Abstract
Cystatins of parasitic nematodes are well-described pathogenicity factors which contribute to downregulation of T-cell proliferation of their hosts and induce anti-inflammatory cytokine responses. We compared the immunomodulatory effects of two cystatins of the filarial nematodes Onchocerca volvulus and Acanthocheilonema viteae with two homologous proteins of the free-living nematode Caenorhabditis elegans. Like filarial cystatins, the C. elegans cystatins (rCysele1 and rCysele2) possessed domains relevant for inhibition of papain-like proteases and were biologically active inhibitors of human cathepsins B, L, and S. However, the inhibition of cathepsin B by C. elegans cystatin was much stronger. C. elegans cystatins lacked a domain involved in inhibition of legumain-like proteases that was present in O. volvulus cystatin. Filarial cystatins suppressed the proliferation of human peripheral blood mononuclear cells (PBMC) and murine spleen cells, while the C. elegans cystatins had this effect to a much lesser extent. Whereas filarial cystatins markedly increased the production of interleukin (IL)-10, C. elegans cystatins increased the production of IL-12 and gamma interferon (IFN-γ) by human PBMC. The cystatins of both the filariae and C. elegans induced an upregulation of inducible nitric oxide by IFN-γ-stimulated murine macrophages. These data suggest that filarial cystatins but not the C. elegans cystatins downregulate proliferative responses of host cells due to characteristics which might reflect an adaptation of filariae to their parasitic life style.
Endoparasitic nematodes are specifically adapted to a life within immunocompetent hosts, where they are continuously exposed to immune effector mechanisms. Their survival depends in part on the evasion of harmful immune responses or the modulation of the immune system. It is thought that secreted immunomodulatory molecules of parasitic nematodes are pathogenicity factors with a key role in balancing the host-parasite relationship (1, 40). This raises the question of whether secreted immunomodulators of parasitic nematodes have characteristics distinct from the molecules of their free-living relatives.
The tissue-dwelling filarial nematodes can be considered extremely well adapted parasites, because they live in direct contact with their host's immune system and nevertheless have life spans of 10 years and more (37). This long persistence is ascribed to modulation of the immune system, which leads to inhibition of inflammatory responses that could eliminate the parasites (23). One characteristic of humans infected with filariae is a distinct cellular hyporeactivity and a polarization of the immune response towards a Th2 type (9, 39). These traits can be explained by the recruitment of alternatively activated macrophages which block cellular proliferation by cell-to-cell contact (19) and the release of recently described immunomodulatory components by the worms.
We and others have shown that a cysteine protease inhibitor (cystatin) downregulates T-cell proliferation and alters macrophage functions (16, 25, 36, 42), and a serine protease inhibitor (serpin) was reported to impair functions of granulocytes (49). Filariae were also shown to produce a functionally active macrophage migration-inhibitory factor (35) as well as a transforming growth factor beta (TGF-β) homologue (10). Furthermore, phosphorylcholine, a phospholipid component associated with several filarial proteins, inhibits cellular proliferation by interfering with signal transduction chains that lead to cellular activation (15). Comparison of such parasite-derived immunomodulators with molecules of free-living nematodes should provide a clue to whether the capacity of these components to interact with the immune system represents a specific adaptation to the parasitic life style.
In this study we chose the secreted filarial immunomodulator cystatin as a model protein for a comparison with homologous proteins of the free-living nematode Caenorhabditis elegans. Filarial cystatin accounts for a major proportion of the immunosuppressive activity of secreted filarial proteins (16) and therefore has to be considered a major pathogenicity factor of filariae. Cystatins are natural, tight-binding cysteine protease inhibitors and represent important regulators of proteolytic processes. Many cystatins possess two distinct inhibitory functions, the inhibition of papain-like proteases (family 1) and of legumain-like proteases (family 13). Several immunomodulatory properties of common cystatins have been described. Human cystatin C inhibits the phagocytic function of monocytes and granulocytes (18), and various members of the cystatin superfamily upregulate the inducible nitric oxide production of murine macrophages (44, 45). In addition, cystatins have the potential to modulate immune responses, as they interfere with steps in the antigen-processing pathway in major histocompatibility complex (MHC) class II antigen presentation (27, 33, 47).
Filarial cystatin is clearly involved in parasite-induced immunomodulation, as it induces cellular hyporeactivity of T cells, modulates the production of cytokines, and downregulates essential costimulatory molecules on macrophages (16, 42). A filarial cystatin was shown to interfere with antigen presentation by inhibiting multiple cysteine protease activities found in endosomes/lysosomes of B cells (25). In vivo experiments revealed that application of filarial cystatin via osmotic pumps results in upregulated production of tumor necrosis factor alpha (TNF-α) and a diminished antigen-specific proliferation of spleen cells in mice (36). Recently, the cystatin of the intestinal nematode Nippostrongylus brasiliensis was also shown to be involved in immune evasion by modulating antigen processing in antigen-presenting cells (5).
Our results reveal distinct differences between cystatins of filarial nematodes and C. elegans in their interference with cellular immune responses in vitro. While filarial cystatins induce a marked suppression of proliferative T-cell responses and production of Th2-like cytokines, the C. elegans cystatins suppress cellular proliferation to a lesser extent and lead to Th1-like cytokine responses. This pattern suggests that the cystatins of parasitic nematodes evolved in a manner that allows very specific modulation of host responses.
MATERIALS AND METHODS
C. elegans culture and preparation of total RNA from C. elegans.
C. elegans worms were cultured on NGM (nematode growth medium) plates seeded with Escherichia coli OP50 and kept at room temperature. The C. elegans mixed culture was harvested, and bacteria were removed by washing three times with M9 buffer (0.02 M KH2PO4, 0.035 M Na2HPO4, 0.085 M NaCl, 1 mM MgSO4). RNA was isolated by guanidinium thiocyanate (Tristar; AGS, Heidelberg, Germany). mRNA was transcribed into cDNA by reverse transcription-PCR (Moloney murine leukemia virus; Promega, Mannheim, Germany).
Cloning and expression of recombinant proteins.
The cDNA of the mature cystatin proteins, without the signal sequence, were amplified by PCR with degenerate primers derived from internal sequences of C. elegans chromosome III, genes K08B4.6 and R01B10.1, showing similarities to cystatin domains (48). The Cysele1 forward primer was CAAATTGCTGGTGGATTG; the Cysele1 reverse primer was AATTTTTTCATCAGGTTTCAC; the Cysele2 forward primer was GGTATGATGACTGGAGGA; and the Cysele2 reverse primer was GAACTGCTCGTCAGCGGT. PCR amplification yielded a 360-bp fragment (rCysele1) and a 372-bp fragment (rCysele2), which were cloned into a T overhang vector (pGEM-T Easy Vector System; Promega, Madison, Wis.). The NotI-restricted fragments from the pGEM vector were undirectionally subcloned into the NotI site of the expression vector pET28a, yielding polypeptides with a six-histidine tag (pET System; Novagen, Madison, Wis.). The recombinant plasmids were transformed into competent E. coli BL21(DE3) and screened for expression by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
rCysele1 and rCysele2 were purified under nondenaturing conditions by affinity chromatography with Ni-nitrilotriacetic acid resin (Qiagen, Hilden, Germany) and dialyzed four times against phosphate-buffered saline. The recombinant filarial cystatins Ov17 (rOv17) (42), a cystatin of the parasitic nematode O. volvulus, and recombinant Av17 (rAv17) (16), a cystatin of the rodent filaria A. viteae, were expressed and purified under identical conditions. The control protein, recombinant Ov33 (rOv33) (20), an O. volvulus protein with homologies to an aspartic protease inhibitor, was expressed and purified under identical conditions. This control protein was chosen in view of its closely related function but different structure.
Because rCysele1, rCysele2, rOv17, rAv17, and rOv33 were expressed in E. coli, endotoxin contamination was evaluated by the quantitative, chromogenic Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.). The endotoxin concentration of different batches of rCysele1 was determined to be 0.3 to 4 ng/of protein per ml, and that of rCysele2 was 0.4 to 5.5 ng of protein per ml. rOv17 and rAv17 and the control protein rOv33 showed endotoxin concentrations of 0.5 to 5 ng of protein per ml. In addition, we used cystatin preparations with added polymyxin B, and we used CeH/HeJ mice to compare with C3H/HeN mice in the experiments.
Human cathepsin inhibition assays.
The inhibitory activity of rCysele1 and rCysele2 to human cathepsins was characterized by Ki value determinations. Human cathepsin B was purchased from Sigma (Deisenhofen, Germany), human cathepsin L was from Calbiochem (Schwalbach, Germany), and human cathepsin S was kindly provided by B. Wiederanders (Institute of Biochemistry, University of Jena, Jena, Germany). Ki values were determined by measuring the activity of human cathepsin B (1.2 nM), cathepsin L (0.1 nM), or cathepsin S (0.1 nM) in the presence of various concentrations of rCysele1 and rCysele2 with the fluorogenic substrates benzyloxycarbonyl (Z)-Arg-Arg-aminoethylcoumarine (AMC) (10 μM; Bachem, Heidelberg, Germany) for cathepsin B, Z-Phe-Arg-AMC (10 μM; Bachem) for cathepsin L, and Z-Val-Val-Arg-AMC (10 μM; Bachem) for cathepsin S (3). The Ki values were calculated with the program GraphPadPrism.
Antigen-driven and polyclonally stimulated proliferation of human PBMC.
The peripheral blood mononuclear cell (PBMC) fraction of healthy donors was isolated from citrate-treated venous blood by density gradient sedimentation with Ficoll-Hypaque (Pharmacia Biotech Products, Freiburg, Germany). Cells were resuspended in RPMI 1640 (Biochrom, Berlin, Germany), supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM glutamine, and 10% fetal bovine serum (Biochrom). Then 3.5 × 105 PBMC/well were cultured in 96-well flat-bottomed plates at 37°C and stimulated with either 10 IU of purified protein derivative (PPD) (Chiron Behring & Co, Marburg, Germany) together with IFN-α (125 U/ml) (46) or anti-CD3 antibody (4 μg/ml) (Orthoclone-Okt3, kind gift from Janssen-Cliag, Neuss, Germany). Recombinant proteins were added at a concentration of 0.5 μM. PPD-stimulated PBMC were cultured for 96 h and polyclonally stimulated for 72 h at 37°C. Proliferation was quantified by [3H]thymidine incorporation (1 μCi/well; Amersham, Buckinghamshire, United Kingdom) during the last 20 h of incubation. All experiments were performed in triplicate. Viability of the cells in the presence of recombinant proteins was controlled by trypan blue exclusion.
Quantification of cytokines in cell culture supernatant.
Cytokine production was determined in the culture supernatants of unstimulated and stimulated PBMC cocultured with 0.5 μM recombinant proteins. Culture supernatants were collected after 24 h and 48 h of incubation. Cytokines were quantified by enzyme-linked immunosorbent assay (ELISA) according to the manufacturers' instructions (OptEIA; Pharmingen, Hamburg, Germany). The assays were performed in triplicate.
Nitric oxide production of murine peritoneal macrophages.
BALB/c mice were killed by ether inhalation. Peritoneal macrophages were harvested by flushing the peritoneal cavity several times with RPMI. Erythrocytes were lysed with lysis buffer. Murine peritoneal macrophages were washed three times with RPMI 1640 (Biochrom, Berlin, Germany), supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM glutamine, and 10% fetal bovine serum (Biochrom) and cultured at a density of 2 × 105 cells/well in 96-well flat-bottomed plates. Murine peritoneal macrophages were allowed to adhere for 2 h at 37°C. Medium and nonadherent cells were removed by washing with RPMI. Murine peritoneal macrophages were stimulated by IFN-γ (100 U/ml) in a final volume of 300 μl. Purified recombinant proteins were added at a concentration of 0.05 to 0.5 μM. After 24 h, 100 μl of supernatant was removed and analyzed by Griess reaction (11). The absorbance was determined at 550 nm. All experiments were performed in triplicate. Arithmetic mean values ± standard deviation are shown.
Antigen-driven and polyclonally stimulated proliferation of murine spleen cells.
Spleen cells of naive BALB/c and ovalbumin receptor transgenic mice (30) (kindly provided by T. Kamradt, Deutsches Rheuma-Forschungszentrum, Berlin, Germany) were cultured in 96-well flat-bottomed plates at a density of 3.5 × 105 cells/well, and proliferation was stimulated with 2 μg of concanavalin A per ml (Sigma, Deisenhofen, Germany) or 40 μM ovalbumin (Sigma). Proliferation of cells was quantified after 72 h by incorporation of [3H]thymidine (1 μCi/well; Amersham, Buckinghamshire, United Kingdom) added to the cultures during the last 20 h of incubation. Purified recombinant proteins were added at a concentration of 0.25 to 0.5 μM to the spleen cells. Viability of T cells in the presence of the recombinant proteins was controlled by trypan blue exclusion. All experiments were performed in triplicate. Arithmetic mean values ± standard deviation are shown.
Statistical analysis.
Statistical analysis of T-cell proliferation and cytokine data was performed with the Mann-Whitney U test. Nitric oxide data were analyzed with the Wilcoxon rank test. Data are presented as means and standard deviations.
RESULTS
Sequence similarities of nematode cystatins and protease-inhibitory activity of Cysele1 and Cysele2.
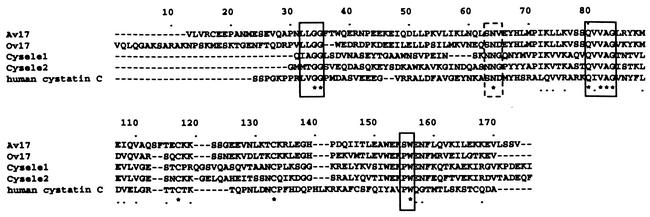
Sequence alignment of filarial cystatins with Cysele1 and Cysele2 showed that the three typical conserved motifs of cysteine protease inhibitors that bind and inhibit proteases of the papain family were present in all nematode cystatins (Fig. 1). However, the N-terminal active domain of both C. elegans cystatins showed only the essential amino acid glycine, whereas the two amino acids in front of the glycine were different (Fig. 1). In contrast, most of the other regions varied considerably between the filarial cystatins and C. elegans cystatins as well as between C. elegans cystatins and human cystatin C. Interestingly, a second active domain, SND, inhibiting legumain-like proteases, enzymes known to be involved in MHC class II antigen processing (2, 24), was lacking in the C. elegans cystatins. However, the corresponding sites were found in mammalian cystatin C as well as in O. volvulus and Brugia malayi cystatins. Another difference between the filarial cystatins and the C. elegans cystatins was the presence of an N-terminal amino acid extension in the filarial cystatins. Theses stretches did not show any homologies to known sequences.
FIG. 1.
Alignment of amino acid sequences of C. elegans cystatin 1 (Cysele1), C. elegans cystatin 2 (Cysele2), A. viteae cystatin (Av17), onchocystatin (Ov17), and human cystatin C. The first amino acid of each sequence is the proposed N terminus of the mature protein. Similar amino acids are marked by dots, and identical amino acids are marked by asterisks. The evolutionarily conserved papain binding sites are boxed. The conserved asparaginyl endopeptidase (AEP) inhibitory domain is boxed with a dashed line. The sequence data are available from GenBank under the following accession numbers: Av17, L43053; Ov17, M37105; Cysele1, AJ310669; Cysele2, AJ310670; and human cystatin C, AAA52164.
To compare the immunomodulatory properties of filarial cystatins with the properties of Cysele1 and Cysele2, full-length mature recombinant proteins (rCysele1 and rCysele2) with an N-terminal six-histidine tag were produced in E. coli. rCysele1 and rCysele2 were tested for their capacity to inhibit the activity of papain-like proteases (family 1 proteases) like the human cysteine proteases cathepsin B, S, and L. Ki determinations showed that rCysele1 and rCysele2 were potent inhibitors of human cathepsin B, L, and S (Table 1). In comparison, recombinant O. volvulus cystatin also strongly inhibited the activity of cathepsins S and L, whereas the activity of human cathepsin B was less efficiently inhibited by O. volvulus cystatin (Table 1) (42). The interaction of filarial cystatins and C. elegans cystatins with human cysteine proteases, which have essential functions in cellular processes, especially in macrophages, indicated that inhibitors of C. elegans and of parasitic nematodes have a similar potential to interfere with the activity of host proteases. The only significant difference between the filarial cystatins and the C. elegans cystatin was observed for cathepsin B, which was significantly more strongly inhibited by the C. elegans cystatins (Table 1).
TABLE 1.
Ki values of nematode cystatins with human cathepsins B, L, and S as cysteine proteases
| Cathepsin |
Ki (nM)
|
||
|---|---|---|---|
| rCysele1 | rCysele2 | rOv17 | |
| B | 38.88 ± 4.06 | 1.18 ± 0.9 | 494.7 ± 154.9 |
| L | 0.0143 ± 0.001 | 0.036 ± 0.004 | 0.038 ± 0.004 |
| S | 0.1214 ± 0.017 | 0.18 ± 0.014 | 0.033 ± 0.003 |
Polyclonal proliferation of murine spleen cells and human PBMC.
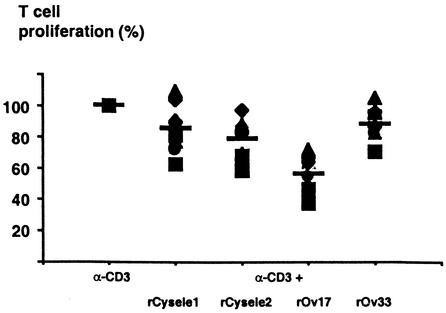
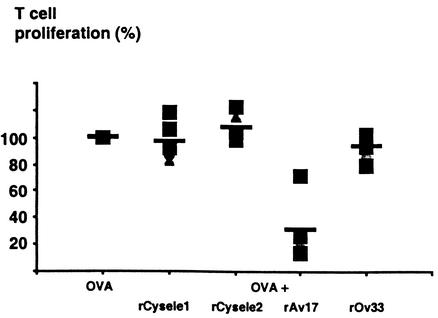
To compare the effects of filarial cystatins and C. elegans cystatins on polyclonal T-cell proliferation, we examined the reaction of human PBMC stimulated with anti-CD3 antibodies, rOv17, the cystatin of the human pathogenic filaria Onchocerca volvulus, and rCysele1 and rCysele2. rOv17 inhibited the anti-CD3-stimulated proliferative responses by 43.6% (P = 0.001, Fig. 2), while the effect of rCysele1 and rCysele2 was much lower (15.1% and 21.6% inhibition, respectively), similar to that of the recombinant control protein rOv33, which also slightly inhibited cellular proliferation (12.1% inhibition). These data suggest that the C. elegans cystatins did not induce cellular hyporeactivity, very much in contrast to the filarial cystatins.
FIG. 2.
T-cell proliferation of human PBMC in the presence of recombinant C. elegans cystatins. Anti-CD3 antibody-stimulated proliferation of PBMC from 10 healthy human donors in the presence of 0.5 μM rCysele1, rCysele2, or rOv17 or of the control protein rOv33. Proliferation of PBMC in the presence of anti-CD3 antibody was set at 100%.
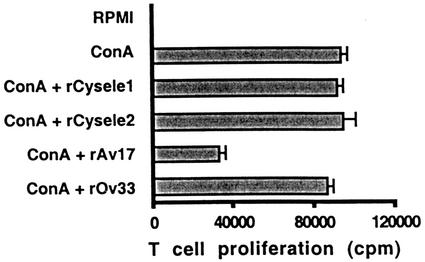
In a parallel approach, we studied the reaction of concanavalin A-stimulated murine spleen cells to rAv17, the cystatin of the rodent filaria A. viteae, in comparison to rCysele1 and rCysele2. While rAv17 significantly suppressed cellular proliferation by 63.5% (P = 0.001), rCysele1 and rCysele2 had no effect on the polyclonally stimulated proliferation of BALB/c spleen cells (Fig. 3). The data from the murine system supported the above results obtained with human cells.
FIG. 3.
Mitogen-induced murine T-cell proliferation in the presence of recombinant nematode cystatins. Concanavalin A (ConA)-stimulated proliferation of BALB/c spleen cells in the presence of a constant amount (0.25 μM) of rCysele1, rCysele2, rAv17, or rOv33. The data shown were obtained with 3.5 × 105 spleen cells from one representative mouse.
Antigen-stimulated proliferation of human PBMC and murine spleen cells.
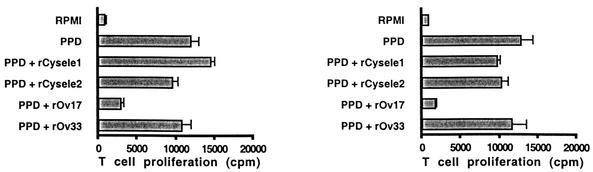
To compare the effects of nematode cystatins on antigen-driven T-cell proliferation, we used purified protein derivative (PPD) of Mycobacterium tuberculosis to stimulate human PBMC. While O. volvulus cystatin induced an inhibition of 72% of the PPD-stimulated proliferation of human PBMC, rCysele1 as well as rCysele2 did not exert a significant effect on the PPD-induced proliferation of human PBMC (Fig. 4). The recombinant O. volvulus control protein rOv33 also did not show a significant effect on the PPD-induced proliferation of the same blood samples.
FIG. 4.
Antigen-driven proliferation of human PBMC in the presence of recombinant nematode cystatins. PPD-stimulated proliferation of PBMC of two representative blood donors in the presence of 0.5 μM rCysele1, rCysele2, or rOv17 or of the control protein rOv33. The data shown were obtained with 3.5 × 105 cells from two representative blood donors.
In parallel, we studied a murine model of cellular proliferation with spleen cells of ovalbumin receptor transgenic mice stimulated with 40 μM ovalbumin. In the presence of rAv17 (0.25 μM), we found an inhibition of cellular proliferation of 68.4% (P = 0.01). In contrast, rCysele1 and rCysele2 as well as the control protein rOv33 had no significant effects on the antigen-driven cellular proliferation of the murine spleen cells (Fig. 5).
FIG. 5.
Antigen-driven proliferation of murine spleen cells in the presence of recombinant nematode cystatins. Ovalbumin (OVA)-stimulated proliferation of spleen cells of ovalbumin receptor transgenic mice in the presence of a constant amount (0.25 μM) of rCysele1, rCysele2, rAv17, or rOv33. The data shown were obtained with spleen cells from five individual mice.
These results demonstrate that the filarial cystatins rOv17 and rAv17 interfere with the antigen-driven cellular proliferation of human PBMC and mouse spleen cells, as we have shown in previous studies, while the cystatins of C. elegans did not share this capacity.
Cytokine production of human PBMC in presence of rCysele1 and rCysele2.
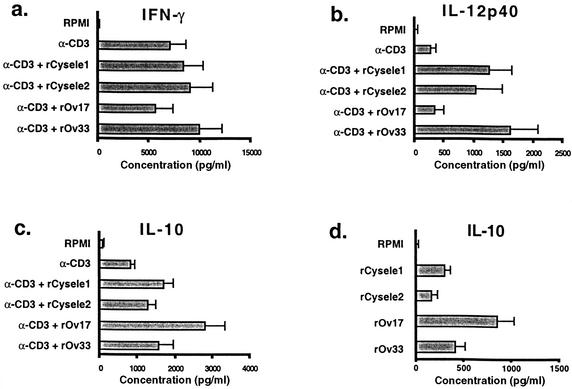
One possible mechanism by which the filarial cystatins mediate the suppression of T cells is interference with cytokine expression by immune cells. Previous studies have shown that the most prominent effect on cytokine production in the presence of the filarial cystatins was an upregulation of IL-10. In contrast, IL-12 and IFN-γ production was decreased in the presence of rOv17 in comparison to the control protein (42). We comparatively analyzed whether this pattern of cytokine production was also induced by C. elegans cystatin.
Tests with anti-CD3-stimulated human PBMC showed that rOv17 induced a significant upregulation of IL-10. PBMC produced in the presence of rOv17, a mean of 2.8 ng of IL-10/ml (P = 0.001), while the IL-10 production by PBMC in the presence of the C. elegans cystatins reached the level of the control protein (1.5 ng/ml) (Fig. 6). The differences in IL-10 production between rOv17 and rCysele1 and rOv17 as well as rCysele2 were significant (P = 0.05).
FIG. 6.
Production of IL-10, IL-12, and IFN-γ by human PBMC in the presence of recombinant nematode cystatins. Anti-CD3 antibody-stimulated production of IL-10 (a), IL-12 p40 (b), and IFN-γ (c) by PBMC from eight healthy donors after 48 h. (d) Spontaneous production of IL-10 in the blood of six donors after 24 h. The concentration of rCysele1, rCysele2, rOv17, and the control protein rOv33 was 0.5 μM.
The IL-12 production of anti-CD3-stimulated human PBMC was not significantly altered by the presence of rOv17, while rCysele1 and rCysele2 significantly (P = 0.001) enhanced the release of IL-12 by about 4.3-fold in comparison to the anti-CD3 stimulation (Fig. 6). The IL-12 levels measured in the presence of the control protein were even higher than those induced by rCysele1 and rCysele2. The IFN-γ production showed a trend to be downregulated in the presence of the O. volvulus cystatin, while rCysele1 and rCysele2 had a tendency to upregulate the production of IFN-γ (Fig. 6), similar to the control protein. The differences between the IFN-γ levels of the cystatins compared to the control protein were not statistically significant. However, differences in the IFN-γ levels between rOv17 and rCysele1 as well as rCysele2 were significant (P = 0.05).
In parallel, we determined the amounts of IL-10 and IL-12p40 in culture supernatant of unstimulated PBMC of six individuals after 24 h. The test revealed that both C. elegans cystatins had no effect on the production of IL-10 and IL-12p40 of unstimulated cells and therefore reacted like the control protein rOv33. In contrast, rOv17 induced a significant increase of the cytokine IL-10 in unstimulated cells (P = 0.001, Fig. 6) but had no influence on the IL-12p40 production in unstimulated cells.
These data show that the filarial cystatins increased IL-10 production significantly better than the C. elegans cystatin. In contrast, C. elegans cystatins enhanced the production of the Th1 cytokines IL-12 and IFN-γ, which was not the case for the parasite cystatins.
Production of inducible nitric oxide by nematode cystatins.
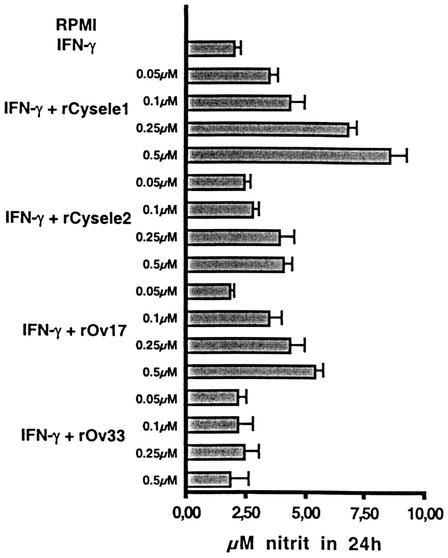
Members of the cystatin superfamily, e.g., chicken cystatin, have been described to cause an upregulation of the inducible nitric oxide production of IFN-γ-activated macrophages. (44), and the same applies to A. viteae and O. volvulus cystatins (17). Therefore, we tested whether C. elegans cystatins also modulate the nitric oxide production of IFN-γ-activated macrophages. Peritoneal macrophages of BALB/c mice were stimulated with IFN-γ for 24 h, and nitric oxide production was measured by the stable degradation product nitrite in the culture supernatant. The culture supernatant of IFN-γ-activated macrophages of BALB/c mice contained 2 μM ± 0.2 μM nitrite, whereas no nitrite was detected in nonactivated cells. The addition of 0.5 μM rCysele1 led to an increase in nitric oxide production up to 8.5 μM ± 0.66 μM nitrite (P = 0.001), while 0.5 μM rCysele2 led to an increased production of nitric oxide of 4 μM ± 0.39 μM nitrite (P = 0.001, Fig. 7). The filarial cystatin rOv17 stimulated nitric oxide production to 5.4 μM ± 0.32 μM in the presence of the molecule at 0.5 μM (P = 0.001), while the recombinant filarial control protein (rOv33) had no effect on the inducible nitric oxide production of macrophages.
FIG. 7.
Nitric oxide production by IFN-γ-activated murine macrophages in the presence of recombinant nematode cystatins. Peritoneal macrophages from BALB/c mice were activated with IFN-γ for 24 h and incubated with various concentrations of rCysele1, rCysele2, rOv17, and rOv33. The nitric oxide production of macrophages was determined in the culture supernatant by measuring nitrite. Data shown are means ± standard deviation of triplicate determinations.
These data show that the capacity to upregulate the inducible nitric oxide production is a common feature of nematode cystatins, irrespective of the life style of the worms.
DISCUSSION
This study compares the immunomodulatory activity of cystatins of parasitic filarial nematodes and of the free-living nematode C. elegans. One of the major characteristics of filarial cystatins shown here is their capacity to interfere with proliferation of host T cells. In contrast, the C. elegans cystatins Cysele1 and Cysele2 used in our experiments had this effect to a much lesser extent. Neither the antigen-driven proliferation of ovalbumin-stimulated spleen cells from ovalbumin receptor transgenic mice nor the mitogen-stimulated proliferation of mice were affected by C. elegans cystatins compared to the cystatin of the rodent filaria A. viteae. This lack of immunomodulatory capacity was also found when human PBMC stimulated with anti-CD3 antibodies or with PPD were exposed to cystatins of C. elegans. In contrast, the cystatin of the human-pathogenic filaria O. volvulus strongly interfered with the proliferation of human PBMC.
These data on filarial cystatins support the results of earlier studies. A. viteae cystatin inhibits murine T-cell proliferation, which coincides with the upregulation of IL-10 (16). O. volvulus cystatin suppresses polyclonally stimulated as well as antigen-driven cellular proliferation of human PBMC, accompanied by downregulation of MHC class II protein and the costimulatory molecule CD86 on monocytes (42). Effects in line with these data were described for Brugia malayi cystatin, which inhibits class II MHC-restricted antigen processing (25). Moreover, application of Litomosoides sigmodontis cystatin to experimental animals reduced the antigen-specific cellular reactivity of murine spleen cells ex vivo (36).
In an attempt to identify differences between the nematode cystatins that could account for the observed differential immunomodulatory properties, we comparatively characterized filarial cystatins and C. elegans cystatins by several approaches. Our Ki value determinations of nematode cystatins performed with the human cathepsins B, L, and S may reflect differences in the amino acid sequences. Differences in the N-terminal active domain of cystatins have been shown to change their inhibitory capacity (4, 13, 14). While the Ki values of O. volvulus cystatin and C. elegans cystatin for cathepsins L and S were in the same range, cathepsin B was significantly better inhibited by C. elegans cystatins than by O. volvulus cystatin. This is of particular interest because cathepsin B was described to play a role in the destructive degradation of antigenic fragments during the process of antigen presentation (7, 27). It has been shown that inhibition of cathepsin B resulted in enhancement rather than inhibition of presentation of specific antigens (7, 26). This suggests that efficient cathepsin B inhibitors like Cysele1 and Cysele2 might protect epitopes from proteolytic destruction and would thus support antigen presentation and antigen-specific proliferation of T cells.
Apart from antigen presentation, protease inhibitors of the papain-like proteases (family 1) also have other important functions. In experimental murine leishmaniasis, it was shown that treatment of mice with a synthetic cathepsin B inhibitor induced a switch of CD4+ T-cell differentiation from Th2 to Th1. CA074, a specific inhibitor of cathepsin B, appeared to block the development of the Th2 response that usually develops in BALB/c mice infected with Leishmania major and, in turn, promoted the development of Th1 cells (21). Further studies with site-directed mutagenesis will show whether the increased inhibition of cathepsin B by C. elegans cystatins is related to the observed amino acid differences in the N-terminal inhibitory domain. However, cathepsins L and S also have essential functions in processing and presentation of antigens (31, 32, 33, 41), and our experiments show that they are similarly inhibited by nematode cystatins.
Our evaluation of the cytokine production of human PBMC in the presence of O. volvulus cystatin confirms earlier studies on the effect of filarial cystatins on murine cells and human cells (16, 42). The comparison with C. elegans cystatins revealed a prominent difference. Human PBMC exposed to O. volvulus cystatin induced TNF-α, followed by a strong production of IL-10. This IL-10 burst would support Th2-like cytokine responses (29). In contrast, both C. elegans cystatins upregulated the production of the Th1 cytokines IL-12 and IFN-γ, after an initial release of TNF-α. In this regard, the C. elegans cystatins behave very much like chicken cystatin, which increases the levels of IL-12 and reduces the levels of IL-4, leading to a switch of CD4+ differentiation from Th2 to Th1, as shown in a study of experimental leishmaniasis (6).
The molecular basis for this differential induction of cytokines is currently unclear. The induction of TNF-α by all nematode cystatins is compatible with a binding of proteins to toll-like receptors of human PBMC, a class of receptors which have been described to mediate responses to microbial ligands (43). An upregulation of TNF-α followed by an upregulation of IL-10 is a characteristic pattern of events evoked by contact of macrophages with bacterial lipopolysaccharide and related substances (28, 38). However, an influence of lipopolysaccharide has been excluded in this and previous studies by careful controls. We therefore assume that cystatins have the inherent property to induce TNF-α and IL-10. The degree to which IL-10 is produced might be determined by the other cytokines produced at the same time as a response to cystatin. The relative lack of IL-10 upregulation by C. elegans cystatin might be due partly to the parallel increased production of the Th1 cytokines IL-12 and IFN-γ.
The theoretical consequences of the IL-10 burst after contact with filarial cystatins coincide well with our observations. IL-10 is a cytokine which causes immunosuppressive effects, leading to reduced T-cell proliferation (8). A combination of IL-10 and TGF-β produced by regulatory T cells is well known to account for immunological tolerance to food antigens (12) and for cellular unresponsiveness in filarial infections (9). Such a combined production of both cytokines did not occur in our context, since the TGF-β responses of human PBMC were not altered after contact with nematode cystatins (our unpublished data). However, IL-10 on its own might have modulatory effects, since cellular hyporeactivity in filarial infections has been associated with this cytokine (22, 34). Therefore, an increased capacity to induce IL-10 might be an adaptation to the parasitic life style.
The enhancement of the inducible nitric oxide production of IFN-γ-stimulated murine macrophages by all tested nematode cystatins confirms earlier studies on filarial cystatins (17). This upregulation of inducible nitric oxide can be considered a common quality of cystatins, as chicken cystatin, human stefin B, and rat kininogen upregulate the nitric oxide production of IFN-γ-activated murine macrophages (44). Interestingly, the upregulation of nitric oxide by cystatin is considered not to be due to its biological activity as a protease inhibitor, as related synthetic protease inhibitors did not cause this effect (17, 44). The induction of nitric oxide by cystatins was suggested to be at least partly due to a modulation of the cytokine production exerted by these molecules (17, 45).
In conclusion, our comparison with C. elegans cystatins reveals that cystatins of the parasitic filarial nematodes seem to exert their effect by at least two distinct mechanisms that act in concert, the inhibition of proteases and the induction of cytokines. The low inhibition by O. volvulus cystatin of cathepsin B together with a strong inhibition of cathepsins L and S is prone to inhibit antigen processing and presentation. In contrast, the strong inhibition of cathepsin B by the C. elegans cystatins will support antigen presentation and at the same time lead to Th1-like responses. These tendencies would be reinforced by the cytokine responses, in that the filarial cystatins induce mainly IL-10, which inhibits antigen presentation and favors the development of Th2-like responses. In contrast, the C. elegans cystatins reinforce the tendency towards a Th1-like response by inducing IL-12 and IFN-γ. This intricate interplay between protease inhibition and induction of cytokine response supposedly leads to the induction of anti-inflammatory responses and contributes to the persistence of filariae in the host.
Acknowledgments
We gratefully acknowledge support of this study by a grant from the Deutsche Forschungsgemeinschaft (HA 2542/1-3) to S.H.
We thank E. Rödel (Institute for Informatics, Humboldt University of Berlin) for help with the statistical analysis.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Allen, J. E., and A. S. MacDonald. 1998. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 20:241-247. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Fernandez, M., A. J. Barrett, B. Gerhartz, P. M. Dando, J. Ni, and M. Abrahamson. 1999. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J. Biol. Chem. 274:19195-19203. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, A. J., and H. Kirschke. 1981. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 80:535-561. [DOI] [PubMed] [Google Scholar]
- 4.Bjork, I., I. Brieditis, and M. Abrahamson. 1995. Probing the functional role of the N-terminal region of cystatins by equilibrium and kinetic studies of the binding of Gly-11 variants of recombinant human cystatin C to target proteinases. Biochem. J. 306:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dainichi, T., Y. Maekawa, K. Ishii, T. Zhang, B. F. Nashed, T. Sakai, M. Takashima, and K. Himeno. 2001. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect. Immun. 69:7380-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das, L., N. Datta, S. Bandyopadhyay, and P. K. Das. 2001. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T-cell response. J. Immunol. 166:4020-4028. [DOI] [PubMed] [Google Scholar]
- 7.Deussing, J., W. Roth, P. Saftig, C. Peters, H. L. Ploegh, and J. A. Villadangos. 1998. Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc. Natl. Acad. Sci. USA 95:4516-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Waal Malefyt, R., J. Haanen, H. Spits, M. G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T-cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doetze, A., J. Satoguina, G. Burchard, T. Rau, C. Loliger, B. Fleischer, and A. Hoerauf. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int. Immunol. 12:623-630. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Escobar, N., E. Lewis, and R. M. Maizels. 1998. A novel member of the transforming growth factor-beta (TGF-beta) superfamily from the filarial nematodes Brugia malayi and B. pahangi. Exp Parasitol. 88:200-209. [DOI] [PubMed] [Google Scholar]
- 11.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 12.Groux, H., and F. Powrie. 1999. Regulatory T cells and inflammatory bowel disease. Immunol. Today 20:442-445. [DOI] [PubMed] [Google Scholar]
- 13.Hall, A., H. Dalboge, A. Grubb, and M. Abrahamson. 1993. Importance of the evolutionarily conserved glycine residue in the N-terminal region of human cystatin C (Gly-11) for cysteine endopeptidase inhibition. Biochem. J. 291:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall, A., K. Hakansson, R. W. Mason, A. Grubb, and M. Abrahamson. 1995. Structural basis for the biological specificity of cystatin C. Identification of leucine 9 in the N-terminal binding region as a selectivity-conferring residue in the inhibition of mammalian cysteine peptidases. J. Biol. Chem. 270:5115-5121. [DOI] [PubMed] [Google Scholar]
- 15.Harnett, W., M. R. Deehan, K. M. Houston, and M. M. Harnett. 1999. Immunomodulatory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 21:601-608. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann, S., B. Kyewski, B. Sonnenburg, and R. Lucius. 1997. A filarial cysteine protease inhibitor down-regulates T-cell proliferation and enhances interleukin-10 production. Eur. J. Immunol. 27:2253-2260. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann, S., A. Schonemeyer, B. Sonnenburg, B. Vray, and R. Lucius. 2002. Cystatins of filarial nematodes up-regulate the nitric oxide production of interferon-gamma-activated murine macrophages. Parasite Immunol. 24:253-262. [DOI] [PubMed] [Google Scholar]
- 18.Leung-Tack, J., C. Tavera, A. Er-Raki, M. C. Gensac, and A. Colle. 1990. Rat cystatin C: inhibitor of granulocyte phagocytic functions. Biol. Chem. Hoppe Seyler 371:255-258. [PubMed] [Google Scholar]
- 19.Loke, P., A. S. MacDonald, A. Robb, R. M. Maizels, and J. E. Allen. 2000. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur. J. Immunol. 30:2669-2678. [DOI] [PubMed] [Google Scholar]
- 20.Lucius, R., N. Erondu, A. Kern, and J. E. Donelson. 1988. Molecular cloning of an immunodominant antigen of Onchocerca volvulus. J. Exp. Med. 168:1199-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maekawa, Y., K. Himeno, H. Ishikawa, H. Hisaeda, T. Sakai, T. Dainichi, T. Asao, R. A. Good, and N. Katunuma. 1998. Switch of CD4+ T-cell differentiation from Th2 to Th1 by treatment with cathepsin B inhibitor in experimental leishmaniasis. J. Immunol. 161:2120-2127. [PubMed] [Google Scholar]
- 22.Mahanty, S., S. N. Mollis, M. Ravichandran, J. S. Abrams, V. Kumaraswami, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1996. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J. Infect. Dis. 173:769-773. [DOI] [PubMed] [Google Scholar]
- 23.Maizels, R. M., D. A. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 24.Manoury, B., E. W. Hewitt, N. Morrice, P. M. Dando, A. J. Barrett, and C. Watts. 1998. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature 396:695-699. [DOI] [PubMed] [Google Scholar]
- 25.Manoury, B., W. F. Gregory, R. M. Maizels, and C. Watts. 2001. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr. Biol. 11:447-451. [DOI] [PubMed] [Google Scholar]
- 26.Manoury-Schwartz, B., G. Chiocchia, V. Lotteau, and C. Fournier. 1997. Selective increased presentation of type II collagen by leupeptin. Int. Immunol. 9:581-589. [DOI] [PubMed] [Google Scholar]
- 27.Medd, P. G., and B. M. Chain. 2000. Protein degradation in MHC class II antigen presentation: opportunities for immunomodulation. Semin. Cell Dev. Biol. 11:203-210. [DOI] [PubMed] [Google Scholar]
- 28.Meisel, C., K. Vogt, C. Platzer, F. Randow, C. Liebenthal, and H. D. Volk. 1996. Differential regulation of monocytic tumor necrosis factor-alpha and interleukin-10 expression. Eur. J. Immunol. 26:1580-1586. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann, T. R. 1994. Properties and functions of interleukin-10. Adv. Immunol. 56:1-26. [PubMed] [Google Scholar]
- 30.Murphy, K. M., A. B. Heimberger, and D. Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250:1720-1723. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa, T., W. Roth, P. Wong, A. Nelson, A. Farr, J. Deussing, J. A. Villadangos, H. Ploegh, C. Peters, and A. Y. Rudensky. 1998. Cathepsin L: critical role in Ii degradation and CD4 T-cell selection in the thymus. Science 280:450-453. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa, T. Y., W. H. Brissette, P. D. Lira, R. J. Griffiths, N. Petrushova, J. Stock, J. D. McNeish, S. E. Eastman, E. D. Howard, S. R. Clarke, E. F. Rosloniec, E. A. Elliott, and A. Y. Rudensky. 1999. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity 10:207-217. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa, T. Y., and A. Y. Rudensky. 1999. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunol. Rev. 172:121-129. [DOI] [PubMed] [Google Scholar]
- 34.Osborne, J., and E. Devaney. 1999. Interleukin-10 and antigen-presenting cells actively suppress Th1 cells in BALB/c mice infected with the filarial parasite Brugia pahangi. Infect. Immun. 67:1599-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pastrana, D. V., N. Raghavan, P. FitzGerald, S. W. Eisinger, C. Metz, R. Bucala, R. P. Schleimer, C. Bickel, and A. L. Scott. 1998. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect. Immun. 66:5955-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaff, A. W., H. Schulz-Key, P. T. Soboslay, D. W. Taylor, K. MacLennan, and W. H. Hoffmann. 2002. Litomosoides sigmodontis cystatin acts as an immunomodulator during experimental filariasis. Int. J. Parasitol. 32:171-178. [DOI] [PubMed] [Google Scholar]
- 37.Plaisier, A. P., G. J. van Oortmarssen, J. Remme, and J. D. Habbema. 1991. The reproductive lifespan of Onchocerca volvulus in West African savanna. Acta Trop. 48:271-284. [DOI] [PubMed] [Google Scholar]
- 38.Platzer, C., C. Meisel, K. Vogt, M. Platzer, and H. D. Volk. 1995. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int. Immunol. 7:517-523. [DOI] [PubMed] [Google Scholar]
- 39.Plier, D. A., K. Awadzi, and D. O. Freedman. 1996. Immunoregulation in onchocerciasis: persons with ocular inflammatory disease produce a Th2-like response to Onchocerca volvulus antigen. J. Infect. Dis. 174:380-386. [DOI] [PubMed] [Google Scholar]
- 40.Pritchard, D. I., C. E. Lawrence, P. Appleby, I. A. Gibb, K. Glover. 1994. Immunosuppressive proteins secreted by the gastrointestinal nematode parasite Heligmosomoides polygyrus. Int. J. Parasitol. 24:495-500. [DOI] [PubMed] [Google Scholar]
- 41.Riese, R. J., P. R. Wolf, D. Bromme, L. R. Natkin, J. A. Villadangos, H. L. Ploegh, and H. A. Chapman. 1996. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity 4:357-366. [DOI] [PubMed] [Google Scholar]
- 42.Schönemeyer, A., R. Lucius, B. Sonnenburg, N. Brattig, R. Sabat, K. Schilling, J. Bradley, and S. Hartmann. 2001. Modulation of human T-cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J. Immunol. 167:3207-3215. [DOI] [PubMed] [Google Scholar]
- 43.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 44.Verdot, L., G. Lalmanach, V. Vercruysse, S. Hartmann, R. Lucius, J. Hoebeke, F. Gauthier, and B. Vray. 1996. Cystatins up-regulate nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages. J. Biol. Chem. 271:28077-28081. [DOI] [PubMed] [Google Scholar]
- 45.Verdot, L., G. Lalmanach, V. Vercruysse, J. Hoebeke, F. Gauthier, and B. Vray. 1999. Chicken cystatin stimulates nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages via cytokine synthesis. Eur. J. Biochem. 266:1111-1117. [DOI] [PubMed] [Google Scholar]
- 46.von Baehr, V., W. Mayer, C. Liebenthal, von R. Baehr, W. Bieger, and H. D. Volk. 2001. Improving the in vitro antigen specific T-cell proliferation assay: the use of interferon-alpha to elicit antigen specific stimulation and decrease bystander proliferation. J. Immunol Methods 251:63-71. [DOI] [PubMed] [Google Scholar]
- 47.Vray, B., S. Hartmann, and J. Hoebeke. 2002. Immunomodulatory properties of cystatins. Cell. Mol. Life Sci. 59:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson, R., R. Ainscough, K. Anderson, C. Baynes, M. Berks, J. Bonfield, J. Burton, M. Connell, T. Copsey, J. Cooper, et al. 1994. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature 368:32-38. [DOI] [PubMed] [Google Scholar]
- 49.Zang, X., M. Yazdanbakhsh, H. Jiang, M. R. Kanost, and R. M. Maizels. 1999. A novel serpin expressed by blood-borne microfilariae of the parasitic nematode Brugia malayi inhibits human neutrophil serine proteinases. Blood 94:1418-1428. [PubMed] [Google Scholar]