Abstract
Immune responses associated with intestinal nematode infections are characterized by the activation of T-helper 2 (Th2) cells. Previous studies demonstrated that during Trichinella spiralis infection, Th2 cells contribute to the development of intestinal muscle hypercontractility and to worm eviction from the gut, in part through signal transducer and activator of transcription factor 6 (Stat6). Interleukin-9 (IL-9), a Th2-cell-derived cytokine, has pleiotropic activities on various cells that are not mediated through Stat6. In this study, we investigated the role of IL-9 in the generation of enteric muscle hypercontractility in mice infected with the intestinal parasite T. spiralis and the cecal parasite Trichuris muris. Treatment of mice with IL-9 enhanced infection-induced jejunal muscle hypercontractility and accelerated worm expulsion in T. spiralis infection. These effects were associated with an up-regulation of IL-4 and IL-13 production from in vitro-stimulated spleen cells. In addition, increases in the level of intestinal goblet cells and in the level of mouse mucosal mast cell protease 1 (MMCP-1) in serum were observed in infected mice following IL-9 administration. However, the neutralization of IL-9 by anti-IL-9 vaccination or by anti-IL-9 antibody had no significant effect on worm expulsion or muscle contraction in T. spiralis-infected mice. In contrast, the neutralization of IL-9 significantly attenuated T. muris infection-induced colonic muscle hypercontractility and inhibited worm expulsion. The attenuated expulsion of the parasite by IL-9 neutralization was not accompanied by changes in goblet cell hyperplasia or the MMCP-1 level. These findings suggest that IL-9 contributes to intestinal muscle function and to host protective immunity and that its importance and contribution may differ depending on the type of nematode infection.
Among the distinct CD4+-T-helper-cell subsets (29), the T-helper 2 (Th2) type of immune response is important in host protective immunity to many intestinal nematode infections and is characterized by the expression of cytokines such as interleukin-4 (IL-4), IL-5, IL-9, and IL-13 (9, 16, 19). The association between host protective immunity and the Th2 type of immune response has been observed in infections with both the small intestinal nematode Trichinella spiralis (9, 20, 24) and the cecal parasite Trichuris muris (10, 11). Consequently, considerable interest has focused on the effector functions induced by Th2 cytokines, including mucosal mastocytosis, eosinophilia, goblet cell hyperplasia, and intestinal muscle hypercontractility.
Infection of mice or rats with nematode parasites is associated with a variety of alterations in intestinal physiology, including enhanced contractility of small intestinal muscle (13, 36; J. M. Goldhill, F. Finkelman, J. Urban, S. Morris, C. R. Maliszewski, and T. Shea-Donohue, Gastroenterology 108, abstr. A286, 1995) and intestinal goblet cell hyperplasia (9, 19, 23). Previously, Vallance et al. demonstrated that nematode infection-induced intestinal muscle hypercontractility is, at least in part, immune mediated, as it was attenuated in infected athymic CD4−/− and major histocompatibility complex class II-deficient mice (38). Recently, Khan et al. also reported that the Th2 cytokines IL-4 and IL-13 are present in the muscularis externa during nematode infection and contribute to changes in intestinal muscle function through a process dependent on signal transducer and activator of transcription factor 6 (Stat6) (24). In contrast, the Th2 cytokine IL-5 contributes little to these responses (37).
There is growing interest in the Th2 cytokine IL-9, which acts on various cell types, including T and B lymphocytes, mast cells, eosinophils, lung epithelial cells, and hematopoietic progenitors (7, 8, 31, 39). IL-9 has been shown to protect mice from gram-negative bacterial shock, and this effect was correlated with the down-regulation of tumor necrosis factor alpha, IL-12, and gamma interferon production and with the up-regulation of IL-10 production (21). The generation of transgenic mice overexpressing IL-9 has suggested a critical role for this cytokine in a variety of immunological responses, including intestinal mastocytosis and enhanced resistance to nematode infection (14, 15). Nevertheless, neither the role of IL-9 in intestinal muscle function nor the precise effector mechanism by which IL-9 contributes to host protection during intestinal nematode infection is known.
In the present study, using two different nematode species, T. spiralis and T. muris, which inhabit the small intestine and the large intestine, respectively, we have assessed the contribution of IL-9 to infection-induced enteric muscle hypercontractility and worm expulsion from the gut. For the first time, we demonstrate that IL-9 influences muscle hypercontractility during nematode parasite infections. While the administration of IL-9 enhanced infection-induced jejunal muscle contraction and worm expulsion in T. spiralis infection, the neutralization of IL-9 had no significant effect on muscle contraction or worm expulsion. In contrast, the neutralization of IL-9 significantly attenuated colonic muscle contraction and worm expulsion in T. muris infection. These results suggest a role for IL-9 in intestinal muscle hypercontractility and highlight differences in the contributions of this cytokine to host protection against different nematode infections.
MATERIALS AND METHODS
Animals.
C57BL/6 and BALB/c mice were obtained from Jackson Laboratories; they were kept in sterilized, filter-topped cages and fed autoclaved food in the animal facilities of McMaster University. Only male mice were used, at the age of 8 to 10 weeks. The protocols used were in direct accordance with guidelines drafted by the McMaster University Animal Care Committee and the Canadian Council on the Use of Laboratory Animals.
Parasitological techniques.
The T. spiralis parasites used in this study originated in the Department of Zoology at the University of Toronto, and the colony was maintained through serial infections alternating between male Sprague-Dawley rats and male CD1 mice. The larvae were obtained from infected rodents 60 to 90 days postinfection (p.i.) by using a modification (40) of the technique described by Castro and Fairbairn (5). Mice were killed at various times p.i. Adult worms were recovered from mice after the intestine had been opened longitudinally, rinsed, and placed in Hanks' balanced salt solution for 3 h at 37°C. Worms were counted under a dissecting microscope.
The techniques used for T. muris maintenance, infection, and recovery were described previously (41). Mice were infected with approximately 100 eggs and killed at various times p.i.
In vivo treatments.
T. spiralis-infected mice were injected intraperitoneally (i.p.) with IL-9 (100 ng/day) which had been purified from the serum of IL-9 transgenic mice on immobilized rat anti-mouse IL-9 antibody. Dose-response studies had revealed that this dose is optimum for in vivo experiments. Control mice received the same volume of buffer (phosphate-buffered saline [PBS] plus 1% mouse serum) as that used for the cytokine injection. Treatment was performed every day from the day of infection until the mice were sacrificed.
For neutralizing IL-9, mice were treated with murine anti-IL-9 monoclonal antibodies (MM9A1 and MM9CI) or were vaccinated against IL-9 with IL-9 chemically complexed to ovalbumin (OVA) to induce neutralizing anti-IL-9 antibodies as described previously (32). For the latter experiments, control mice were immunized with OVA only. In studies with anti-IL-9 antibodies, mice were treated with 1 mg of antibody or control mouse immunoglobulin G (IgG) on day 0 and then with 0.5 mg on every alternate day until day 20 p.i.
Measurement of muscle contraction in intestinal muscle strips.
Preparation of intestinal muscle strips for muscle contractility experiments and analysis of carbachol-induced contraction were described previously (36). Briefly, the jejunum or colon was removed and placed in oxygenated (95% O2-5% CO2) Krebs solution, and 1-cm sections of whole gut were cut from the jejunum or from the colon. The lumen of each segment was flushed with Krebs buffer prior to the insertion of short (2- to 3-mm) lengths of Silastic tubing (outer diameter, 0.065 in.; inner diameter, 0.030 in.; Dow Corning, Midland, Mich.) into the open ends of the gut segments. Tubing was then tied in place with surgical silk. The insertion of the tubing was found to maintain the patency of the gut segments over the course of the experiments. Segments were then hung in the longitudinal axis and attached at one end to an FT03C force transducer (Grass, Quincy, Mass.), and responses were recorded on a Grass 7D polygraph. Tissues were equilibrated for 30 min at 37°C in Krebs buffer that had been oxygenated with 95% O2- 5% CO2 before the start of the experiment. The previously identified optimal tension (400 mg for jejunum and 800 mg for colon) was then applied in carbachol dose-response experiments before the addition of the first dose of carbachol (36). Previous experiments had indicated that these values were the optimal tensions for determining the maximal responsiveness of both control and inflammed tissues. After the application of tension, the gut segments were exposed to different concentrations of carbachol. After the maximal response to each dose was obtained, the tissues were rinsed twice and equilibrated in fresh Krebs solution for 15 min before the addition of the next dose. Contractile responses to carbachol were expressed as milligrams of tension per cross-sectional area as described previously (36). For each mouse, the mean tension was calculated from at least three segments.
Measurement of contraction and relaxation in dispersed smooth muscle cells.
Muscle cells were isolated from longitudinal muscle of the myenteric plexus (LMMP) in the jejunum of noninfected C57BL/6 mice by a method similar to that used by Bitar and Makhlouf to prepare smooth muscle cells from the guinea pig stomach (4). Briefly, the jejunum was removed and placed in Dulbecco modified Eagle medium with 1% antibiotic-antimycotic agent. LMMP was prepared by careful dissection, preincubated with 10 ng of IL-9/ml or with PBS overnight in a 5% CO2 incubator, and then incubated for two successive 10-min periods at 31°C in 10 ml of HEPES medium (98.1 mM NaCl, 3.87 mM KCl, 2.42 mM NaH2PO4 · H2O, 4.86 mM l-glutamic acid, 4.86 mM fumaric acid, 4.86 mM pyruvate, 11.17 mM glucose, 1.79 mM CaCl2, 1.2 mM MgSO4 · 7H2O, 23.5 mM HEPES [pH 7.4]) containing 10 mg of collagenase (CLS type 1; Sigma, St. Louis, Mo.)/ml, bovine serum albumin (Sigma), and trypsin inhibitor. After incubation, the partly digested LMMP was washed with enzyme-free HEPES medium and reincubated in 10 ml of fresh HEPES medium to allow the cells to disperse spontaneously. Cells were then harvested by filtration through 210-μm polyester mesh.
Dispersed cells were stimulated by the addition of an 0.8-ml aliquot of the cell suspension to 0.1 ml of the test agent and incubation at room temperature for 30 s, because we previously found that carbachol induced the maximal contractile response in jejunal longitudinal smooth muscle cells after 30 s of incubation. The reaction was interrupted by the addition of acrolein to a final concentration of 1%. The median cell length of 50 cells on each slide was measured with a microscope by using image-splitting micrometry, and the percent decrease in the mean cell length relative to that of the control was determined.
MMCP-1 ELISA.
The levels of mouse mucosal mast cell protease 1 (MMCP-1) in serum were measured by using an MMCP-1 enzyme-linked immunosorbent assay (ELISA) kit purchased from Moredun Animal Health (Penicuik, United Kingdom) as previously described (22). Briefly, rabbit anti-MMCP-1 was used as a capture antibody. Tenfold serial dilutions of serum were made from 1/10 to 1/10,000. Horeseradish peroxidase-conjugated rabbit anti-mouse MMCP-1 was then added, and quantification was carried out by reference to purified MMCP-1.
Evaluation of in vitro cytokine production from spleen cells.
Single-cell suspensions of spleen tissues were prepared in RPMI 1640 containing 10% fetal calf serum, 5 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 25 mM HEPES, and 0.05 mM 2-mercaptoethanol (all from Gibco-BRL). Cells (107) were incubated in the presence of concanavalin A (ConA) (5 μg/ml) or 50 μg of T. spiralis antigen/ml. Culture supernatants were harvested after 48 h and stored at −20°C. Cytokines in the supernatants were measured by ELISAs with commercially available kits purchased from R & D Systems (Minneapolis, Minn.).
Histological analysis.
A segment (1 cm in length) of small intestine or cecum tip was fixed in 10% neutral buffered formalin and processed by standard histological techniques. The neutral buffered formalin-fixed sections were stained with periodic acid-Schiff stain for counting intestinal goblet cells. The number of goblet cells was expressed per 10 villus crypt units.
Statistical analysis.
Data were analyzed by using Student's t test, with a P value of <0.05 being considered significant. All results are expressed as the mean and standard error of the mean (SEM).
RESULTS
IL-9 administration to normal C57BL/6 mice stimulated intestinal muscle contractility.
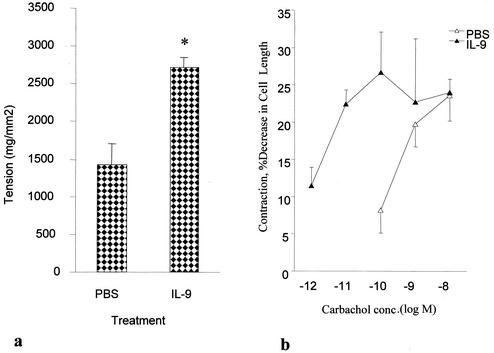
The administration of IL-9 to noninfected C57BL/6 mice significantly increased jejunal muscle contractility. As shown in Fig. 1a, carbachol-induced jejunal muscle contractility increased significantly in IL-9 treated mice compared to that in PBS-treated mice after 10 days of daily IL-9 treatment. To determine the locus of action of IL-9, we investigated the ability of IL-9 to contract dispersed jejunal smooth muscle cells. Strips of muscle were preincubated with the cytokine prior to dispersion of the cells and subsequent stimulation by carbachol. Preincubation with IL-9 did not alter mean cell length; cell lengths were 44.5 ± 3.4 μm in IL-9-exposed cells and 42.4 ± 6.2 μm in control cells. Carbachol (0.1 nM)-induced contraction of cells exposed to IL-9 was 28.6% ± 0.3%, a significant increase over the value of 8.2% ± 3.1% seen in control cells exposed to PBS (Fig. 1b).
FIG. 1.
IL-9-stimulated carbachol-induced contraction of jejunal muscle strips and dispersed muscle cells in noninfected C57BL/6 mice. (a) Maximum tension generated by jejunal muscle strips in response to 1 μM carbachol. Noninfected C57BL/6 mice were treated i.p. with IL-9 (100 ng/day) or sterile PBS in 1% normal serum for 10 days and killed to investigate carbachol-induced muscle contraction in the jejunal muscle strips. Values are means and SEMs for five animals. An asterisk indicates a significant difference compared to the value obtained in PBS-treated mice. (b) Dose-response curve for carbachol-induced jejunal muscle cell contraction. LMMP from the jejunum was preincubated with PBS or IL-9 (10 ng/ml) overnight prior to dispersion of the cells. Carbachol-induced contraction in dispersed muscle cells was measured. Values are means and SEMs from four or five experiments. conc., concentration.
IL-9 enhanced jejunal muscle contractility and intestinal worm expulsion in T. spiralis-infected mice.
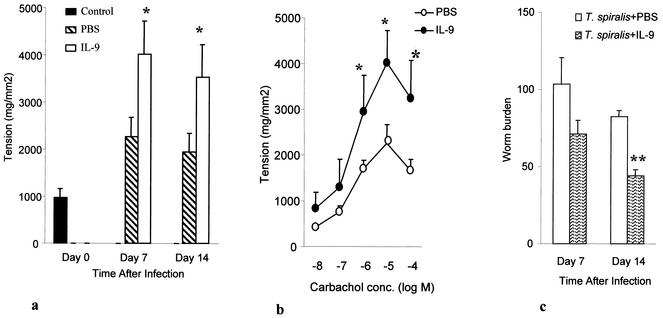
We next investigated the effect of exogenous IL-9 on intestinal muscle contraction during T. spiralis infection of mice. As shown in Fig. 2a, carbachol-induced jejunal muscle contractility increased significantly in IL-9-treated infected mice compared to that in PBS-treated infected mice at days 7 and 14 p.i. This result was not a reflection of the carbachol dose used in these experiments, as differences between IL-9-treated mice and PBS-treated mice were observed for several doses (Fig. 2b).
FIG. 2.
IL-9-enhanced jejunal muscle contraction and T. spiralis worm expulsion in C57BL/6 mice. Mice were infected with T. spiralis and then treated with IL-9 (100 ng/day) or sterile PBS in 1% normal serum. Mice were killed at days 7 and 14 p.i. to investigate muscle contraction and worm recovery. (a) Maximum tension generated by jejunal muscle strips taken from T. spiralis-infected mice in response to carbachol. Day 0 shows data for control noninfected mice. (b) Dose-response relationships for carbachol-induced contraction of jejunal muscle strips from IL-9 treated infected and PBS-treated infected mice on day 7 p.i. conc., concentration. (c) Worm recovery on days 7 and 14 after T. spiralis infection. Values are means and SEMs for five animals. A single asterisk indicates a value significantly higher than that in PBS-treated infected mice. A double asterisk indicates a value significantly lower than that in PBS-treated infected mice.
To investigate the effect of IL-9 on T. spiralis expulsion, infected mice were treated with IL-9 and sacrificed on different days p.i. to count worm numbers in the small intestine. Repeated treatment of mice with IL-9 accelerated worm expulsion in mice infected with T. spiralis. We recovered significantly lower numbers of worms from IL-9-treated mice than from control mice on days 7 and 14 p.i. (Fig. 2c).
IL-9 enhanced serum MMCP-1 levels and intestinal goblet cell numbers in T. spiralis infection.
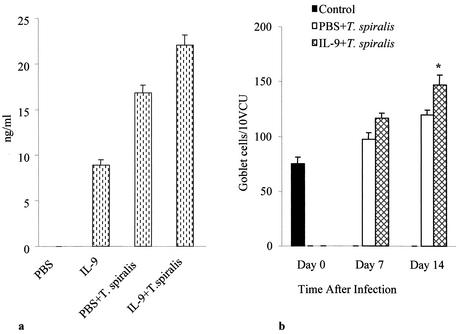
MMCP-1 levels were undetectable in noninfected mice. Infection with T. spiralis caused a clear increase in serum MMCP-1 concentrations (from <0.1 to 16.85 ± 0.8 ng/ml). IL-9 administration to both noninfected and T. spiralis-infected mice resulted in a significant up-regulation of the enzyme (Fig. 3a).
FIG. 3.
IL-9-enhanced serum MMCP-1 levels and intestinal goblet cell numbers in T. spiralis infection. (a) T. spiralis-infected and noninfected mice were treated with IL-9 (100 ng/day) or with sterile PBS in 1% normal serum. Mice were killed on day 14 p.i. to investigate serum MMCP-1 levels. Values are means and SEMs for four animals. (b) Intestinal goblet cell response in T. spiralis infection. Mice were infected with T. spiralis and then treated with IL-9 (100 ng/day) or sterile PBS in 1% normal serum. Mice were killed on days 7 and 14 p.i. to investigate periodic acid-Schiff-stained intestinal goblet cell numbers. Day 0 shows data for control noninfected mice. Values are means and SEMs for five animals. An asterisk indicates a significant difference compared to the value obtained in PBS-treated infected mice. VCU, villus crypt unit.
The administration of IL-9 during T. spiralis infection also increased the numbers of intestinal goblet cells. As shown in Fig. 3b, there was a significant elevation of goblet cell numbers at day 14 p.i. in T. spiralis-infected mice, and IL-9 administration significantly enhanced the development of this infection-induced goblet cell hyperplasia on day 14 p.i.
Administration of IL-9 up-regulates IL-4 and IL-13 production from in vitro-stimulated spleen cells.
To evaluate the influence of IL-9 on the production of IL-4 and IL-13 in T. spiralis infection, spleen cells collected from IL-9- or PBS-treated infected mice on day 8 p.i. were stimulated with ConA or with T. spiralis antigen. The production of both IL-4 and IL-13 in cultures of ConA-stimulated spleen cells collected from IL-9 treated mice was significantly increased (Table 1). A similar trend was also observed after in vitro stimulation with T. spiralis antigen.
TABLE 1.
Cytokines produced by in vitro-stimulated spleen cellsa
| Treatment used with T. spiralis infection | Stimulation | Level (pg/ml) of:
|
|
|---|---|---|---|
| IL-4 | IL-13 | ||
| PBS | ConA | 135.5 ± 14.3 | 392.7 ± 23.5 |
| IL-9 | ConA | 313.9 ± 60.9 | 752.9 ± 95.7 |
| PBS | T. spiralis antigen | 63.9 ± 12.8 | 156 ± 18.2 |
| IL-9 | T. spiralis antigen | 138.4 ± 17.3 | 315.9 ± 56.9 |
C57BL/6 mice were infected with T. spiralis orally and treated i.p. with PBS or IL-9 (100 ng/daily) from the day of infection. Mice were killed on day 8 p.i., spleen cells were stimulated with ConA or T. spiralis antigen for 48 h, and the levels of IL-4 and IL-13 present in the supernatants were investigated by ELISAs. Each value represents the mean and SEMs for four mice.
Anti-IL-9 antibody administration or anti-IL-9 vaccination did not alter jejunal muscle contractility or worm expulsion in T. spiralis-infected mice.
Although the administration of IL-9 to T. spiralis-infected mice enhanced jejunal muscle contractility and worm expulsion, we could not establish a role for endogenous IL-9 in the development of muscle hypercontractility in T. spiralis-infected mice. The neutralization of IL-9 by anti-IL-9 monoclonal antibody or by anti-IL-9 vaccination had no significant effect on intestinal muscle contractility (Fig. 4a) or on worm expulsion (Fig. 4b) in T. spiralis infection.
FIG. 4.
Neutralization of IL-9 does not affect jejunal muscle contraction or worm expulsion in T. spiralis infection. (a) Carbachol-induced maximum tension generated by jejunal muscle strips after neutralization of IL-9 activity. Mice were infected with T. spiralis and treated with anti-IL-9 monoclonal antibody or with mouse IgG (1 mg on day 0 and then 0.5 mg on every alternate day until day 20 p.i.). Mice were killed at days 14 and 21 p.i. to investigate jejunal muscle contraction. Day 0 shows data for control noninfected mice. (b) Worm recovery from T. spiralis-infected mice following neutralization of IL-9 by anti-IL-9 vaccination or by anti-IL-9 antibody. Mice were immunized with IL-9- OVA or treated with anti-IL-9 antibody and then infected with T. spiralis. Mice were killed on day 21 p.i. to investigate intestinal worm burden. Values are means and SEMs for five animals.
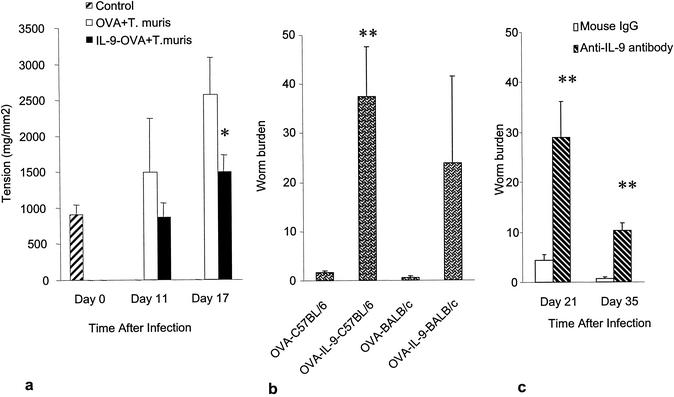
Anti-IL-9 vaccination attenuated colonic muscle contractility and inhibited worm expulsion in T. muris infection.
To investigate the effect of the neutralization of IL-9 in another nematode parasite, which resides in a different location of the gut, the cecum, mice were vaccinated against IL-9 and infected with T. muris. In contrast to the findings observed with T. spiralis, the neutralization of IL-9 significantly inhibited worm expulsion and attenuated colonic muscle hypercontractility in T. muris infection. As shown in Fig. 5a, we observed a significantly lower degree of colonic muscle contractility on days 11 and 17 p.i. in mice immunized against IL-9 with the IL-9- OVA complex but not in mice treated with OVA alone. This lower degree of muscle contractility correlated with the presence of higher numbers of worms in the cecum. We recovered substantially higher number of worms on day 34 p.i. from the ceca of both C57BL/6 and BALB/c mice, which were given the IL-9- OVA complex to neutralize IL-9 activity, than from the ceca of OVA-treated control mice (Fig. 5b). Similar results were also observed with anti-IL-9 monoclonal antibody treatment in BALB/c mice on days 21 and 35 p.i. (Fig. 5c).
FIG. 5.
IL-9 immunoneutralization attenuates colonic muscle contraction and inhibits worm expulsion in T. muris infection. C57BL/6 or BALB/c mice vaccinated against IL-9 or treated with anti-IL-9 antibody were infected with 100 eggs of T. muris orally. Mice were killed on different days p.i. to investigate colonic muscle contraction and worm recovery. (a) Maximum tension generated by colonic muscle in C57BL/6 mice in response to 1 μM carbachol on days 11 and 17 p.i. Day 0 shows data for control noninfected mice. (b) Worm recovery on day 34 p.i. from C57BL/6 and BALB/c mice after anti-IL-9 vaccination. (c) Worm recovery from BALB/c mice after anti-IL-9 antibody (MM9A1) treatment. A single asterisk indicates a value significantly lower in IL-9- OVA-treated mice than in OVA-treated mice. A double asterisk indicates a value significantly higher than that in OVA-treated mice. Values are means and SEMs for four animals.
Blocking of IL-9 is not associated with significant changes in serum MMCP-1 levels or in goblet cell numbers during T. spiralis or T. muris infection.
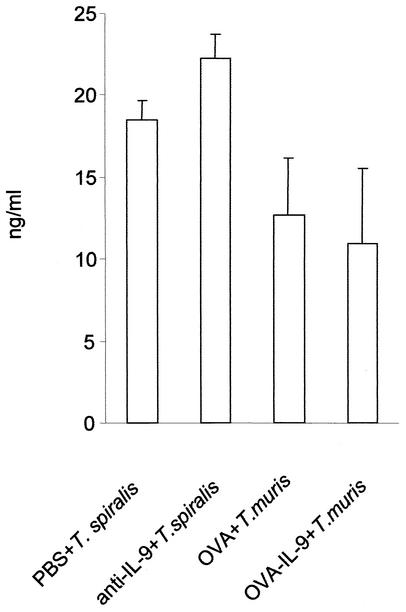
Blocking of IL-9 with either anti-IL-9 antibody or the IL-9- OVA vaccination procedure had no effect on serum MMCP-1 levels in either T. spiralis- or T. muris-infected mice (Fig. 6). To verify that the failure of anti-IL-9 antibody to block MMCP-1 induction after infection was not the result of excessive IL-9 production, noninfected mice were treated with 10 μg of anti-IL-9 antibody/mouse (a dose 50 times lower than that used for infected mice) prior to two daily injections of IL-9 (200 ng/mouse). Serum MMCP-1 levels measured 24 h after IL-9 administration rose from less than 1 to 36.1 ± 17.8 ng/ml in mice given IL-9 only but remained undetectable when IL-9 was given after anti-IL-9 treatment.
FIG. 6.
Neutralization of IL-9 does not alter serum MMCP-1 levels in T. spiralis and T. muris infections. In a T. spiralis infection, mice were treated with anti-IL-9 monoclonal antibody or with mouse IgG (1 mg on day 0 and then 0.5 mg on every alternate day) and then killed on day 14 p.i. to investigate serum MMCP-1 levels by ELISAs. In a T. muris infection, mice were immunized with IL-9- OVA complex or OVA and then killed on day 17 p.i. to investigate MMCP-1. Values are means and SEMs for four animals.
Anti-IL-9 antibody or anti-IL-9 vaccination also had no significant effect on jejunal goblet cell numbers in T. spiralis-infected mice or on colonic goblet cell numbers in T. muris-infected mice (data not shown).
DISCUSSION
The present study demonstrates that IL-9 induces hypercontractility of jejunal smooth muscle, acting at the level of intestinal musculature. In mice infected with T. spiralis, this effect was accompanied by accelerated worm expulsion. These results emphasize the importance of muscle hypercontractility for host defense through facilitation of the eviction of the parasite from the gut and the potential use of IL-9 to stimulate this response. Endogenous IL-9 does not, however, appear to play a critical role in the hypercontractility observed in unmanipulated T. spiralis-infected C57BL/6 mice, since anti-IL-9 antibody did not interfere with worm clearance. As it was previously shown that hypercontractility induced by T. spiralis is mediated largely through Stat6, we interpret the action of IL-9, which is not a Stat6 activator, to reflect its ability to enhance IL-4 and IL-13 activities.
In contrast to the observations made with T. spiralis, IL-9 plays a critical role in mediating the hypercontractility of colonic muscle during T. muris infection. Taken together, these results indicate that the roles of individual cytokines in the generation of Th2-mediated muscle hypercontractility differ according to the type of nematode infection: while IL-4 and IL-13 are required and sufficient for hypercontractility induced by T. spiralis infection, IL-9 is required for an optimal response to T. muris infection. In both situations, however, increasing IL-9 levels accelerates worm clearance.
T. spiralis is a parasite that dwells in the small intestine, and studies with animal models have demonstrated that, like other nematode infections, infection with T. spiralis is associated with enhanced contractility of small intestinal muscle (6, 33, 36). A previous study from our laboratory showed that mouse strains expelling this parasite rapidly (e.g., NIH Swiss) exhibit a greater degree of small intestinal muscle contractility than B10.BR mice, which expel this parasite slowly (36). Recent work has shown that jejunal muscle hypercontractility is attenuated in mice deficient in Stat6, suggesting an important role for Th2 cytokines (24). Very recently, it was also shown that IL-4 and IL-13 enhance carbachol-induced contraction in dispersed smooth muscle cells isolated from the jejunum of mice (1). It was also shown that the transfer and overexpression of the IL-12 gene during Th2-based T. spiralis infection shift the immune response toward Th1 and inhibit worm expulsion. In addition, this shift in the immune response attenuates infection-induced muscle hypercontractility (26). Taken together, these results clearly indicate that the Th2 type of immune response is critical for the development of intestinal muscle hypercontractility in this infection and also that the processes underlying muscle hypercontractility and parasite expulsion may share a common immunological basis and may be causally related. In the present study, the IL-9-mediated enhancement of muscle contractility in infected mice might have occurred as the result of an additive effect of this cytokine on other Th2 cytokines or simply through an increase in the production of IL-4 and IL-13, both of which were produced in higher amounts from spleen cells from IL-9-treated mice. IL-9 induction of IL-4, IL-5, and IL-13 expression in lungs was also shown recently by Temann et al. (34).
To assess the contribution of IL-9 in T. spiralis infection, we also studied the effect of IL-9 treatment on muscle contractility and worm expulsion in Stat6-deficient mice infected with T. spiralis. Consistent with previous findings (24), significant attenuation of infection-induced intestinal muscle contractility and inhibition of worm expulsion occurred in Stat6-deficient T. spiralis-infected mice. In spite of the acceleration of worm expulsion and the enhancement of muscle contractility in wild-type C57BL/6 mice, IL-9 treatment had no significant effect on muscle contractility or on worm expulsion in Stat6-deficient mice on days 7 and 14 p.i. (data not shown). On the basis of these results, it seems likely that the effect that we observed on muscle contractility was due to increased production of IL-4 and IL-13. The failure to inhibit the development of muscle hypercontractility and worm expulsion by using two different methods of IL-9 neutralization may have been due to a dominant effect of IL-4 or IL-13 in this infection, a situation similar to that recently observed in the context of worm expulsion in IL-4-deficient mice (2). Previous studies suggested that blocking of both IL-4 and IL-13 is required for efficient inhibition of worm expulsion in T. spiralis infection but that blocking of either IL-4 or IL-13 is sufficient to inhibit worm expulsion in T. muris infection (17). It is possible that in T. spiralis infection, immunoneutralization of IL-9 failed to affect worm expulsion and muscle contractility due to a compensatory action of IL-4 or IL-13 or both.
The nematode parasite T. muris inhabits the large intestine of mice and is closely related at the morphological, physiological, and antigenic levels to Trichuris trichuria, the causative agent of chronic trichuriasis in humans (12). Different inbred strains of mice exhibit differential immune responsiveness to T. muris infection. Resistant strains (C57BL/6, BALB/c, and BALB/K) mount a Th2 type of response and expel the parasite rapidly from the intestine, while susceptible strains (AKR and B10.BR) develop a chronic infection with the activation of a nonprotective Th1 type of response (11). Previous studies with T. muris demonstrated that IL-9 transgenic mice, which constitutively overexpress IL-9, expel worms more quickly than wild-type mice (15) and that vaccination against IL-9 inhibits worm expulsion (32) from the intestine. Although the importance of IL-9 in the generation of a host protective immune response in this infection was well documented in these studies, the ultimate effector mechanisms by which IL-9 mediates the eviction of the parasite from the gut remain to be determined. In this study, we clearly observed an attenuation of T. muris infection-induced colonic muscle contraction in mice which were immunized against IL-9. This finding correlated with the inhibition of worm expulsion from the large intestine. These findings clearly indicate that IL-9 plays a critical role in worm expulsion and muscle hypercontractility during this nematode infection. In addition, as IL-9 activates Stat3 (42), the results of the present study indicate that in addition to the Stat6 pathway, other pathways, such as the Stat3 pathway, may also be involved in intestinal muscle function.
Characteristic components of the cellular immune response to nematode infections also include mucosal mastocytosis and goblet cell hyperplasia (9, 19). Here, we further investigated the extent to which these responses are IL-9 dependent and are involved in host protection against these two nematode infections. Mast cells are generally considered important for host protective immunity in T. spiralis infection, although some controversy exists (27, 35). To investigate the mast cell response, we quantified the levels in serum of MMCP-1, a protease known to be produced by mucosal mast cells in vivo (30). The administration of exogenous IL-9 increased MMCP-1 levels in T. spiralis infection. As IL-9 is a growth factor for mast cells, this increment in MMCP-1 levels reflects the efficacy of in vivo IL-9 treatment. Moreover, the suppression of MMCP-1 by anti-IL-9 antibody treatment in noninfected mice suggested that the antibody effectively inhibited the activity of IL-9 in vivo. Nevertheless, the absence of any significant effects of anti-IL-9 antibody treatment on MMCP-1 levels and worm expulsion in T. spiralis-infected mice indicates that other cytokines compensate for the absence of IL-9 in maintaining MMCP-1 levels in this infection. Recently, we found that MMCP-1 levels are decreased in T. spiralis-infected IL-4 knockout mice immunized against IL-9 (unpublished observation), suggesting that effective inhibition of MMCP-1 is attainable by blocking both IL-4 and IL-9 but not by blocking IL-9 only.
While IL-9 immunoneutralization inhibited worm expulsion, it had no significant effect on serum MMCP-1 levels in T. muris-infected mice. This observation supports a previous observation that immunization against IL-9 had no significant effect on mucosal mast cell numbers in the cecum in this nematode infection compared to a control (32). These observations do not support a major role for mast cells in the expulsion process of T. muris, an observation similar to one recently made for anti-IL-3 antibody treatment in this parasite infection (3).
Hyperplasia of mucin-secreting intestinal goblet cells has also been observed in a number of nematode infections, including T. spiralis and T. muris infections (9, 18, 23, 25). Mucins from goblet cells may play an important role in trapping and removing intestinal nematodes from the gut (28). In this study, we observed significantly higher numbers of goblet cells in IL-9-treated mice than in PBS-treated mice infected with T. spiralis. This enhancement of the goblet cell response may also be due to the additive effects of IL-9 on other Th2 cytokines. Although exogenous IL-9 treatment increased goblet cell numbers in the intestine, neutralization of this cytokine had no significant effect on these cells in T. spiralis infection. In T. muris infection, although goblet cell numbers decreased in mice which were immunized against IL-9, the reduction was not statistically different from that in controls. Thus, IL-9-mediated host protective immunity in T. muris infection correlated more with colonic muscle hypercontractility than with goblet cells.
In conclusion, the present data indicate that IL-9 influences intestinal muscle function during enteric nematode infection and that the involvement of this cytokine in host protective immunity in nematode infection differs in infections with different species of nematodes. The results suggest that when IL-9 stimulates intestinal muscle contraction, it is associated with an acceleration of worm clearance, and that anti-IL-9 neutralization inhibits worm clearance when it is associated with an attenuation of muscle contraction. While IL-9 seems to be critical in the development of colonic muscle hypercontractility and worm expulsion in T. muris infection, it is not essential for the development of hypercontractility in T. spiralis-infected mice, although the addition of IL-9 enhanced hypercontractility and worm expulsion, presumably by an additive affect of its action together with those of IL-4 and IL-13. This study provided evidence for a role of IL-9 in immune-mediated alterations in intestinal muscle function and generated interesting data on the contribution of the same cytokine to host protection in different species of nematode parasites.
Acknowledgments
This study was supported by the grant from the Canadian Institutes for Health Research (CIHR) and in part by the Belgian Federal Service for Scientific Technical and Cultural Affairs and the Actions de Recherche Concertées, Communauté Française de Belgique, Direction de la Recherche Scientifique.
M.R. is a scientific associate at Télévie.
Editor: J. M. Mansfield
REFERENCES
- 1.Akiho, H., P. Blennerhasset, Y. Deng, and S. M. Collins. 2002. Role of IL-4, IL-13 and Stat6 in inflammation induced hypercontractility of murine smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G226-G233. [DOI] [PubMed]
- 2.Bancroft, A. J., D. Artis, D. D. Donaldson, J. P. Sypek, and R. K. Grencis. 2000. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. Eur. J. Immunol. 30:2083-2091. [DOI] [PubMed] [Google Scholar]
- 3.Betts, C. J., and K. J. Else. 1999. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 21:45-52. [DOI] [PubMed] [Google Scholar]
- 4.Bitar, K. N., and G. M. Makhlouf. 1982. Receptors on smooth cells: characterization by contraction and specific antagonists. Am. J. Physiol. 242:G400-G407. [DOI] [PubMed]
- 5.Castro, G. A., and D. Fairbairn. 1969. Carbohydrates and lipids in Trichinella spiralis larvae and their utilization. J. Parasitol. 55:51-58. [PubMed] [Google Scholar]
- 6.Collins, S. M. 1996. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 111:1683-1689. [DOI] [PubMed] [Google Scholar]
- 7.Demoulin, J. B., and J.-C. Renauld. 1998. Interleukin 9 and its receptor: an overview of structure and function. Int. Rev. Immunol. 16:345-364. [DOI] [PubMed] [Google Scholar]
- 8.Dong, Q., J. Louahed, A. Vink, C. D. Sullivan, C. J. Messler, Y. Zhen, A. Haczku, F. Huax, M. Arras, K. J. Holroyd, J. C. Renauld, R. C. Levitt, and N. C. Nicoloides. 1999. IL-9 induces chemokine expression in lung epithelial cells and baseline airway eosinophilia in transgenic mice. Eur. J. Immunol. 29:2130-2139. [DOI] [PubMed] [Google Scholar]
- 9.Else, K. J., and F. D. Finkelman. 1998. Intestinal nematode parasites, cytokines and effector mechanisms. Int. J. Parasitol. 28:1145-1158. [DOI] [PubMed] [Google Scholar]
- 10.Else, K. J., L. Hultner, and R. K. Grencis. 1992. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of Th-cell subsets in resistant and susceptible mice. Immunology 75:232-237. [PMC free article] [PubMed] [Google Scholar]
- 11.Else, K. J., and R. K. Grencis. 1991. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology 72:508-513. [PMC free article] [PubMed] [Google Scholar]
- 12.Else, K. J., and R. K. Grencis. 1991. Helper T-cell subsets in mouse trichuriasis. Parasitol. Today 7:313-316. [DOI] [PubMed] [Google Scholar]
- 13.Farmer, S. G. 1981. Propulsive activity of the rat small intestine during infection with the nematode Nippostrongylus brasiliensis. Parasite Immunol. 3:227-234. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner, H., N. Humphreys, J.-C. Renauld, J. Van Snick, and R. K. Grencis. 1997. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur. J. Immunol. 27:2536-2540. [DOI] [PubMed] [Google Scholar]
- 15.Faulkner, H., J.-C. Renauld, J. Van Snick, and R. K. Grencis. 1998. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect. Immun. 66:3832-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelman, F. D., T. Shea-Donohue, C. A. Goldhill, J. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal helminths: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 17.Finkelman, F. D., T. A. Wynn, D. D. Donaldson, and J. F. Urban. 1999. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infection. Curr. Opin. Immunol. 11:420-426. [DOI] [PubMed] [Google Scholar]
- 18.Garside, P., R. K. Grencis, and A. McMowat. 1992. T lymphocyte dependent enteropathy in murine Trichinella spiralis infection. Parasite Immunol. 14:217-225. [DOI] [PubMed] [Google Scholar]
- 19.Grencis, R. K. 1997. Th2-mediated host protective immunity to intestinal nematode infections. Philos. Trans. R. Soc. Lond. B 352:1377-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grencis, R. K., L. Hultner, and K. J. Else. 1991. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology 74:329-332. [PMC free article] [PubMed] [Google Scholar]
- 21.Grohmann, U., J. Van Snick, F. Campanile, S. Silla, A. Giampietri, C. Vacca, J. C. Renauld, M. C. Fioretti, and P. Puccetti. 2000. IL-9 protects mice from Gram-negative bacterial shock: suppression of TNF-α, IL-12, and IFN-γ, and induction of IL-10. J. Immunol. 164:4197-4203. [DOI] [PubMed] [Google Scholar]
- 22.Huntley, J. F., C. Gooden, G. F. J. Newlands, A. Mackeller, D. A. Lammas, D. Wakelin, N. Tuohy, R. G. Woodbury, and H. R. P. Miller. 1990. Distribution of intetinal mast cell proetinase in blood and tissues of normal and Trichinella-infected mice. Parasite Immunol. 12:85-95. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa, N., D. Wakelin, and Y. Mahida. 1997. Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 113:542-549. [DOI] [PubMed] [Google Scholar]
- 24.Khan, W. I., B. A. Vallance, P. A. Blennerhasset, Y. Deng, E. F. Verdue, K. I. Matthaei, and S. M. Collins. 2001. Critical role for signal transducer and activator of transcription factor 6 in mediating intestinal muscle hypercontractility and worm expulsion in Trichinella spiralis-infected mice. Infect. Immun. 69:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan, W. I., P. A. Blennerhasset, C. Ma, K. I. Matthaei, and S. M. Collins. 2001. Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol. 23:39-42. [DOI] [PubMed] [Google Scholar]
- 26.Khan, W. I., P. A. Blennerhasset, Y. Deng, J. Gauldie, B. A. Vallance, and S. M. Collins. 2001. IL-12 gene transfer alters gut physiology and host immunity in nematode-infected mice. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G102-G110. [DOI] [PubMed]
- 27.Lawrence, C. E., J. C. M. Paterson, L. M. Higgins, T. T. Mcdonald, M. W. Kennedy, and P. Garside. 1998. IL-4 regulated enteropathy in an intetinal nematode infection. Eur. J. Immunol. 28:2672-2684. [DOI] [PubMed] [Google Scholar]
- 28.Miller, H. R. P. 1987. Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitology 94:S77-S100. [DOI] [PubMed]
- 29.Mossman, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 30.Newlands, G. F. J., S. Gibson, D. P. Knox, R. Grencis, D. Wakelin, and H. R. P. Miller. 1987. Characterization and mast cell origin of a chymotrysin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology 62:629-634. [PMC free article] [PubMed] [Google Scholar]
- 31.Renauld, J.-C., F. Houssiau, J. Louahed, A. Vink, J. Van Snick, and C. Uyttenhove. 1993. Interleukin-9. Adv. Immunol. 54:79-91. [DOI] [PubMed] [Google Scholar]
- 32.Richard, M., R. K. Grencis, N. E. Humphreys, J.-C. Renauld, and J. Van Snick. 2000. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc. Natl. Acad. Sci. USA 97:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukhdeo, M. V. K., and N. A. Croll. 1981. Gut propulsion in mice infected with Trichinella spiralis. J. Parasitol. 67:906-910. [PubMed] [Google Scholar]
- 34.Temann, U. A., P. Ray, and R. A. Flavell. 2002. Pulmonary overexpression of IL-9 induces Th2 cytokine expression leading to immune pathology. J. Clin. Investig. 109:29-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uber, C. L., R. L. Roth, and D. A. Levy. 1980. Expulsion of Nippostrongylus brasiliensis by mice deficient in mast cells. Nature 287:226-228. [DOI] [PubMed] [Google Scholar]
- 36.Vallance, B. A., P. A. Blennerhassett, and S. M. Collins. 1997. Increased intestinal muscle contractility and worm expulsion in nematode infected mice. Am. J. Physiol. Gastrointest. Liver Physiol. 272:G321-G327. [DOI] [PubMed]
- 37.Vallance, B. A., P. A. Blennerhassett, Y. Deng, K. I. Matthaei, I. G. Yong, and S. M. Collins. 1999. IL-5 contribute to worm expulsion and muscle hypercontractility in primary T. spiralis infection. Am. J. Physiol. Gastrointest. Liver Physiol. 277:G400-G408. [DOI] [PubMed]
- 38.Vallance, B. A., F. Galeazzi, S. M. Collins, and D. P. Snider. 1999. CD4 T cells and major histocompatibility complex class II expression influence worm expulsion and increased intestinal muscle contraction during Trichinella spiralis infection. Infect. Immun. 67:6090-6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Snick, J., A. Goethals, J.-C. Renauld, E. Van Roost, C. Uyttenhove, M. R. Rubira, R. L. Moritz, and R. J. Simpson. 1989. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40). J. Exp. Med. 169:363-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vermillion, D. L., and S. M. Collins. 1998. Increased responsiveness of jejunal longitudinal muscle in Trichinella-infected rats. Am. J. Physiol. Gastrointest. Liver Physiol. 254:G124-G129. [DOI] [PubMed]
- 41.Wakelin, D. 1967. Acquired immunity to Trichuris muris in the alnino laboratory mouse. Parasitology 57:515-517. [DOI] [PubMed] [Google Scholar]
- 42.Yin, T., S. R. Keller, F. W. Quelle, B. A. Witthuhn, M. L. Tsang, G. E. Lienhard, J. N. Ihle, and Y.-C. Yang. 1995. Interleukin-9 induces tyrosine phosphorylation of insulin receptor substrate-1 via JAK kinases. J. Biol. Chem. 270:20497-20502. [DOI] [PubMed] [Google Scholar]