutants expressing this antigen at a lower level or not all. This suggests that Lex may be involved in H. pylori internalization. However, the role of other carbohydrate antigens cannot be ruled out and needs to be further investigated by testing strains with various Lewis antigenic phenotypes. All H. pylori strains tested to date were able to invade human epithelial cells in vitro (8, 19, 28). This is in concordance with our in vitro and in vivo findings, and may be related to the fact that most H. pylori strains tested to date express Lex/y.
It has been shown that the expression of Lex/y by H. pylori is higher at pH 7 than at pH 5 (16). In humans, the antral juxtamucosal pH varies between 5 and almost 7 (9, 20). This may induce optimal expression of Lex for adherence and subsequent internalization. Moreover, in xenografts, colonization was always related, as previously shown (13), to an increase of the gastric juice pH. This might also enhance the expression of Lex and explain why no Le antigens phase variation was observed in isolates obtained from the mucus.
We could not detect any significant influence of the bacterial Lewis status on colonization. These results are similar to those recently obtained with mice by Takata et al. (25) with H. pylori 26695 and isogenic mutants expressing variable amounts of Lex/y. Heneghan et al. (10) demonstrated that bacterial Lex expression was associated with higher bacterial density, as assessed by semiquantitative histological examination, in patients with chronic gastritis but not in patients with ulcers. However, only about 20% of H. pylori cells adhere to gastric epithelial cells, whereas the majority of the bacteria are free-living in the mucus (12). Thus, it is conceivable, as shown in our study, that the total number of viable bacteria present in gastric biopsy specimens, which may include the deeper mucous layer, may not be altered even if the number of adherent bacteria is reduced. Among H. pylori Lewis antigens, only Lex has been shown to be conclusively involved in adherence to the gastric epithelium (7, 17). However, other adhesins such as BabA have also been shown to be involved in H. pylori adherence (5, 6). Thus, even if the role of Lex in adherence seems very likely, it is unclear what role Lewis antigens play in colonization and internalization. Strains that do not express other adhesins or strains colonizing hosts that do not express the corresponding receptors may utilize Lex antigens for their adherence. It appears as though H. pylori may require a number of different adhesins for colonization depending on the host bacteria interaction (1). We have demonstrated that Lewis antigens are involved with internalization of the bacteria. It is also conceivable that Lex may increase the frequency or affinity of intimate contact between H. pylori and gastric epithelial cells without increasing the bacterial density of adherent bacteria or the colonization density. Moreover, more intimate contact may increase host cell responses and promote the subsequent H. pylori internalization. However, in the present study, transmission electron microscopy analyses showed that intimate contacts occurred in vivo between H. pylori and gastric cells with all strains tested, including H. pylori that did not internalize to a high degree (H. pylori 26695fucTa− and H. pylori UA948 fucT2−). Further quantitative ultrastructural studies are necessary to elucidate whether this phenomenon is less frequent with strains expressing Lex than with isogenic mutants that do not express this antigen.
Acknowledgments
D.E.T. and D.A.R. were supported by a grant from the Alberta Heritage Foundation for Medical Research (AHFMR).
Editor: V. J. DiRita
REFERENCES
- 1.Appelmelk, B. J., and C. M. J. E. Vandenbroucke-Grauls. 2000. H. pylori and Lewis antigens. Gut 47:10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale, G., P. Malfertheiner, and H. Ditschuneit. 1987. Campylobacter-like organisms in the duodenal mucosa in patients with active duodenal ulcer. Klin. Wochenschr. 65:144-146. [DOI] [PubMed] [Google Scholar]
- 3.Björkholm, B., V. Zhukhovitsky, C. Löfman, K. Hultén, H. Enroth, M. Block, R. Rigo, P. Falk, and L. Engstrand. Helicobacter pylori entry into human gastric epithelial cells: a potential determinant of virulence, persistence, and treatment failures. Helicobacter 5:148-154. [DOI] [PubMed]
- 4.Bode, G., P. Malfertheiner, and H. Ditschuneit. 1990. Pathogenetic implications of ultrastructural findings in Campylobacter pylori related gastroduodenal disease. Scand. J. Gastroenterol. 23(Suppl. 142):25-39. [PubMed] [Google Scholar]
- 5.Boren, T., S. Normark, and P. Falk. 1994. Helicobacter pylori: molecular basis for host recognition and bacterial adherence. Trends Microbiol. 2:221-228. [DOI] [PubMed] [Google Scholar]
- 6.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards, N. J., M. A. Monteiro, G. Faller, E. J. Walsh, A. P. Moran, I. S. Roberts, and N. J. High. 2000. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Mol. Microbiol. 35:1530-1539. [DOI] [PubMed] [Google Scholar]
- 8.Evans, D. G., D. J. Evans, and D. Y. Graham. 1992. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology 102:1557-1567. [DOI] [PubMed] [Google Scholar]
- 9.Frieri, G., G. De Petris, A. Aggio, D. Santarelli, E. Ligas, R. Rosoni, and R. Caprilli. 1995. Gastric and duodenal juxtamucosal pH and Helicobacter pylori. Digestion 56:107-110. [DOI] [PubMed] [Google Scholar]
- 10.Heneghan, M. A., C. F. McCarthy, and A. P. Moran. 2000. Relationship of blood group determinants on Helicobacter pylori lipopolysaccharide with host Lewis phenotype and inflammatory response. Infect. Immun. 68:937-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazi, J. L., R. Sinniah, V. Zaman, M. L. Ng, N. A. Jafarey, S. M. Alam, S. J. Zuberi, and A. M. Kazi. 1990. Ultrastructural study of Helicobacter pylori-associated gastritis. J. Pathol. 161:65-70. [DOI] [PubMed] [Google Scholar]
- 12.Lee, A., J. Fox, and S. Hazell. 1993. Pathogenicity of Helicobacter pylori: a perspective. Infect. Immun. 61:1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozniewski, A., F. Muhale, R. Hatier, A. Marais, M. C. Conroy, D. Edert, A. Le Faou, M. Weber, and A. Duprez. 1999. Human embryonic gastric xenografts in nude mice: a new model of Helicobacter pylori infection. Infect. Immun. 67:1798-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteiro, M. A., K. H. N. Chan, D. A. Rasko, D. E. Taylor, P. Y. Zheng, B. J. Appelmelk, H. P. Wirth, M. Yang, M. J. Blaser, S. O. Hynes, A. P. Moran, and M. B. Perry. 1998. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. J. Biol. Chem. 19:11533-11543. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro, M. A., B. J. Appelmelk, D. A. Rasko, A. P. Moran, S. O. Hynes, L. L. McLean, K. H. Chan, F. S. Michael, S. M. Logan, J. O'Rourke, A. Lee, D. E. Taylor, and M. B. Perry. 2000. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H. pylori Sydney strain, H. pylori P466 carrying sialyl Lewis Lewis X, and H. pylori UA915 expressing Lewis B: classification of H. pylori lipopolysaccharides into glycotype families. Eur. J. Biochem. 267:305-320. [DOI] [PubMed] [Google Scholar]
- 16.Moran, A. P., Y. A. Knirel, S. N. Senchenkowa, G. Widmalm, S. O. Hynes, and P. E. Jansson. 2002. Phenotypic variation in molecular mimicry between Helicobacter pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J. Biol. Chem. 277:5785-5795. [DOI] [PubMed] [Google Scholar]
- 17.Osaki, T., H. Yamaguchi, H. Taguchi, M. Fukuda, H. Kawakami, H. Hirano, S. Watanabe, A. Takagi, and S. Kamiya. 1998. Establishment and characterization of a monoclonal antibody to inhibit adhesion of Helicobacter pylori to gastric epithelial cells. J. Med. Microbiol. 47:505-512. [DOI] [PubMed] [Google Scholar]
- 18.Papadogiannakis, N., R. Willén, B. Carlén, S. Sjöstedt, T. Wadström, and A. Gad. 2000. Modes of adherence of Helicobacter pylori to gastric surface epithelium in gastroduodenal disease: a possible sequence of events leading to internalisation. APMIS 108:439-447. [DOI] [PubMed] [Google Scholar]
- 19.Petersen, A. M., K. Sørensen, J. Blom, and K. A. Krogfelt. 2001. Reduced intracellular survival of Helicobacter pylori vacA mutants in comparison with their wild-types indicates the role of VacA in pathogenesis. FEMS Immunol. Med. Microbiol. 30:103-108. [DOI] [PubMed] [Google Scholar]
- 20.Quigley, E. M., and L. A. Turnberg. 1987. pH of the microclimate lining human gastric and duodenal mucosa in vivo. Studies in control subjects and in duodenal ulcer patients. Gastroenterology 92:1876-1884. [DOI] [PubMed] [Google Scholar]
- 21.Rasko, D. A., G. Wang, M. A. Monteiro, M. M. Palcic, and D. E. Taylor. 2000. Synthesis of mono- and di-fucosylated type I Lewis blood group antigens by Helicobacter pylori. Eur. J. Biochem. 267:6059-6066. [DOI] [PubMed] [Google Scholar]
- 22.Rasko, D. A., G. Wang, M. M. Palcic, and D. E. Taylor. 2000. Cloning and characterization of the α(1,3/4) fucosyltransferase of Helicobacter pylori. J. Biol. Chem. 275:4988-4994. [DOI] [PubMed] [Google Scholar]
- 23.Rasko, D. A., T. J. M. Wilson, D. Zopf, and D. E. Taylor. 2000. Lewis antigen expression and stability in Helicobacter pylori isolated from serial gastric biopsies. J. Infect. Dis. 181:1089-1095. [DOI] [PubMed] [Google Scholar]
- 24.Russel, D. G. 2000. Where to stay inside the cell: a homesteader's guide to intracellular parasitism, p. 131-152. In P. Cossart, P. Boquet, S. Normark, and R. Rappuoli (ed.), Cellular microbiology. ASM Press, Washington, D.C.
- 25.Takata, T., E. El-Omar, M. Camorlinga, S. A. Thompson, Y. Minohara, P. B. Ernst, and M. J. Blaser. 2002. Helicobacter pylori does not require Lewis X or Lewis Y expression to colonize C3H/HeJ mice. Infect. Immun. 70:3073-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor, D. E., D. A. Rasko, R. Sherburne, C. Ho, and L. D. Jewell. 1998. Lack of correlation between Lewis antigen expression by Helicobacter pylori and gastric epithelial cells in infected patients. Gastroenterology 115:1113-1122. [DOI] [PubMed] [Google Scholar]
- 27.Tricottet, V., P. Bruneval, O. Vire, and J. P. Camilleri. 1986. Campylobacter-like organisms and surface epithelium abnormalities in active chronic gastritis in humans: an ultrastructural study. Ultrastruct. Pathol. 10:113-122. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson, S. M., J. R. Uhl, B. C. Kline, and F. R. Cockerill, III. 1998. Assessment of invasion frequencies of cultured HEp-2 cells by clinical isolates of Helicobacter pylori using an acridine orange assay. J. Clin. Pathol. 51:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, Z., L. Jin, G. Champion, K. B. Seydel, and S. L. Stanley, Jr. 2001. Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect. Immun. 69:3240-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]