Abstract
Intimin-conjugated fluorescent beads bind to spleen CD4 T cells and Peyer's patch, mesenteric lymph node, and cecal follicle lymphocytes, with less binding to lamina propria T cells and intraepithelial lymphocytes. Intimin costimulates proliferation of spleen CD4 T cells and cells from organized lymphoid tissues but does not costimulate cells from the lamina propria of normal or inflamed colon.
The first gene to be associated with the attaching and effacing activity of enteropathogenic Escherichia coli (EPEC) was the intimin gene (15). Five different intimin types (α, β, γ, δ, and ɛ) have been identified (1, 19). The best-characterized receptor for intimin is the translocated intimin receptor (Tir), which EPEC, enterohemorrhagic E. coli (EHEC), and Citrobacter rodentium inject into the enterocyte by using a type III secretion system (16). Intimin also binds to eukaryotic receptors. Purified intimin binds to HEp-2 cells, and cell-binding activity localizes to the C-terminal 280 amino acids (Int280) (8). A specific cysteine residue in EPEC intimin (Cys937) is essential for the binding activity (8, 11). Intimin α also binds to β1 integrins in vitro (9). Recently, intimin γ from EHEC 0157 has been shown to bind cell surface nucleolin on HEp-2 cells (24).
These data show that intimin interacts not only with Tir but also with the host cell intimin receptor(s), but the functional consequences of intimin binding to mammalian receptors is not well understood. Latex beads coated with intimin induce the formation of villi-like structures on HEp2 cells (20). Intimin, like invasin of Yersinia spp., also potently stimulates T-cell proliferation (2, 6, 14). Since EPEC, EHEC, and C. rodentium are gut pathogens, intimin has the potential to interact with cells of the gut immune system in vivo. In this study, Int280 interactions with the mucosal and systemic immune system have been characterized by using flow cytometry and proliferation assays.
Maltose binding protein (MBP)-Int280α and MBP-Int280CS (MBP-Int280 in which Cys937 was replaced with Ser) were made as previously described (7). Two hundred microliters of fluorescent carboxylate-modified latex beads (1-μm diameter; Sigma product L3155 red or L4655 green; 2.5% solid) were mixed with 20 μg of MBP-Int280 and MBP-Int280CS in the presence of 1.6 mg of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide prediluted in 80 μl of 15 mM sodium acetate buffer (pH 5.0). Following overnight mixing at room temperature, 1/10 of the volume of 1 M glycine was added (approximately 30 μl) and the samples were mixed for a further 30 min at room temperature. To terminate the reaction, the beads were washed four times in freshly prepared 50 mM phosphate buffer (pH 7.4) (1.9 ml of 200 mM NaH2PO4, 8.1 ml of 200 mM Na2HPO4, 30 ml of H2O) containing 0.9% NaCl. The beads were resuspended and stored in 500 μl of 50 mM phosphate buffer-1% bovine serum albumin. Prior to use, 1-μl volumes were overlaid onto HEp2 cells to confirm binding.
Six-week-old female BALB/c mice obtained from A. Tuck & Sons, Southend-on-Sea, United Kingdom, were used in all experiments. TNBS colitis in mice was induced as previously described (13). Suspensions of spleen cells, Peyer's patch lymphocytes, mesenteric lymph node lymphocytes, cecal lymphoid follicle lymphocytes, colonic intestinal intraepithelial lymphocytes (IEL), and lamina propria lymphocytes (LPL) were prepared as previously described (27). CD4+ T cells from spleen cell suspensions were purified by using a magnetic-activated cell sorter system (Miltenyi Biotec, Auburn, Calif). Lymphocyte proliferation assays using concanavalin A (ConA) and anti-CD3 were carried out exactly as described previously (12, 14). Cells were also stimulated with purified Int280 (14) or with MBP-intimin. Preliminary experiments indicated that both intimins costimulated anti-CD3-activated spleen cells (data not shown).
From Pharmingen, we obtained fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated anti-CD3ɛ monoclonal antibody (mAb) 145-2C11, FITC-conjugated anti-CD4 mAb RM4-5, PE-conjugated anti-CD8α mAb 53-6.7, FITC-conjugated isotype control mAb R35-95, and PE-conjugated isotype control mAb R-PE. The integrin α4 chain mAb PS/2 was kindly provided by P. Kilshaw, Babraham Institute, Cambridge, United Kingdom. Lymphocytes were resuspended in phosphate-buffered saline-0.1% (wt/vol) sodium azide supplemented with 0.2% (wt/vol) fetal calf serum at a final concentration of 2.5 × 105 cells/tube. Cells were incubated with FITC- or PE-CD3, FITC-CD4, PE-CD8, intimin fluorescent beads, and integrin antibodies for 1 h on ice. Unconjugated antibodies were detected with an FITC-rabbit anti-rat antibody (Sigma). Cells were then washed with fluorescence-activated cell sorter buffer at 1,500 rpm for 10 min and analyzed by using a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.). Results were analyzed using Cell Quest software (Becton Dickinson). The percentage of cells which bound beads was the percentage which gave a signal above that of untreated lymphocytes.
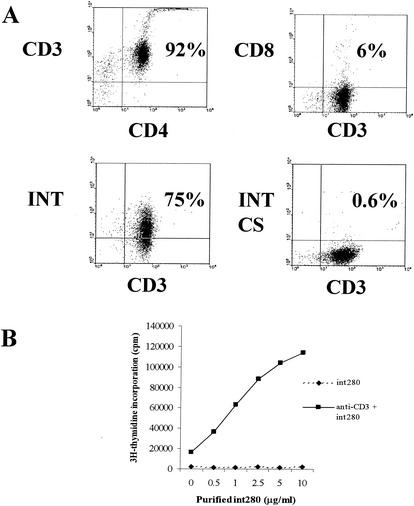
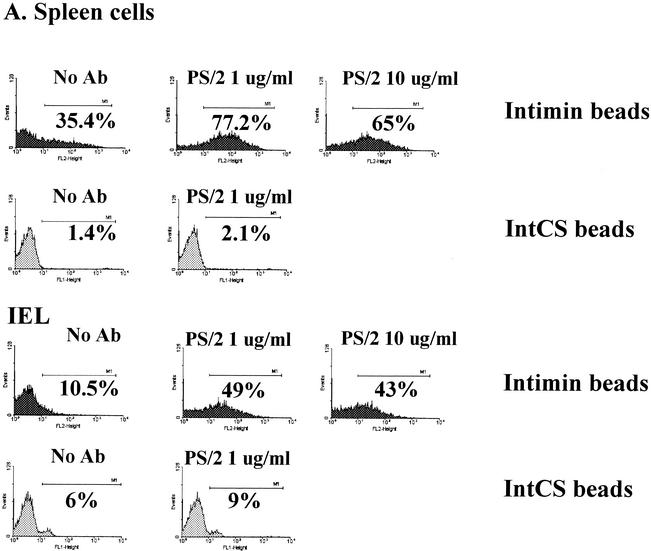
When double labeled with intimin beads and CD3, approximately 75% of the purified CD4 cells showed binding of intimin (Fig. 1A). Beads conjugated to intimin CS showed no binding. The addition of graded doses of intimin α markedly enhanced the proliferative response of purified CD4 cells (Fig. 1B). The binding to Int280 was highest for splenocytes and mesenteric lymph node cells (44%) and lowest for IEL (10%) (Table 1). α4β1 Integrin expression has been shown previously to be high in splenocytes and low in IEL (4, 10). Splenocytes and IEL were incubated with the blocking anti-α4 PS/2 antibody (23) for 1 h at 4°C, washed, and then incubated with the intimin fluorescent beads. PS/2 showed binding to IEL (Fig. 2B) and spleen cells (data not shown); however, antibody pretreatment increased intimin binding to both IELs and spleen cells (Fig. 2A). These results suggest that intimin binds to integrins at a site different from that involved in VCAM and fibronectin binding (5).
FIG. 1.
(A) Dual-parameter flow cytometry on purified spleen CD4 cells. The upper left and right panels show that the magnetic-activated cell sorter-purified cells were highly enriched for CD4 cells. The bottom two panels show that 75% of CD3+ cells (in the CD4 purified cells) also bound intimin. The ratio of intimin beads to cells is 5:1. This experiment was repeated three times, and representative results are shown. (B) Anti-CD3 stimulation of splenic CD4+ T lymphocytes in the presence of purified Int280 (dose response from 0.5 to 10 μg/ml). Incubation was for 64 h. Results shown are from one experiment that is representative of two. Values are means ± standard errors of the mean (SEM). Int280 costimulates CD4+ T lymphocytes in a dose-dependent manner.
TABLE 1.
Summary of fluorescent MBP-Int280 and MBP-Int280CS bead binding to mucosal and splenic lymphocytes
| Lymphocyte | % Bindinga
|
|
|---|---|---|
| Int280 | Int280CS | |
| Splenic | 44 ± 6 | 1 ± 0.1 |
| Mesenteric lymph node | 44 ± 5 | 1 ± 0.5 |
| Peyer's patch | 36 ± 3 | 1 ± 0.3 |
| LPL | 24 ± 7 | 1 ± 0.5 |
| IEL | 10 ± 2 | 1.3 ± 0.3 |
Bead-to-cell ratio of 2:1. Results are means ± 1 SE of three experiments. Binding to LPL and IEL was significantly lower than binding to Peyer's patch, mesenteric lymph node, and spleen cells (P < 0.05; Mann-Whitney U test). A greater percentage of spleen cells and IEL were positive when the ratio of beads to cells was raised to 5:1, but the same pattern was seen in different tissues.
FIG. 2.
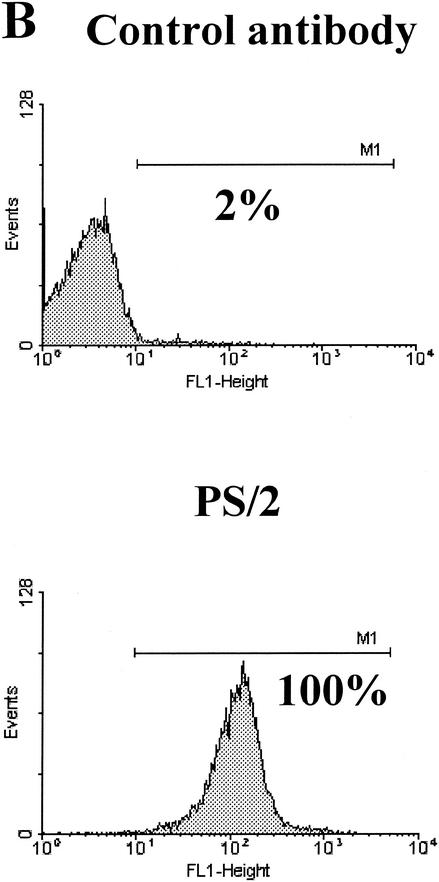
(A) Flow cytometry plots for splenocytes and IEL incubated with PS/2 at 1 and 10 μg/ml and then intimin beads at a cell-to-bead ratio of 1:2. Ab, antibody. (B) The PS/2 antibody stains all IEL, showing that α4β1 integrins are present on the cell surface.
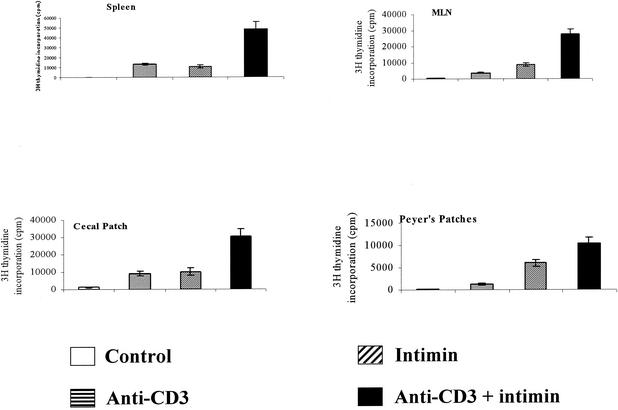
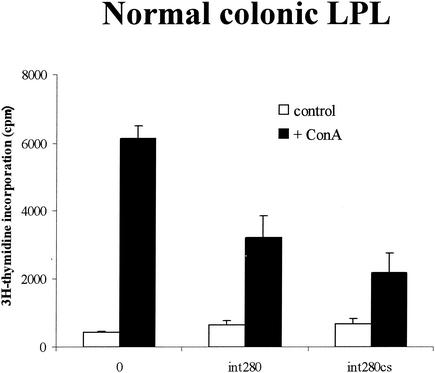
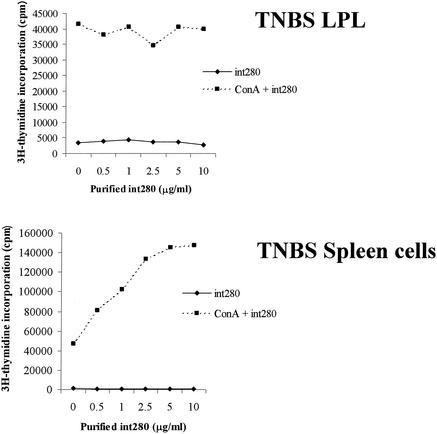
On their own, anti-CD3 or intimin elicited weak responses in cells from organized lymphoid tissues (Fig. 3). However, when they were added in combination, there was marked enhancement of T-cell proliferative responses. Colonic lamina propria cells did not respond to CD3 stimulation in vitro (data not shown) nor did they respond to Int280 or Int280CS alone, but they did give a weak proliferative response to ConA (5 μg/ml) (Fig. 4). Enhancement by Int280 or Int280CS was not seen (Fig. 4). To induce a nonspecific influx of T cells into the mucosa, as might happen during natural EPEC, EHEC, or C. rodentium infection, we injected TNBS in 50% ethanol intracolonically into mice. Lamina propria T cells from TNBS-colitic mice responded vigorously to ConA, but the addition of intimin had no costimulatory effects. Splenocytes from the same mice responded well to ConA, and intimin massively boosted the response (Fig. 5). Repeated attempts to elicit anti-CD3 or ConA proliferative responses from colonic IEL were unsuccessful (data not shown).
FIG. 3.
Anti-CD3 stimulation of splenocytes, mesenteric lymph node (MLN) lymphocytes, cecal lymphoid follicle lymphocytes, and Peyer's patch lymphocytes in the presence of MBP-Int280 at 5 μg/ml. Incubation was for 64 h. Results are expressed as means ± SEM. This experiment was repeated and gave identical results. Although only a single concentration of intimin is shown, all responses showed a linear increase over the range of 0.5- to 10-μg of intimin/ml.
FIG. 4.
ConA (5 μg/ml) stimulation of colonic LPLs from normal mice in the presence of purified Int280 and Int280CS (10 μg/ml). Cells were cultured for 64 h. Results are expressed as means ± SEM. This experiment was repeated three times.
FIG. 5.
ConA (5 μg/ml) stimulation of colonic LPL (top) and splenocytes (bottom) taken from TNBS-colitic mice in the presence of purified Int280 (dose response from 0.5 to 10 μg/ml). Mice were killed 4 days after induction of colitis. Cells were cultured for 64 h. Results are expressed as means ± SEM and are from one experiment that is representative of two.
In this work, we show unequivocally that intimin binds to approximately 75% of splenic CD4+ T cells. The tissues showing the highest percentage of lymphocytes binding intimin were the spleen, mesenteric lymph node, and Peyer's patches. In contrast, there was markedly reduced binding to colonic lamina propria T cells and IEL. Finally, whereas intimin is a potent costimulator of T-cell responses of cells from all organized lymphoid tissues tested, it had no effect on lamina propria T cells.
These results therefore extend the notion that intimin(s) has a mammalian receptor(s) (8, 17, 20). Diebel et al. (3) also showed that intimin β bound eukaryotic cells, but only the C-terminal domain and not the full-length molecule. Frankel et al. have also shown that intimin binds to isolated α4β1 and α5β1 integrins (9). Finally, it has recently been shown that intimin γ binds to nucleolin (24).
Based on our group's previous work (9), we therefore considered that intimin might be binding to β1 integrins. However, when we attempted to block binding by using antibodies to the α4 integrin, we actually enhanced binding. The antibodies clearly bound to the surface of T cells, as shown by flow cytometry, so we are at a loss to explain these results mechanistically, unless of course blocking integrin binding allowed intimin to bind strongly to another ligand on the lymphocyte surface. We could not block the binding of lymphocytes to intimin with PS/2 antibody which recognizes the α4 chain of the α4β1 heterodimer (18), even though PS/2 has been shown to block binding to VCAM-1 and fibronectin (23).
When we attempted to costimulate LPL responses from normal mice or mice with an inflamed colon with intimin, we were unsuccessful. We can only speculate about the mechanism for this failure to costimulate, especially since in humans, anti-β1 integrin antibodies strongly costimulate anti-CD3 responses of LPL (25). It is, however, well established that LPL differ from systemic T cells in their activation requirements (21, 22, 26).
Intimin clearly has the potential to interact with cells of the immune system, but the consequences are not clear. We demonstrated that dead C. rodentium injected into the colon of mice elicited a mucosal lamina propria Th1 response and colonic hyperplasia, identical to what occurs with live infection (13, 14). We hypothesized that this was due to activation of resident LPL by intimin; however, the situation is clearly more complex. Other molecules present on the bacteria may be needed to activate lamina propria T cells. Alternatively, activation using CD3 and ConA may not reflect the in vivo situation. LPL probably have specificity for antigens of the bacterial flora. When the epithelial barrier is broken either by ethanol or by live C. rodentium infection, antigens of the flora may enter the lamina propria and, together with intimin, may be sufficient to activate the cells and cause cytokine secretion.
Acknowledgments
This work was supported by the European Union Training and Mobility of Researchers Programme (ERBFMRXCT9) and by the Wellcome Trust. Nathalie S. Goncalves was supported by the Crohn's in Childhood Research Association (CICRA).
We thank Stephen Reece for assistance.
Editor: A. D. O'Brien
REFERENCES
- 1.Adu-Bobie, J., G. Frankel, C. Bain, A. G. Goncalves, L. R. Trabulsi, G. Douce, S. Knutton, and G. Dougan. 1998. Detection of intimins α, β, γ and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J. Clin. Microbiol. 36:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brett, S. J., A. V. Mazurov, I. G. Charles, and J. P. Tite. 1993. The invasion protein of Yersinia spp. provides costimulatory activity to human T cells through interaction with β1 integrins. Eur. J. Immunol. 23:1608-1614. [DOI] [PubMed] [Google Scholar]
- 3.Diebel, C., P. Dersch, and F. Ebel. 2001. Intimin from Shiga toxin-producing Escherichia coli and its isolated C-terminal domain exhibit different binding properties for Tir and a eukaryotic surface receptor. Int. J. Med. Microbiol. 290:683-691. [DOI] [PubMed] [Google Scholar]
- 4.Ebert, E. C., and A. I. Roberts. 1996. Human intestinal intraepithelial lymphocytes bind to mucosal mesenchymal cells through VLA-4 and CD11a. Cell. Immunol. 167:108-114. [DOI] [PubMed] [Google Scholar]
- 5.Elices, M. J., L. Osborn, Y. Takada, C. Crouse, S. Luhowskyj, M. Hemer, and R. R. Lobb. 1990. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 60:577-584. [DOI] [PubMed] [Google Scholar]
- 6.Ennis, E., R. R. Isberg, and Y. Shimizu. 1993. Very late antigen 4-dependent adhesion and costimulation of resting human T cells by the bacterial β1 integrin ligand invasion. J. Exp. Med. 177:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frankel, G., D. C. Candy, P. Everest, and G. Dougan. 1994. Characterization of the C-terminal domains on intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect. Immun. 62:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel, G., D. C. A. Candy, E. Fabiani, J. Adu-Bobie, S. Gil, M. Novakova, A. D. Phillips, and G. Dougan. 1995. Molecular characterization of a carboxy-terminal eukaryotic-cell-binding domain of intimin from enteropathogenic Escherichia coli. Infect. Immun. 63:4323-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, S. G. Kachalsky, D. Candy, L. Cahalon, M. J. Humphries, and G. Dougan. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to β1 integrins. J. Biol. Chem. 271:20359-20364. [DOI] [PubMed] [Google Scholar]
- 10.Hamann, A., D. P. Andrew, D. Jablonski-Westrich, B. Holzmann, and E. C. Butcher. 1994. Role of alpha-4 integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 152:3282-3293. [PubMed] [Google Scholar]
- 11.Hicks, S., G. Frankel, J. B. Kaper, G. Dougan, and A. D. Phillips. 1998. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 66:1570-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins, L. M., S. A. C. McDonald, N. Whittle, N. Crockett, J. G. Shields, and T. T. MacDonald. 1999. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interaction: prevention of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J. Immunol. 162:486-493. [PubMed] [Google Scholar]
- 13.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to murine inflammatory bowel disease. Infect. Immun. 67:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalvez, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588-591. [DOI] [PubMed] [Google Scholar]
- 15.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 17.McKee, M. L., and A. D. O'Brien. 1996. Truncated enterohemorrhagic Escherichia coli (EHEC) 0157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to Hep2 cells. Infect. Immun. 64:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake, K., I. L. Weismann, J. S. Greenberger, and P. W. Kincade. 1991. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J. Exp. Med. 173:599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oswald, E., H. Schmidt, S. Morabito, H. Karch, O. Marches, and A. Caprioli. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips, A. D., J. Giron, S. Hicks, G. Dougan, and G. Frankel. 2000. Intimin from enteropathogenic Escherichia coli mediates remodelling of the eukaryotic cell surface. Microbiology 146:1333-1344. [DOI] [PubMed] [Google Scholar]
- 21.Pirzer, U. C., G. Schürmann, S. Post, M. Betzler, and S. C. Meuer. 1990. Differential responsiveness to CD3-Ti vs. CD2-dependent activation of human intestinal T lymphocytes. Eur. J. Immunol. 20:2339-2342. [DOI] [PubMed] [Google Scholar]
- 22.Qiao, L., G. Schürmann, M. Betzler, and S. C. Meuer. 1991. Activation and signaling status of human lamina propria T lymphocytes. Gastroenterology 101:1529-1536. [DOI] [PubMed] [Google Scholar]
- 23.Ruegg, C., A. A. Postigo, E. E. Sikorski, E. C. Butcher, R. Pytela, and D. J. Erle. 1992. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J. Cell Biol. 117:179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinclair, J. F., and A. D. O'Brien. 2002. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-γ of enterohemorrhagic Escherichia coli 0157:H7. J. Biol. Chem. 277:2876-2885. [DOI] [PubMed] [Google Scholar]
- 25.Stallmach, A., T. Giese, K. Pfister, B. M. Wittig, S. Kunne, M. Humphries, M. Zeitz, and S. Meuer. 2001. Activation of β1 integrins mediates proliferation and inhibits apoptosis of intestinal CD4-positive lymphocytes. Eur. J. Immunol. 31:1228-1238. [DOI] [PubMed] [Google Scholar]
- 26.Targan, S. R., R. L. Deem, M. Liu, S. Wang, and A. Nel. 1995. Definition of lamina propria T cell responsive state: enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J. Immunol. 154:664-675. [PubMed] [Google Scholar]
- 27.Viney, J. L., and T. T. MacDonald. 1992. Lymphokine secretion and proliferation of intraepithelial lymphocytes from murine small intestine. Immunology 77:19-24. [PMC free article] [PubMed] [Google Scholar]