Abstract
The increasing death toll from malaria, due to the decreasing effectiveness of current prophylactic and therapeutic regimens, has sparked a search for alternative methods of control, such as vaccines. Although several single proteins have shown some promise as subunit vaccines against sexual blood stages in experimental systems, it is clear that multicomponent vaccines are required. Many logistic difficulties make such an approach prohibitively expensive. In an effort to try to overcome some of these issues, we examined the possibility of oral immunization as a route for inducing host protective immunity. We report here that oral feeding of a malaria protein induced serum antibody levels similar to those induced by intraperitoneal immunization with Freund's adjuvant. Further, responses to conformational epitopes were induced. In the rodent challenge system, significant levels of protection to lethal challenge with malaria were induced in mice. The protective efficacy was highly correlated with antibody levels, which depended on the antigen dosage and required cholera toxin subunit B as an oral adjuvant. These findings offer new approaches to the development of a malaria vaccine and provide justification for the investigation of transgenic plants as a means of vaccine delivery.
Malaria, caused by infection with protozoa of the genus Plasmodium, is the most important parasitic disease of humans. It remains one of the major causes of human death and disease in the developing world and is responsible for several million deaths a year. An affordable vaccine against malaria would greatly benefit the people living in those regions and would foster economic growth. Much interest has been focused on the identification of potential vaccine candidates as well as the development of vaccine production and delivery systems. Several proteins have been identified as inducing a level of protective efficacy, including merozoite surface protein 1 (MSP1) (8), apical membrane antigen 1 (18), erythrocyte binding antigen 175 (2), and merozoite surface protein 4 (MSP4) (16). MSP4 is a small integral membrane protein with an epidermal growth factor (EGF)-like domain in its carboxyl terminus (16). MSP4 is immunogenic both in laboratory animals (22, 23) and during natural malaria infection (24), and antibodies raised to MSP4 can inhibit parasite growth in vitro (T. Wu, personal communication). The homologue of MSP4 in the rodent malaria species Plasmodium yoelii, PyMSP4/5, is highly effective at protecting mice against challenge with lethal strains of malaria (13, 14).
Although all of these vaccine molecules show clear evidence of protective efficacy, none of them is considered to provide sufficient protection for use in isolation in humans. Factors such as lack of protection against strains expressing variant forms of these proteins, insufficient immunogenicity with available adjuvants, and short duration of protection all limit the utility of individual proteins to various degrees. One solution to this problem is the combination of several proteins into multiple-subunit (multisubunit) vaccines; however, such multisubunit vaccines are subject to considerable logistic difficulties in formulation and testing. Previously validated single-subunit vaccines must be combined and reformulated, often with changes in adjuvants; they must then be taken through the entire vaccine development pathway, including testing for toxicity, stability, and immunogenicity. The expense and time required for this process place major limits on the speed with which a malaria vaccine can be developed and deployed. Methods of vaccine delivery that allow rapid combination of antigens and that have the potential to be delivered at low cost would be highly advantageous. One potential method is oral immunization; however, it is not clear whether this method of immunization would be capable of inducing immune responses active against a parasite resident in the circulation.
Here we report that oral immunization of mice with recombinant MSP4 or PyMSP4/5 in the presence of cholera toxin subunit B (CTB) induces antibody responses comparable to those achieved with parenteral immunization. The antibody responses induced are predominantly immunoglobulin G1 (IgG1) and are directed to multiple epitopes, including conformational epitopes. Challenge of mice with a lethal dose of P. yoelii showed that the induced immune responses could protect mice against death.
MATERIALS AND METHODS
Parasites and animals.
P. yoelii YM parasites were kindly supplied by Michael F. Good (Queensland Institute of Medical Research, Brisbane, Queensland, Australia). Female BALB/c mice, 6 to 8 weeks old, were purchased from the Central Animal Services of Monash University, Clayton, Victoria, Australia.
Recombinant proteins and CTB.
The expression and purification of recombinant full-length MSP4 and PyMSP4/5 in Escherichia coli (EcMSP4 and EcMSP4/5, respectively) were described previously (13, 23). EcMSP4 contains the entire coding sequence of mature MSP4 but lacks the N-terminal secretion signal and the C-terminal glycosylphosphatidylinositol anchor attachment signal sequences. Similarly, EcMSP4/5 contains the full-length PyMSP4/5 gene sequence but lacks the N-terminal secretion signal and the C-terminal glycosylphosphatidylinositol anchor attachment signal sequences. Both proteins contain a hexahistidine tag at the C terminus. For measurement of epitope specificity, four glutathione S-transferase (GST) fusion proteins, each containing approximately one-quarter of mature MSP4 (22), and three GST fusion proteins, each containing one-third of mature PyMSP4/5 (L. Kedzierski, unpublished data), were used. CTB was purchased from Sigma Chemical Co. (St. Louis, Mo.).
Oral immunization and challenge infection.
Groups of four to eight mice were deprived of food and water for 2 h and then orally immunized with 0.25 ml of phosphate-buffered saline containing purified recombinant protein and/or CTB. Oral immunization was performed under anesthetic conditions by intubation with an animal-feeding needle (Popper & Sons, Inc., New Hyde Park, N.Y.). Six immunizations were given at weeks 0, 1, 2, 3, 6, and 8. Sera were collected prior to the initial immunization and 10 days after the sixth immunization. At 2 weeks after the sixth immunization, mice were challenged intraperitoneally with 105 P. yoelii YM-parasitized red blood cells as previously described (14). Blood was collected each day from days 3 to 30 postinfection, and parasitemia was monitored microscopically by using Giemsa-stained thin blood smears.
Parenteral immunization.
Parenteral immunization of mice with either EcMSP4 or EcMSP4/5 was carried out as described previously (14, 23).
Antibody assays.
Antibodies in sera were measured with an enzyme-linked immunosorbent assay as described previously (23). The optical density (OD) was read at 405 nm, and the background OD values obtained from phosphate-buffered saline- or GST-coated plates were subtracted from the values obtained from antigen-coated plates. To assess the antibody reactivity induced to conformational epitopes, the recombinant proteins were reduced and alkylated as previously described (22), and the treated proteins were used to coat microtiter plates in parallel with nonreduced proteins. For the determination of antibody subclasses, a panel of alkaline phosphatase-conjugated anti-mouse immunoglobulin subclasses (IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA) (Southern Biotechnology Associates, Inc., Birmingham, Ala.) was used for secondary antibodies.
Statistical analysis.
Statistical analysis was performed with Graphpad Prism Software (Graphpad Software Inc.). Fisher's exact probability test was used to compare the numbers of surviving animals in different groups, and the Mann-Whitney U test was used to compare peak levels of parasitemia between two groups. Wilcoxon and Mann-Whitney U tests were used to compare antibody levels between groups for paired and unpaired data, respectively. Spearman's rank correlation test was used to assess associations between antibody levels and peak levels of parasitemia.
RESULTS
Antibody responses following oral immunization with P. falciparum MSP4.
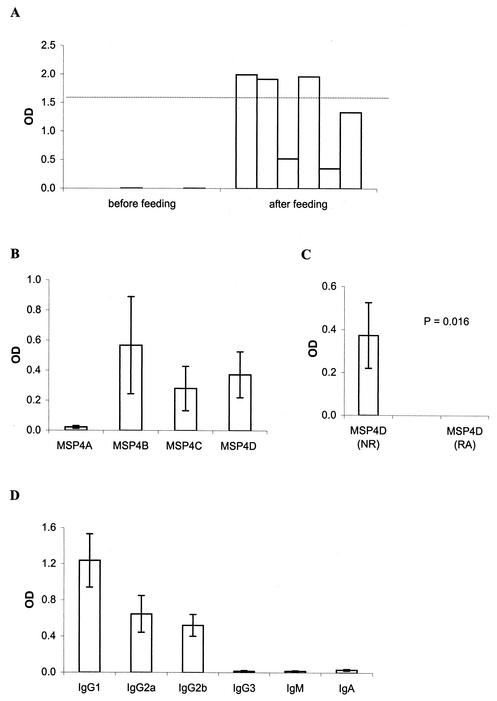
In order to establish whether P. falciparum antigens could be administered orally and induce systemic antibody responses, a group of six mice were gavage fed with 25 μg of EcMSP4 mixed with 10 μg of CTB, and the resultant antibodies were measured with an enzyme-linked immunosorbent assay. After six oral immunizations, all mice developed MSP4-specific antibodies (Fig. 1A); the levels of the antibodies in four of the mice were comparable to those induced by intraperitoneal injections with the same doses of antigens emulsified in complete Freund's adjuvant (CFA) and incomplete Freund's adjuvant (IFA) (23). The antibodies reacted with the four fragments of MSP4, MSP4A, MSP4B, MSP4C, and MSP4D, each of which contained a sequence spanning approximately one-quarter of the mature molecule and each of which contained an epitope recognized by sera from people exposed to malaria (Fig. 1B). Antibody reactivity to MSP4A was relatively lower than that induced to the other fragments; however, it was significantly higher than the background (P = 0.025). Of interest was the observation that oral feeding with MSP4 could induce antibodies to at least one conformational epitope within MSP4D, which contains the EGF-like domain. Reduction of MSP4D, either alone or in combination with alkylation, significantly decreased its reactivity with antibodies (Fig. 1C). Reduction and alkylation of EcMSP4 also decreased its reactivity with antibodies induced by oral immunization (data not shown); however, a substantial amount of reactivity remained, suggesting the presence of responses to both linear epitopes and conformational epitopes. Examination of the isotypes of the MSP4-specific antibodies induced by oral immunization showed that they were predominantly IgG1, with lower IgG2a and IgG2b responses (Fig. 1D), a pattern identical to that induced by parenteral immunization (23).
FIG. 1.
Characteristics of antibodies raised in mice by oral immunization with 25 μg of EcMSP4 and 10 μg of CTB. (A) Antibody level. (B) Epitope specificity. (C) Sensitivity of reduction. (D) Isotype distribution. All sera were measured at a 1:10,000 dilution. In panel A, the dotted line shows the mean antibody level induced by intraperitoneal immunization with the corresponding protein emulsified in Freund's adjuvant; in panels B, C, and D, the results are represented as the averages for six individually tested serum samples per group, and the error bars indicate the standard errors of the means. In panel C, antibody reactivities to nonreduced MSP4D (NR) and reduced and alkylated MSP4D (RA) are compared by using a Wilcoxon matched-pair test.
Protection of orally immunized mice against lethal challenge with P. yoelii.
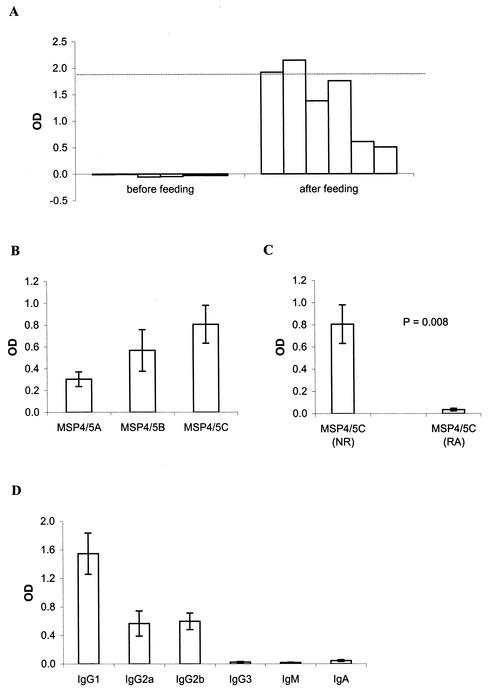
In order to study the protective efficacy of the antibodies induced by oral immunization, we used the P. yoelii challenge model and performed oral feeding and challenge experiments with BALB/c mice. Oral immunization with 25 μg of EcMSP4/5 and 10 μg of CTB induced PyMSP4/5-specific antibodies in all six immunized mice (Fig. 2A). In three of the mice, the levels of the antibodies were comparable to those achieved by intraperitoneal injections in a standard parenteral immunization protocol with CFA and IFA (14). The antibodies reacted with all three fragments of PyMSP4/5, MSP4/5A, MSP4/5B, and MSP4/5C, each of which contained one-third of the molecule (Fig. 2B), and a significant proportion of antibodies to the EGF-like domain recognized conformational epitopes (Fig. 2C). The predominant isotype of the PyMSP4/5-specific antibodies was IgG1, with lower IgG2a and IgG2b responses (Fig. 2D).
FIG. 2.
Characteristics of antibodies raised in mice by oral immunization with 25 μg of EcMSP4/5 and 10 μg of CTB. (A) Antibody level. (B) Epitope specificity. (C) Sensitivity of reduction. (D) Isotype distribution. All sera were measured at a 1:10,000 dilution. In panel A, the dotted line shows the mean antibody level induced by intraperitoneal immunization with the corresponding protein emulsified in Freund's adjuvant; in panels B, C, and D, the results are represented as the averages for six individually tested serum samples per group, and the error bars indicate the standard errors of the means. In panel C, antibody reactivities to nonreduced MSP4/5C (NR) and reduced and alkylated MSP4/5C (RA) are compared by using a Wilcoxon matched-pair test.
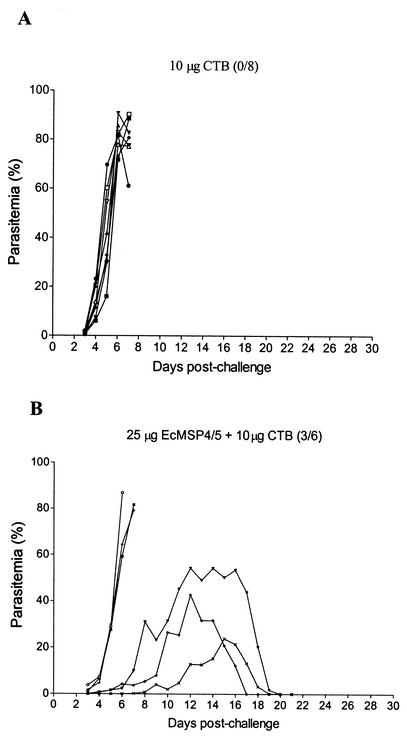
The immunized mice were challenged with a lethal dose of 105 P. yoelii YM parasites. Eight mice that were immunized with 10 μg of CTB were also challenged in the same protocol. All eight mice developed fulminating infections and died between days 6 and 7 postchallenge, with a peak parasitemia level of 77.8 to 90.6% (Fig. 3A). In contrast, three of the six mice immunized with 25 μg of EcMSP4/5 and 10 μg of CTB showed clear evidence of protective immunity and survived the challenge (Fig. 3B). There was a significant difference in the numbers of surviving mice in the groups (P value, 0.05, as determined by Fisher's exact probability test), and a significant difference was also observed in peak parasitemia levels between the two groups (P value, 0.002, as determined by the Mann-Whitney U test). These results demonstrated that 25 μg of EcMSP4/5 administered orally in the presence of 10 μg of CTB could partially protect mice against a lethal challenge with P. yoelii.
FIG. 3.
Blood-stage parasitemia of mice orally immunized with 10 μg of CTB (A) or 25 μg of EcMSP4/5 in the presence of 10 μg of CTB (B) prior to challenge with P. yoelii YM. The survival rate (number of surviving mice/total number of mice) is shown at the top of each graph.
Dose-dependent antibody responses and protective efficacy.
To replicate and extend these studies, groups of four to eight mice were immunized orally with differing amounts (25, 5, 1, and 0.2 μg) of EcMSP4/5 in the presence of 10 μg of CTB. An additional group of eight mice was immunized with 25 μg of EcMSP4/5 alone to investigate the effect of CTB on oral immunogenicity. As shown in Table 1, the combination of 25 μg of EcMSP4/5 and 10 μg of CTB induced higher, although not statistically significant (P value, >0.05, as determined by the Mann-Whitney U test), levels of antibodies than 25 μg of EcMSP4/5 alone, demonstrating the adjuvant effect of CTB. In the presence of 10 μg of CTB, a dose-dependent antibody response was observed, with larger amounts of EcMSP4/5 inducing higher levels of antibodies; however, the difference was not statistically significant (P value, >0.05, as determined by analysis of variance) due to the small numbers of animals in the groups. CTB-specific antibody responses were equally high in all mice immunized with10 μg of CTB, regardless of the dose of EcMSP4/5. Mice immunized with 25 μg of EcMSP4/5 alone did not produce antibodies to CTB, confirming that all mice were adequately and properly dosed with the oral antigen.
TABLE 1.
Comparison of antibody responses in mice orally immunized with different amounts of EcMSP4/5 with or without 10 μg of CTB
| Antigen (μg) | No. of mice tested | Level (mean ± SD OD) of antibodies to:
|
|
|---|---|---|---|
| PyMSP4/5a | CTBb | ||
| CTB (10) | 8 | 0.002 ± 0.003 | 0.686 ± 0.366 |
| EcMSP4/5 (25) | 6 | 1.195 ± 0.514 | 0.005 ± 0.006 |
| EcMSP4/5 (25) + CTB (10) | 7 | 1.539 ± 0.510 | 0.771 ± 0.323 |
| EcMSP4/5 (5) + CTB (10) | 4 | 0.704 ± 0.751 | 0.665 ± 0.311 |
| EcMSP4/5 (1) + CTB (10) | 4 | 0.527 ± 0.406 | 0.680 ± 0.439 |
| EcMSP4/5 (0.2) + CTB (10) | 4 | 0.173 ± 0.342 | 0.705 ± 0.613 |
PyMSP4/5-specific antibodies were measured against EcMSP4/5 at a serum dilution of 1:10,000.
CTB-specific antibodies were measured against CTB at a serum dilution of 1:10,000.
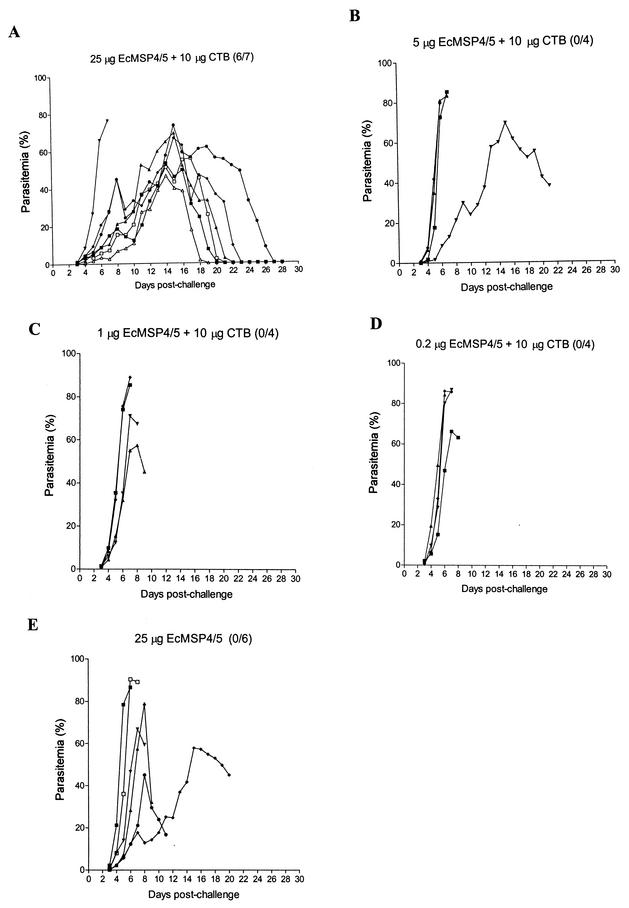
When mice were challenged with a lethal dose of P. yoelii, six out of the seven mice that were immunized with 25 μg of EcMSP4/5 and 10 μg of CTB were protected against the challenge (Fig. 4A). The results of this second trial demonstrated significant protection, as measured by survival rates and peak parasitemia levels, compared to that in CTB-immunized mice (P value, 0.001, as determined by Fisher's exact probability test; P value, 0.0003, as determined by the Mann-Whitney U test). The degree of protection was similar to that obtained in the first trial (P value, >0.05, as determined by Fisher's exact probability test; P value, >0.05, as determined by the Mann-Whitney U test). None of the mice that were given 5, 1, or 0.2 μg of EcMSP4/5 in the presence of 10 μg of CTB or 25 μg of EcMSP4/5 alone survived the parasite challenge (Fig. 4B to E). Most of these mice developed fulminant infections similar to those observed in mice immunized with CTB. The exceptions were one mouse immunized with 25 μg of EcMSP4/5 alone and one mouse immunized with 5 μg of EcMSP4/5 and 10 μg of CTB, which had peak parasitemia levels comparable to those in the protected mice but succumbed to infection when the parasitemia levels were 38.0 and 45.0%, respectively (Fig. 4B and E). Compared to the mice that were immunized with CTB alone, no significant difference was observed either in peak parasitemia levels or in survival rates in these other groups, indicating that 25 μg of EcMSP4/5 and a mucosal adjuvant, such as CTB, is required in an oral vaccine for the induction of protective immunity.
FIG. 4.
Blood-stage parasitemia of mice orally immunized with different amounts of EcMSP4/5 with or without CTB prior to challenge with P. yoelii YM. The amounts of EcMSP4/5 and/or CTB and the survival rate (number of surviving mice/total number of mice) are shown at the top of each graph.
DISCUSSION
Oral feeding with recombinant MSP4 and PyMSP4/5 induced polyspecific antibody responses against multiple epitopes in both proteins. For MSP4, at least four different specificities were induced, and for PyMSP4/5, at least three different specificities were induced. It was previously shown that antibodies raised to fragments of MSP4 or PyMSP4/5 react with their corresponding full-length recombinant proteins and vice versa (22; Kedzierski, unpublished). In addition, antibodies raised to both the fragments and the full-length proteins react with the native proteins in parasite lysates, and both the fragments and the full-length proteins are recognized by antisera induced during the course of parasite infections (22; Kedzierski, unpublished). Finally, sera from immunized mice in this study reacted with parasite proteins in immunoblotting analyses (data not shown). Collectively, these results indicate that the fragments and the full-length proteins contain at least some epitopes with the same conformations as those in the native proteins and that these are clearly able to induce antibodies following oral immunization. Of interest was the observation that both MSP4 and PyMSP4/5 could induce antibodies to at least one conformational epitope formed by disulfide bonding. As previous studies have indicated that the correct folding of the EGF-like domain is crucial for the proper antigenicity of the MSP4 molecule (22), this observation suggests that this domain of the protein is resistant to intestinal proteolysis and is presented to B cells in a relatively intact form. The responses induced were predominantly IgG subclasses; very little IgA was detected. The levels of antibodies induced compared well with those found after parenteral immunization with a combination of CFA and IFA (14, 23).
Our results demonstrated that CTB is an effective mucosal adjuvant for oral immunization with malaria antigens. Immunization with EcMSP4/5 alone could induce an immune response that was boosted by the addition of CTB to levels above the protective threshold. The presence of CTB elevated the antibody titer but did not affect the spectrum of epitopes recognized or the capacity to raise antibodies to conformational epitopes (data not shown). CTB is the pentameric subunit of cholera toxin that binds to the intestinal GM1 ganglioside receptor (19). It enhances the immunogenicity of coadministered antigen, either mixed with or conjugated to CTB, when given by the mucosal route, and has been extensively used in oral vaccination studies (9). CTB appears to be nontoxic due to the absence of toxigenic cholera toxin subunit A (19), and it can be safely administered to humans in the form of a registered oral cholera vaccine (12). Recombinant CTB secreted by the gram-positive bacterium Bacillus brevis has been shown to have properties similar to those of native CTB with respect to GM1 binding activity and adjuvant effect (26). Unlike commercial CTB, which contains a trace amount of contaminating cholera toxin subunit A, recombinant CTB has shown no toxicity in safety studies (6) and is currently being tested in human trials (10, 11). Cholera infection is reasonably common in Africa, where malaria is a severe health problem. It is possible that previous exposure to cholera toxin and subsequent mucosal immunity may limit the effectiveness of CTB as an oral adjuvant in some individuals. Such issues will need to be addressed by appropriate studies; however, our results are not likely to be dependent on the use of a particular adjuvant.
We have demonstrated that feeding with recombinant EcMSP4/5 offers protection against challenge with a lethal dose of murine malaria P. yoelii. Survival rates in two separate trials were 50 and 86%, with all mice developing patent parasitemia. When death was considered as the readout, the levels of protection induced by oral and parenteral immunizations were comparable; no significant difference was observed between the survival rates (9 of 13 mice for oral immunization and 28 of 33 mice for parenteral immunization; P value, >0.05, as determined by Fisher's exact probability test). The time course of infection, with respect to the onset of patency, days to clearance, and the duration of infection, was similar to that seen after parenteral immunization of mice with a regimen that included CFA (14). One difference was that peak parasitemia levels were, on average, higher in mice that were orally immunized.
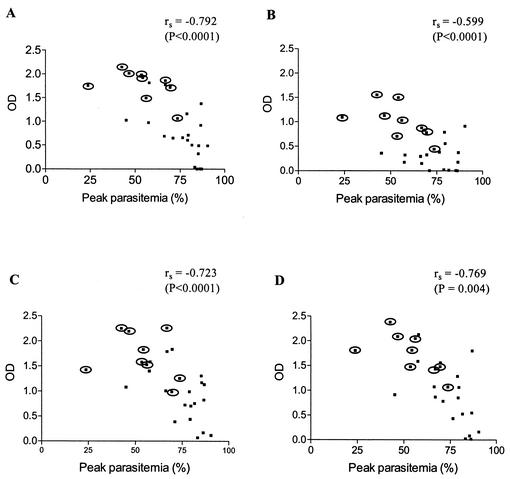
There was a good correlation between the PyMSP4/5-specific antibodies in the prechallenge sera and protective efficacy (Fig. 5). In general, mice with higher antibody responses showed better protection, whereas mice with lower antibody levels succumbed to infection. There appeared to be a threshold level of prechallenge antibody responses to EcMSP4/5 that was required for survival and full recovery from infection. The correlation between the EcMSP4/5-specific antibodies and peak parasitemia was significant; Spearman's rank correlation coefficient was −0.792 (P < 0.001) (Fig. 5A). In contrast, no correlation was observed between the CTB-specific antibodies and protective immunity against parasite infection (P > 0.05). This observation was in agreement with the results of a previous study of parenteral immunization (14) and suggested titer-dependent, antibody-mediated protection when PyMSP4/5 was administered orally. Significant correlations were also observed between protective efficacy and antibodies directed to the three different regions of PyMSP4/5, including the conformation-dependent EGF-like domain (Fig. 5B to D), as well as various isotypes of the PyMSP4/5-specific antibodies (data not shown).
FIG. 5.
Scatter diagram showing the correlation between PyMSP4/5-specific antibody levels (OD) in prechallenge sera and peak levels of parasitemia in 31 mice orally immunized with different amounts of EcMSP4/5 with or without CTB. (A) Antibodies to full-length PyMSP4/5. (B, C, and D) Antibodies to MSP4/5A, MSP4/5B, and MSP4/5C, respectively. The sera were measured at dilutions of 1:10,000 against full-length PyMSP4/5 and 1:1,000 against the three PyMSP4/5 fragments. Circled points indicate mice that survived parasite challenge. Spearman's rank correlation coefficient (rs) and the associated P value are shown at the top of each graph.
Mucosal immunization with malaria proteins has not been extensively studied, and no successful studies using oral administration of recombinant proteins to protect against malaria have been reported. Intranasal immunization with recombinant P. yoelii MSP119, the carboxyl terminus of MSP1, induced some protective immunity to blood-stage malaria infection in mice (7). Intranasal inoculation is generally thought to be superior to oral administration (4, 25), but in the previous study, the level of antibody obtained and the subsequent degree of protection attained were much lower than those seen following parenteral immunization (7), even though a relatively high dose of mucosal adjuvant (50 μg of CTB) was used. Plasmodial antigens have been expressed in live attenuated Salmonella and immune responses have been induced; in some studies, protection against infection has been demonstrated following intranasal or oral immunization (20, 21, 25). This approach is not likely to be useful for the expression of many of the malaria surface proteins that contain a complex disulfide knot or EGF-like domains, such as MSP1, apical membrane antigen 1, MSP4, MSP5, and MSP8 (1, 3, 15, 22, 25), because of the reducing environment of the Salmonella cytoplasm.
Oral administration of antigens offers a number of theoretical advantages for the development of a malaria vaccine. The difficulties of deployment are considerably ameliorated over those of a parenteral vaccine, and an oral vaccine appears to offer a relatively straightforward means of combining antigens into a multivalent formulation. Antigen combination is currently thought to be an essential requirement for an efficacious malaria vaccine, but the costs of the development of such a combination with a requirement for reformulation and retesting are very high. The demonstrated feasibility of inducing protective immunity by oral immunization provides a rationale for the development of oral vaccines based on transgenic plant expression of malaria antigens. Transgenic plants offer the possibility of low-cost immunization by a distributed network of local health care providers (17). There is already an initial report of the successful expression of MSP119 in transgenic plants (5), but no immunogenicity or protection data are available. We have constructed transgenic plants expressing immunoreactive PyMSP4/5 (L. Wang, unpublished data) and have shown that this protein reacts with a number of antisera that recognize the native protein in parasites. Determination of the oral immunogenicity and protective efficacy of the transgenic plant material is currently under way, as is the construction of plants expressing P. falciparum MSP4 and MSP5.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council (NH&MRC), the U.S. Agency for International Development (USAID), the Howard Hughes Medical Institute International Scholars Program, and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
Editor: J. M. Mansfield
REFERENCES
- 1.Black, C. G., T. Wu, L. Wang, A. R. Hibbs, and R. L. Coppel. 2001. Merozoite surface protein 8 of Plasmodium falciparum contains two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 114:217-226. [DOI] [PubMed] [Google Scholar]
- 2.Camus, D., and T. J. Hadley. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230:553-556. [DOI] [PubMed] [Google Scholar]
- 3.Crewther, P. E., M. L. Matthew, R. H. Flegg, and R. F. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galen, J. E., O. G. Gomez-Duarte, G. A. Losonsky, J. L. Halpern, C. S. Lauderbaugh, S. Kaintuck, M. K. Reymann, and M. M. Levine. 1997. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine 15:700-708. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh, S., P. Malhotra, P. V. Lalitha, S. Guha-Mukherjee, and V. S. Chauhan. 2002. Expression of Plasmodium falciparum C-terminal region of merozoite surface protein (PfMSP119), a potential malaria vaccine candidate, in tobacco. Plant Sci. 162:335-343. [Google Scholar]
- 6.Goto, N., J. Maeyama, Y. Yasuda, M. Isaka, K. Matano, S. Kozuka, T. Taniguchi, Y. Miura, K. Ohkuma, and K. Tochikubo. 2000. Safety evaluation of recombinant cholera toxin B subunit produced by Bacillus brevis as a mucosal adjuvant. Vaccine 18:2164-2171. [DOI] [PubMed] [Google Scholar]
- 7.Hirunpetcharat, C., D. Stanisic, X. Q. Liu, J. Vadolas, R. A. Strugnell, R. Lee, L. H. Miller, D. C. Kaslow, and M. F. Good. 1998. Intranasal immunization with yeast-expressed 19 kD carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein-1 (yMSP119) induces protective immunity to blood stage malaria infection in mice. Parasite Immunol. 20:413-420. [DOI] [PubMed] [Google Scholar]
- 8.Holder, A. A., and R. R. Freeman. 1984. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J. Exp. Med. 160:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmgren, J., N. Lycke, and C. Czerkinsky. 1993. Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine 11:1179-1184. [DOI] [PubMed] [Google Scholar]
- 10.Jertborn, M., C. Ahren, and A. M. Svennerholm. 2001. Dose-dependent circulating immunoglobulin A antibody-secreting cell and serum antibody responses in Swedish volunteers to an oral inactivated enterotoxigenic Escherichia coli vaccine. Clin. Diagn. Lab. Immunol. 8:424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jertborn, M., I. Nordstrom, A. Kilander, C. Czerkinsky, and J. Holmgren. 2001. Local and systemic immune responses to rectal administration of recombinant cholera toxin B subunit in humans. Infect. Immun. 69:4125-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1996. Intestinal and systemic immune responses in humans after oral immunization with a bivalent B subunit-O1/O139 whole cell cholera vaccine. Vaccine 14:1459-1465. [DOI] [PubMed] [Google Scholar]
- 13.Kedzierski, L., C. Black, and R. L. Coppel. 2000. Characterisation of the merozoite surface protein 4/5 gene of Plasmodium berghei and Plasmodium yoelii. Mol. Biochem. Parasitol. 105:137-147. [DOI] [PubMed] [Google Scholar]
- 14.Kedzierski, L., C. G. Black, and R. L. Coppel. 2000. Immunization with recombinant Plasmodium yoelii merozoite surface protein 4/5 protects mice against lethal challenge. Infect. Immun. 68:6034-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. 16:63-67. [DOI] [PubMed] [Google Scholar]
- 16.Marshall, V. M., A. Silva, M. Foley, S. Cranmer, L. Wang, D. J. McColl, D. J. Kemp, and R. L. Coppel. 1997. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect. Immun. 65:4460-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mor, T. S., M. A. Gomez-Lim, and K. E. Palmer. 1998. Perspective: edible vaccines—a concept coming of age. Trends Microbiol. 6:449-453. [DOI] [PubMed] [Google Scholar]
- 18.Peterson, M. G., V. M. Marshall, J. A. Smythe, P. E. Crewther, A. Lew, A. Silva, R. F. Anders, and D. J. Kemp. 1989. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol. Cell. Biol. 9:3151-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rappuoli, R., M. Pizza, and G. Dougan. 1999. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 20:493-500. [DOI] [PubMed] [Google Scholar]
- 20.Sadoff, J. C., W. R. Ballou, L. S. Baron, W. R. Majarian, R. N. Brey, W. T. Hockmeyer, J. F. Young, S. J. Cryz, J. Ou, G. H. Lowell, and J. D. Chulay. 1988. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science 240:336-338. [DOI] [PubMed] [Google Scholar]
- 21.Toebe, C. S., J. D. Clements, L. Cardenas, G. J. Jennings, and M. F. Wiser. 1997. Evaluation of immunogenicity of an oral Salmonella vaccine expressing recombinant Plasmodium berghei merozoite surface protein-1. Am. J. Trop. Med. Hyg. 56:192-199. [DOI] [PubMed] [Google Scholar]
- 22.Wang, L., C. G. Black, V. M. Marshall, and R. L. Coppel. 1999. Structural and antigenic properties of merozoite surface protein 4 of Plasmodium falciparum. Infect. Immun. 67:2193-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, L., J. G. T. Menting, C. G. Black, A. Stowers, D. C. Kaslow, S. L. Hoffman, and R. L. Coppel. 2000. Differences in epitope recognition, isotype and titer of antisera to Plasmodium falciparum merozoite surface protein 4 raised by different modes of DNA or protein immunization. Vaccine 19:816-824. [DOI] [PubMed] [Google Scholar]
- 24.Wang, L., T. L. Richie, A. Stowers, D. H. Nhan, and R. L. Coppel. 2001. Naturally acquired antibody responses to Plasmodium falciparum merozoite surface protein 4 in a population living in an area of endemicity in Vietnam. Infect. Immun. 69:4390-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu, S., M. Beier, M. B. Sztein, J. Galen, T. Pickett, A. A. Holder, O. G. Gomez-Duarte, and M. M. Levine. 2000. Construction and immunogenicity in mice of attenuated Salmonella typhi expressing Plasmodium falciparum merozoite surface protein 1 (MSP-1) fused to tetanus toxin fragment C. J. Biotechnol. 83:125-135. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda, Y., K. Matano, T. Asai, and K. Tochikubo. 1998. Affinity purification of recombinant cholera toxin B subunit oligomer expressed in Bacillus brevis for potential human use as a mucosal adjuvant. FEMS Immunol. Med. Microbiol. 20:311-318. [DOI] [PubMed] [Google Scholar]