Abstract
Cells of the dendritic cell (DC) lineage, by their unique ability to stimulate naive T cells, may be of crucial importance in the development of protective immune responses to Leishmania parasites. The aim of this study was to compare the impact of L. major infection on DCs in BALB/c (susceptible, developing Th2 responses), C57BL/6 (resistant, developing Th1 responses), and tumor necrosis factor (TNF)−/− C57BL/6 mice (susceptible, developing delayed and reduced Th1 responses). We analyzed by immunohistochemistry the phenotype of infected cells in vivo. Granulocytes (GR1+) and macrophages (CD11b+) appear as the mainly infected cells in primary lesions. In contrast, cells expressing CD11c, a DC specific marker, are the most frequently infected cells in draining lymph nodes of all mice tested. These infected CD11c+ cells harbored a particular morphology and cell surface phenotype in infected C57BL/6 and BALB/c mice. CD11c+ infected cells from C57BL/6 and TNF−/− C57BL/6 mice displayed a weak parasitic load and a dendritic morphology and frequently expressed CD11b or F4/80 myeloid differentiation markers. In contrast, some CD11c+ infected cells from BALB/c mice were multinucleated giant cells. Giant cells presented a dramatic accumulation of parasites and differentiation markers were not detectable at their surface. In all mice, lymph node CD11c+ infected cells expressed a low major histocompatibility complex II level and no detectable CD86 expression. Our results suggest that infected CD11c+ DC-like cells might constitute a reservoir of parasites in lymph nodes.
Leishmania spp. are protozoan parasites belonging to the Trypanosomatidae family. They are transmitted by phlebotomine sand flies to several mammals, including humans (reviewed in references 8 and 31). Leishmania promastigotes, the extracellular flagellated forms of the parasite in the insect vector, gain access to host tissues during sand-fly bite, where they are internalized by macrophages. They then transform into amastigotes which replicate by binary fission until they are released by the rupture of the host cell. Leishmania sp. induce a large spectrum of diseases in humans, from cutaneous lesions to progressive fatal visceralizing diseases. Clinical manifestations depend on the parasite species, immune response, and genetics of the host. A lot of information on these factors has been drawn from the murine models of Leishmania major infection. Clearance of L. major parasites in infected cells implicates effector mechanisms (7, 20) positively regulated by gamma interferon-producing CD4+ T cells (Th1 cells) and downregulated by interleukin-4 (IL-4)- or IL-10-producing CD4+ T cells (Th2 cells) (reviewed in references 31 and 32). Most inbred mouse strains (including C3H and C57BL/6 mice) develop a protective immune Th1 response and are able to control infection. The key role played by IL-12 in the Th1 differentiation has been extensively documented in Leishmania infection (28, 35). In contrast, BALB/c mice develop an IL-4-mediated Th2 response and a progressive fatal disease (19).
Cells of the dendritic cell (DC) lineage are professional antigen-presenting cells. They take up antigens, generate major histocompatibility complex (MHC)-peptide complexes, migrate from the sites of antigen capture to secondary lymphoid organs, and finally stimulate T cells after a physical interaction with them (reviewed in reference 21). It has been shown that amastigotes, but not promastigotes, efficiently infected DC in vitro (37), suggesting that during the course of infection skin DC might be infected by amastigotes released from macrophages. Recent studies suggest that DC might be critical in the initiation and regulation of a protective immune response to Leishmania: (i) DCs have the unique ability to transport viable amastigotes from the primary lesion to the draining lymph node (25); (ii) in vitro and in vivo experiments suggest that DCs, rather than macrophages, might be the major source of IL-12 during the early stages of Leishmania infection (16); (iii) antigen-pulsed epidermal DCs protect BALB/c susceptible mice from infection with L. major (12); and (iv) pretreatment with the recombinant hematopoietic cytokine Flt3, that induces the expansion of DCs, partially protects against progressive leishmaniasis in susceptible mice (23).
Several works have demonstrated in vitro that L. major infection impairs the antigen-presenting function of macrophages (15) and their ability to produce IL-12 (9), making infected macrophages an unlikely candidate to prime naive T cells in draining lymph nodes. Actually, the impact of L. major infection in vivo on DC populations is unknown. The aim of this study was to compare in vivo the impact of L. major infection on cells of the DC lineage in susceptible and resistant mice. BALB/c and C57BL/6 (B6.WT) strains of mice are classically used as models of mice susceptible and resistant to L. major, respectively. However, as described below, these strains develop, due to genetic difference, a very different class of immune response (Th2 and Th1, respectively) against L. major parasite rending the interpretation of data complex. Thus, we used C57BL/6 mice lacking tumor necrosis factor (TNF) (B6.TNF−/−) mice as a model of susceptible mice in the C57BL/6 genetic background. In these mice susceptibility to L. major is not associated with the development of Th2 response but is correlated with a delayed and reduced Th1 response (38). We analyzed in situ the cell surface phenotype of infected cells in primary footpad lesions and draining lymph nodes of BALB/c, B6.WT, and B6.TNF−/− mice. Our results show that cells displaying DC-like phenotype are the most frequently infected cells in the draining lymph node and harbor different parasitic loads and cell surface markers in susceptible and resistant mice.
MATERIALS AND METHODS
Mice and parasite.
Six- to eight-week-old female BALB/c and C57BL/6 mice were purchased from Charles River Laboratories. C57BL/6 lacking TNF-α were as previously described (38) and were bred in our animal facility. The maintenance and care of mice complied with the guidelines of the ULB Ethics Committee for the humane use of laboratory animals. Promastigotes of L. major (MHOM/IR/−/173 strain) were propagated in vitro at room temperature in RPMI 1640 medium (Life Technologies, Gaithersburg, Md.), supplemented with 10% fetal calf serum (Life Technologies), penicillin G (100 U/ml), and streptomycin (100 μg/ml). Parasites harvested in the stationary phase after 8 to 10 days of culture were centrifuged (2,500 × g, 10 min, 4°C) and then washed three times in RPMI before being used to inoculate animals.
Leishmania infection, lesion monitoring, and tissue processing.
Mice were infected subcutaneously in the rear left hind footpad with 5 × 106 stationary-phase promastigotes of L. major in a final volume of 25 μl (in RPMI medium). The contralateral right footpad received an identical volume of RPMI medium without parasites as an internal control. The thickness of infected and uninfected footpads was regularly measured with a vernier caliper, and the difference between both measurements corresponded to the size of lesions. At selected time points, some mice were killed by cervical dislocation. Footpad lesions (or normal tissue in controls) were cut tangentially to the bone ground. Popliteal homolateral draining lymph nodes were collected to perform immunohistochemical studies (see below). The distribution and enumeration of amastigotes and infected cells were determined in organ sections stained with hematoxylin.
Immunohistochemistry.
In the present study, we used Immunohistowax processing, a new fixation and embedding method for light microscopy, initially developed for DC identification in situ, which preserves antigen immunoreactivity and morphological structures (27). Briefly, primary lesions and draining lymph nodes were fixed for 3 days in Immunohistofix (Aphase, Gosselies, Belgium), followed by dehydratation in a graded series of ethanol solution (30, 50, 70, 90, and 100%) for 30 min each at room temperature. Tissues were embedded in Immunohistowax (Aphase) and cut into 3- to 6-μm sections, deembedded by a wash in acetone for 10 min, and transferred to phosphate-buffered saline (PBS). The tissue sections were treated for 30 min with blocking reagent (1% in PBS; BoehringerMannheim, Mannheim, Germany) to saturate the sites of nonspecific reactions. The endogenous peroxidase activity was neutralized by 3% H2O2 in PBS for 60 min. The slides were then incubated with one of the following biotinylated antibodies (10 μg/ml in 0.5% PBS-blocking reagent): 2G9 (anti-I-A/I-E monoclonal antibody [MAb]; BD Pharmingen, San Diego, Calif.), C1:A3-1 (anti-F4/80 MAb; Serotec, Oxford, United Kingdom), GL1 (anti-CD86 MAb; American Type Culture Collection), M1/70 (anti-CD11b MAb; BD Pharmingen), HL3 (anti-CD11c MAb; BD Pharmingen), RA3-6B2 (anti-CD45R/B220 MAb; BD Pharmingen), RB6-8C5 (anti-GR1 MAb; BD Pharmingen), RM4-5 (anti-CD4 MAb; Pharmingen), and 53-6.7 (anti-mouse-CD8α MAb; BD Pharmingen). They were further incubated, as indicated in Results, with either (i) avidin-biotin-peroxidase complex (Vectastain ABC kit; Vector Laboratories, Burlingame, Calif.) and stained with a solution of diaminobenzidine tetrahydrochloride (DAB tablets, giving brown precipitates; Sigma, St. Louis, Mo.) or a solution of 3-amino-9-ethylcarbazole (AEC tablets, giving red precipitates; Sigma) or (ii) avidin-biotin-alkaline phosphatase complex (Vectastain ABC kit, AK-5000; Vector Laboratories) and stained with alkaline phosphatase substrates (SK-5300, giving blue precipitates; Vector Laboratories). In case of double staining of cells, the excess of biotin from the first antibodies was blocked with the Vector blocking kit (Vector) before incubation of the sections with a second biotinated antibody. Digitized images were captured by using a charge-coupled device color camera (Ikegami Tsushinki, Tokyo, Japan) and analyzed by using CorelDraw 7 software (Corel, Ottawa, Ontario, Canada).
RESULTS
Large number of CD11c+ cells are present in the footpad lesions of susceptible mice infected with L. major.
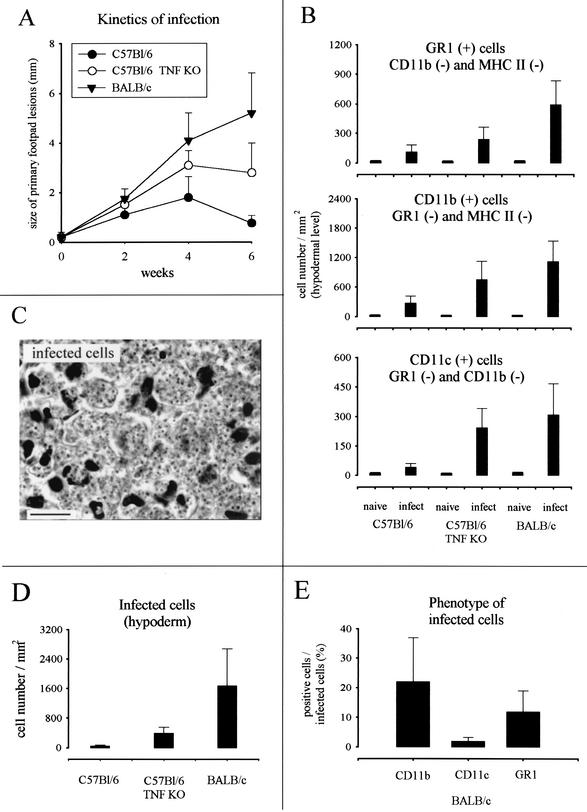
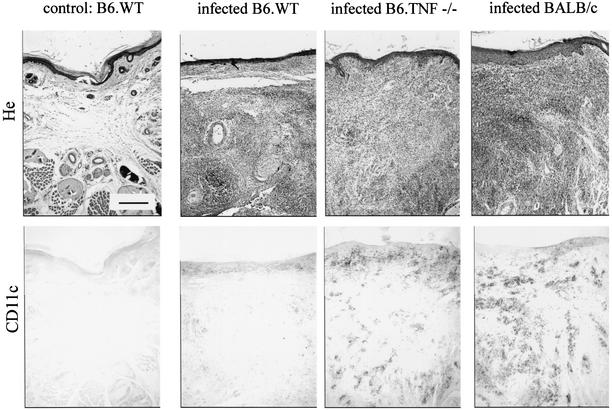
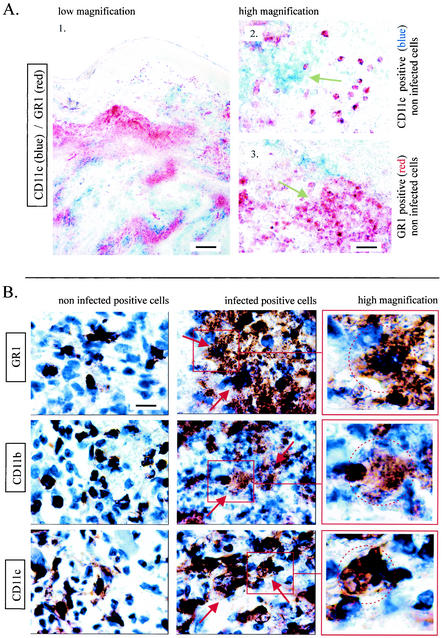
In agreement with previous studies (26, 38), the maximal lesion size in B6.WT and B6.TNF−/− infected mice was observed around the fourth week (Fig. 1A). At this time, the lesions in BALB/c infected mice were well developed without mutilation as observed later in the course of infection (data not shown). Mice infected with 5 × 106 L. major were therefore killed 4 weeks after inoculation for immunohistochemistry analysis. Three major populations were observed and enumerated in lesion by using antibodies recognizing GR1, CD11b, and CD11c (Fig. 1B and 2). Using these antibodies in combinations, we did not observe any double staining in the different control or infected mouse strains. This result demonstrates that each marker identified a distinct population. An example of such a combination is shown Fig. 3A (GR1/CD11c combination). Accordingly to previous studies (11, 17, 22, 33), CD11c+ CD11b− GR1− cells were identified as cells of the DC lineage (Langerhans cells and dermal DCs), CD11c− CD11b+ GR1− as macrophages, and CD11c− CD11b− GR1+ cells were identified as granulocytes. Infected B6.TNF−/− and BALB/c susceptible mice presented a dramatic infiltration and accumulation of these three cell populations in their lesions (Fig. 1B), which was mainly present at the hypodermal level of the skin (see Fig. 2 for CD11c distribution). In comparison, infected resistant B6.WT mice displayed only a moderate recruitment of these cells.
FIG. 1.
(A) Size of primary mouse footpad lesions during the course of L. major infection of B6.WT and B6.TNF−/− mice. The results are from one representative experiment performed with eight animals of each strain and are expressed as the means ± the standard error of the mean. Two other experiments yielded similar results. (B) Numbers of GR1+, CD11b+, and CD11c+ cells present after 4 weeks of infection in the footpad. Each value represents the mean ± the standard deviation (SD) cell counts/mm2 of tissue of 20 sections from four mice. (C) High magnification view of amastigotes stained by hematoxylin. Scale bar, 10 μm. (D) Numbers of infected cells present after 4 weeks of infection in the footpad. Each value represents the mean ± the SD of cell counts/mm2 of tissue of 10 sections from four mice. (E) Percentage of infected cells expressing CD11b, CD11c, or GR1 in the footpad. Each value represents the mean percentage ± the SD of cell counts/mm2 of tissue of 20 sections from four mice.
FIG. 2.
Immunoperoxidase staining (brown precipitates) of embedded serial sections of lesions from uninfected B6.WT mice and from B6.WT, B6.TNF−/−, and BALB/c mice infected for 4 weeks. Uninfected tissues from all strains used in the present study appeared closely similar. Serial sections were counterstained with hematoxylin or stained with MAb recognizing CD11c as indicated in the figure. Scale bar, 200 μm.
FIG. 3.
(A) Alkaline phosphatase (blue = CD11c) and immunoperoxidase (red = GR1) staining of embedded sections of lesions from BALB/c mice infected for 4 weeks. (B) Immunoperoxidase staining (brown precipitates) of embedded sections of lesions from BALB/c mice infected for 4 weeks. Sections were stained with MAbs recognizing GR1, CD11b, or CD11c as indicated in the figure and counterstained with hematoxylin. Scale bars: A1, 300 μm; A2, 30 μm; B, 10 μm.
CD11b+ cells and GR1+ cells are the most frequently infected cells in footpad lesions.
A high-magnification view of amastigotes stained by hematoxylin in infected cells from BALB/c lesion is shown in Fig. 1C. As expected, BALB/c mice in comparison to other mice displayed higher numbers of infected cells in lesions (Fig. 1D). Amastigotes were detected in CD11c+, CD11b+, and GR1+ cells by using hematoxylin staining. CD11b+ cells and GR1+ cells appeared to be the most frequently infected cells compared to CD11c+ cells in BALB/c mice (Fig. 1E). Figure 3B show the morphology of uninfected and infected CD11b+, CD11c+, and GR1+ cells. The intense aggregation of infected cells prevented a precise estimation of the number of amastigotes per cell. Nevertheless, our observations suggest that they were numerous and at roughly similar amounts in CD11b+, CD11c+, and GR1+ cells. Coexpression of CD4, CD8, or CD86 markers were not detected in infected cells (data not shown). Similar qualitative results were observed in infected cells from B6.WT and B6.TNF−/− mice (data not shown).
Distribution of infected cells in draining lymph node of infected mice.
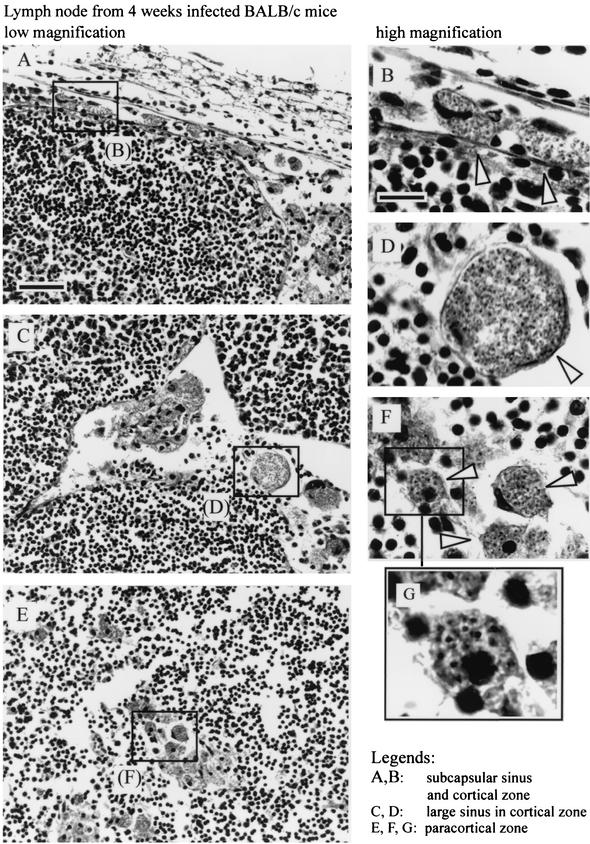
Immunohistochemical analysis showed important modifications of lymph node organization during the course of L. major infection. At 4 weeks after parasite inoculation, the paracortical region of the draining lymph nodes of infected mice, but not of control mice, presented multiple large lobules containing T cells (identified as CD90.2+) and B cells (identified as CD45R+ and MHC-II+) separated by large sinus (data not shown). In BALB/c mice, which presented the highest level of infected cells (Fig. 4A), cells containing amastigotes were localized in the subcapsular region (Fig. 5A and B), in the cortical and paracortical sinus (Fig. 5C and D), and in the paracortical region (Fig. 5E to G). In B6.WT and B6.TNF−/− mice, infected cells were observed mainly in the paracortical region of lymph node (data not shown).
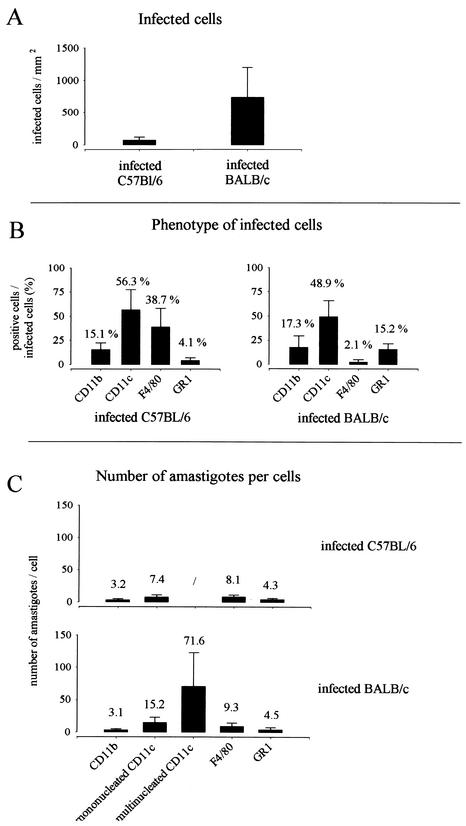
FIG. 4.
(A) Numbers of infected cells present in the lymph nodes after 4 weeks of infection. Each value represents the mean ± the SD of cell counts/mm2 of tissue of 20 sections from four mice. (B) Percentages of infected cells expressing CD11b, CD11c, F4/80, or GR1 in the lymph node. Each value represents the mean of percentage ± the SD of cell counts/mm2 of tissue of 10 sections from four mice. (C) Number of parasites in cells expressing CD11b, CD11c, F4/80, or GR1 present in the lymph nodes at 4 weeks of infection. Each value represents the mean ± the SD of cell counts/mm2 of tissue of 10 sections from four mice.
FIG. 5.
Sections of lymph nodes from BALB/c mice infected for 4 week were counterstained with hematoxylin and eosin. Arrows show the presence of amastigotes and infected cells. Scale bars: A, 50 μm; B, 10 μm.
Amastigotes are massively accumulated in CD11c+ cells in draining lymph nodes of BALB/c mice.
In comparison to uninfected DCs (CD11c+) displaying classical dendritic morphology (Fig. 6B1 and C1), the morphology of infected CD11c+ cells was strongly modified. In infected B6.WT mice, large aggregates of CD11c+ cells with dendritic morphology were observed in paracortical region (Fig. 6A2). Few parasites were detected in these cells (Fig. 6B2 and B3). A similar but enhanced phenomenon was observed in infected B6.TNF−/− mice (Fig. 6A3). In contrast, in lymph nodes from infected BALB/c, infected CD11c+ cells presenting a massive accumulation of parasites were observed in subcapsular region (Fig. 6A4), cortical and paracortical sinus (Fig. 6A5), and paracortical region (Fig. 6A6). It is interesting that CD11c+ infected cells present in subcapsular, cortical and paracortical sinus expressed frequently an intermediate level of CD11c (Fig. 6C3) in comparison to CD11c+ infected cells of paracortical region (Fig. 6C4).
FIG.6.
Immunoperoxidase staining (brown precipitates) of embedded sections of the draining lymph nodes from uninfected C57BL/6 mice (A1) and from B6.WT (A2 and B), B6.TNF−/− (A3), and BALB/c (A4, A5, A6, and C) mice infected for 4 weeks. Sections were stained with MAbsrecognizing CD11c and counterstained with hematoxylin. (B1) C1 CD11c+ uninfected cells; (B2 and C4) mononucleated CD11c+ infected cells; (B3) aggregate of CD11c+ infected cells; (C2) CD11c− infected cells; (C3) mononucleated infected cell expressing intermediate level of CD11c; (C5) multinucleated CD11c+ infected cells. Scale bars: A, 50 μm; B, 10 μm.
The analysis of the phenotype expressed by the infected cells (Fig. 4B) showed that CD11c+ cells were the most frequently infected in lymph nodes of C57BL/6 and BALB/c mice compared to GR1+ and CD11b+ cells. Comparison of the number of intracellular amastigotes showed that the highest parasite levels were observed in mononucleated CD11c+ cells from BALB/c lymph nodes (Fig. 4C) and overall in giant multinucleated CD11c+ cells (Fig. 4C and 6C5). These latter cells were not present in infected B6.WT mice. In summary, CD11c+ cells appear as the most frequently infected cells in lymph nodes from infected mice. CD11c+ from BALB/c mice, but not from other mice, displayed a more intense multiplication of amastigotes.
Infected CD11c+ cells from lymph nodes of C57BL/6, but not BALB/c mice, express the CD11b and F4/80 myeloid markers.
The existence of several subpopulations of DCs in blood (10) and lymphoid organs (4, 17, 22) based on the expression of CD4, CD8, CD11b, CD45R (B220), CD90.2, and F4/80 markers has been well established. In order to determine the phenotype of infected CD11c+ cells in both strains of mice, we performed systematic double staining with the anti-CD11c antibody and another antibody recognizing one of the above listed markers. Our results (Fig. 7A) show that some aggregated infected CD11c+ cells from C57BL/6 mice, but not highly infected cells from BALB/c mice, expressed CD11b or F4/80 myeloid markers. These cells did not express at a detectable level lymphoid markers such as CD4, CD8, CD45R, and CD90.2 (data not shown). CD11c+ infected cells from draining lymph nodes of B6.WT and B6.TNF−/− mice displayed similar phenotype (data not shown). The frequent coexpression of CD11c and F4/80 in infected B6.WT might explain the higher number of F4/80+ infected cells observed in B6.WT compared to BALB/c mice (Fig. 4B).
FIG.7.
(A and B) Immunoalkaline phosphatase (blue = F4/80 and CD11b as indicated) and immunoperoxidase (red = CD11c) staining of embedded sections of lymph nodes from BALB/c and B6.WT mice infected for 4 weeks, as indicated in the figure. Arrows show the presence of double staining. (C) Immunoperoxidase staining (brown precipitates) of embedded serial sections of the draining lymph nodes from BALB/c mice infected for 4 weeks. Sections were stained with MAbs recognizing MHC-II and CD11c, as indicated in the figure, and counterstained with hematoxylin. Scale bars: A, 150 μm; B; 10 μm; C, 50 μm.
Infected CD11c+ cells from the lymph nodes of both mouse strains express low levels of MHC-II, but no CD86 molecules were detected.
Lymph node DCs are frequently described as expressing high surface levels of MHC-II (33). In both strains of mice, we observed that noninfected CD11c+ cells expressed MHC-II at high levels (data not show). In contrast, the large majority of highly infected CD11c+ cells from BALB/c and aggregated infected cells from B6.WT mice express only low levels of surface MHC-II (inferior to that observed on B cells) (Fig. 7C). CD86 expression was not detected on infected CD11c+ cells from lymph nodes of B6.WT or BALB/c mice, whereas it was frequently detected on CD45R+ cells (B cells) of lymph nodes from infected BALB/c mice (data not shown)
DISCUSSION
We analyzed here the in vivo phenotype of L. major-infected cells in the primary site of infection and the draining lymph node of B6.WT, B6.TNF−/− and BALB/c mice 4 weeks after parasite inoculation. Previous works have demonstrated that Leishmania parasites can infect macrophages (31), granulocytes (24), and DCs (5, 34, 37). However, as far as we know the importance of this phenomena has not been estimated quantitatively in vivo during the peak of infection in both resistant and susceptible mice. Moreover, the expression of functionally important markers, such as MHC-II and costimulatory molecules, and differentiation markers at the surface of infected cells has not been analyzed previously.
Our results show that granulocytes and macrophages, identified as CD11b− CD11c− GR1+ and CD11b+ CD11c− GR1−, respectively, are the main infected cells in primary lesions. In contrast, cells expressing a high level of CD11c, a DC specific marker, are the most frequently infected in draining lymph nodes of infected mice. Qualitatively similar results have been obtained at 6 and 9 weeks of infection (data not shown). The preferential infection of DCs by intracellular pathogens has been reported previously in other models. Dengue virus, a flavivirus causing febrile illness, inoculated into human skin, like Leishmania parasites, by mosquitoes, has been reported to infect 10-fold more DCs than monocytes or macrophages (39). DCs have also been described as the major reservoir of human immunodeficiency virus in human lymph nodes (14).
CD11c+ infected cells detected in lymph nodes might (i) have migrated from lesion to draining lymph node or (ii) be infected in the lymph node with parasites released by other cells. The presence of infected cells expressing an intermediate or high level of CD11c marker in the subcapsular sinus of lymph nodes indicates that at least a portion of infected cells that have migrated from the lesion to the lymph node are CD11c+ cells. Recently, it has been demonstrated that all DC populations can be derived from only one population of DC precursors (10). However, during inflammation, DCs can also differentiate from monocytes (30). For this reason, distinguishing between monocytes/macrophages and DCs is difficult, particularly during the course of an infection. Randolph et al. (30) have previously reported that macrophages (identified as CD11b+ CD11c−) recruited in the footpad after an inflammatory stimulus can differentiate into “monocyte-derived DCs” (acquiring CD11c marker) and migrate into the lymph nodes. In our study, we observed that a few CD11c+ cells located in lesions were infected by L. major. Thus, CD11c+ infected cells observed in the subcapsular sinus of the draining lymph node might derive from both monocyte-derived DCs and DCs infected in the footpad.
Compared to BALB/c mice, the lymph nodes of B6.WT mice contained low number of infected cells displaying a moderate parasitic load and DC morphology. In contrast, in lymph nodes of BALB/c mice, infected CD11c+ cells presented high parasitic loads, and we frequently observed CD11c+ multinucleated giant cells harboring a dramatic accumulation of parasites. Indeed, multinucleated giant cells have been previously described in lymph node biopsies from Leishmania-infected patients, but their cell surface phenotype has not been reported (36). B6.TNF−/− mice, due to a reduced and delayed Th1 response, are susceptible to L. major infection (38). In agreement with an earlier study (38), we observed in these mice an increased frequency of infected cells. However, lymph node infected cells from these mice presented weak parasitic loads and no syncytium was observed. Thus, we can conclude that syncytium formation appears as a consequence of high parasitic loads associated with Th2 response in BALB/c mice. It is interesting that, in contrast to macrophages, DCs do not produce NO in response to L. major infection (6), suggesting that these cells are not able to eliminate amastigotes or prevent their multiplication. Thus, these data and our study suggest that DCs can potentially constitute a reservoir of parasites in the case of a nonadapted Th2 immune response.
Another interesting observation was that lymph node CD11c+ infected cells from B6.WT and B6.TNF−/−, but not from BALB/c mice, frequently expressed the CD11b and F4/80 myeloid differentiation markers. The reason for this difference is not known. The similar cell phenotype observed in resistant B6.WT and susceptible B6.TNF−/− mice suggests that the genetic background or the profile of immune response (Th1) might be involved. Whereas the phenotype of DC subsets in lymph node mice has been described in naive mice (17), this has not been reported during the course of infection, making it difficult to associate the phenotype of some CD11c+ infected (CD4−CD8−, CD11b+, or F4/80+) cells from the lymph nodes of B6.WT mice to a classical subset of DCs. However, the absence of detectable CD4 and CD8 expression suggests that these cells might be skin migrating cells (17). The expression of the myeloid markers CD11b and/or F4/80 also suggests that these cells are similar to monocyte-derived DCs, as described by Randolph et al. (30). Interestingly, a recent work (18) has demonstrated that splenic myeloid DCs (CD4+, CD8−, CD11b+, and F4/80+ [22]) rather than other splenic DC subsets are preferentially infected in vitro by L. major.
We detected low expression of MHC-II but no expression of CD86 costimulatory molecule on CD11c+ infected cells from lymph nodes of B6.WT or BALB/c mice, suggesting that the antigen-presenting function of these cells is downregulated. This is not due to technical problems since these markers were clearly detected at the surface of noninfected cells on the same tissue sections. Conflicting results have been reported on the ability of Leishmania species to activate cells of the DC lineage. It has been shown in vitro that bone marrow-derived DCs do not mature in response to L. mexicana infection (3). Similarly, a functional impairment of in vitro T-cell stimulatory properties of splenic DCs at 60 days postinfection, correlating with a reduced surface expression of MHC-II, has been reported during the course of L. donovani infection in BALB/c mice (2). In contrast, Qi et al. (29) have observed in vitro that L. amazonensis induced the upregulation of costimulatory molecules on bone marrow-derived DCs, and von Stebut and al. (37) showed that in vitro infection of fetal-skin-derived DCs from both BALB/c and B6.WT mice infected with L. major induced the upregulation of costimulatory molecules and the release of IL-12. These contrasting results obtained in vitro might be due to the Leishmania species used and the origin of the DCs (bone marrow, spleen, and fetal skin). We have observed in situ that lymph node CD11c+ infected cells from susceptible mice presented high numbers of parasites, which were clearly greater than the parasitic loads observed in cells infected in vitro (18). This suggests that in vivo infection might more deeply affect the physiology of cells. Moreover, the duration of infection and/or environmental factors present in vivo might also influence the phenotype and the T-cell stimulatory properties of DCs. Thus, it is possible that a short time of infection in vitro with L. major activates DC maturation, as reported by von Stebut et al. (37) but that, conversely, long-term infection induces a downregulation of the typical markers of antigen presenting function on DCs, as observed with L. donovani infection (2). Moll et al. (25) reported that lymph node DCs from B6.WT that have recovered from infection with L. major harbor viable parasites. These authors observed that lymph node cells from these mice can efficiently activate Leishmania-specific T cells but that the depletion of DCs impairs this activation. These authors concluded that infected DCs efficiently present Leishmania antigen to specific T cells. These results do not exclude the possibility that infected DCs release antigen that can be presented in vitro to T cells by noninfected competent DCs. Due probably to their large size, isolation of CD11c+ infected cells by a standard procedure has not been possible in our laboratory, and the subsequent analysis of their antigen-presenting cell functions has not been performed. Thus, we cannot actually determine whether infected cells are functionally able to present antigen. However, the downregulation of antigen-presenting markers at their cell surface suggests strongly that presentation is performed by noninfected DCs. Leishmania parasites reside and multiply into parasitophorous vacuoles. These organelles of phagolysosomal origin have similar properties to vacuoles where peptide-MHC-II molecule complexes are formed before their expression at the cell surface. In vitro studies have shown that MHC-II molecules are associated with the parasitophorous vacuoles of macrophages infected by L. major, L. amazonensis, and L. mexicana (1, 13). Some Leishmania species, such as L. amazonensis and L. mexicana, internalize and degrade MHC-II molecules (1, 13), suggesting that Leishmania parasites might affect antigen presentation.
In summary, our analysis of the cell surface phenotype of L. major-infected cells show that cells presenting the CD11c DC marker are the most frequently infected cells in the draining lymph nodes of resistant and susceptible mice. In susceptible BALB/c mice, these cells constitute an important reservoir of parasites and present a reduced expression of antigen presentation markers.
Acknowledgments
We thank Dominique Le Ray (Institute of Tropical Medicine, Antwerp, Belgium) for providing the MHOM/IR/−/173 strain of L. major. We thank Jean-Claude Antoine for critical review of the manuscript and Alain Wathelet and Ella Omasta for diligent technical assistance.
This work was supported by grants from the Belgian Ministry of Scientific Policy (Action de Recherche Concertée), the Fonds National de la Recherche Scientifique (FNRS; Crédit aux Chercheurs [Belgium]), and the Université Libre de Bruxelles. E.M. is supported by the FNRS, and C.D.T. is supported by the Fonds pour la Recherche dans l'Industrie et l'Agriculture (Belgium).
Editor: J. M. Mansfield
REFERENCES
- 1.Antoine, J.-C., T. Lang, E. Prina, N. Courret, and R. Hellio. 1999. H-2M molecules, like MHC II molecules, are targeted to parasitophorous vacuoles of Leishmania-infected macrophages and internalized by amastigotes of L. amazonensis and L. mexicana. J. Cell Sci. 112:2559-2570. [DOI] [PubMed] [Google Scholar]
- 2.Basu, A., G. Chakrabarti, A. Saha, and S. Bandyopadhyay. 2000. Molulation of CD11c+ splenic dendritic cell functions in murine visceral leishmaniasis: correlation with parasite replication in the spleen. Immunology 99:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, C. L., A. Misslitz, L. Colledge, T. Aebischer, and C. C. Blackburn. 2001. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 31:876-883. [DOI] [PubMed] [Google Scholar]
- 4.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98:3520-3526. [DOI] [PubMed] [Google Scholar]
- 5.Blank, C., H. Fuchs, K. Rappersberger, M. Rollinghoff, and H. Moll. 1993. Parasitis of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J. Infect. Dis. 167:418-425. [DOI] [PubMed] [Google Scholar]
- 6.Blank, C., C. Bogdan, C. Bauer, K. Erb, and H. Moll. 1996. Murine epidermal Langerhans cells do not express inducible nitric oxide synthase. Eur. J. Immunol. 26:792-796. [DOI] [PubMed] [Google Scholar]
- 7.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 8.Bradley, D. J. 1987. Genetics of susceptibility and resistance in the vertebrate host, p. 551-581. In W. Peters and R. Killick-Kendrick (ed.), The leishmaniases in biology and medicine. Academic Press, Ltd., London, United Kingdom.
- 9.Carrera, I., R. T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D. L. Sacks. 1996. Leishmania promastigotes selectively inhibit interleukin-12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Hoyo, G. M., P. Martin, H. H. Vargas, S. Ruiz, C. F. Arias, and C. Ardavin. 2002. Characterization of a common precursor population for dendritic cells. Nature 415:1043-1047. [DOI] [PubMed] [Google Scholar]
- 11.Duraiswamy, N., Y. Tse, C. Hammerberg, S. Kang, and K. D. Cooper. 1994. Distinction of class II MHC+ Langerhans cell-like interstitial dendritic antigen-presenting cells in murine dermis from dermal macrophages. J. Investig. Dermatol. 103:678-683. [DOI] [PubMed] [Google Scholar]
- 12.Flohé, S. B., C. Bauer, S. Flohe, and H. Moll. 1998. Antigen-pulsed epidermal Langerhans cells protect susceptible mice from infection with the intracellular parasite Leishmania major. Eur. J. Immunol. 28:3800-3811. [DOI] [PubMed] [Google Scholar]
- 13.Flohé, S., T. Lang, and H. Moll. 1997. Synthesis, stability, and subcellular distribution of major histocompatibility complex class II molecules in Langerhans cells infected with Leishmania major. Infect. Immun. 65:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannan, L. D. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncitia at the mucosal surface of the adenoid. Science 272:115-117. [DOI] [PubMed] [Google Scholar]
- 15.Fruth, U., N. Solioz, and J. A. Louis. 1993. Leishmania major interferes with antigen presentation by infected macrophages. J. Immunol. 150:1857-1864. [PubMed] [Google Scholar]
- 16.Gorak, P. M. A., C. R. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28:687-695. [DOI] [PubMed] [Google Scholar]
- 17.Henri, S., D. Vremec, A. Kamath, J. Waithman, S. Williams, C. Benoist, K. Burnham, S. Saeland, Handman, E., and K. Shortman. 2001. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167:741-748. [DOI] [PubMed] [Google Scholar]
- 18.Henri, S., J. Curtis, H. Hochrein, D. Vremec, K. Shortman, and E. Handman. 2002. Hierarchy of susceptibility of dendritic cell subsets to infection by Leishmania major: inverse relationship to interleukin-12 production. Infect. Immun. 70:3874-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himmelrich, H., P. Launois, I. Maillard, T. Biedermann, F. Tacchini-Cottier, R. M. Locksley, M. Rocken, and J. A. Louis. 2000. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 164:4819-4825. [DOI] [PubMed] [Google Scholar]
- 20.Huang, F. P., D. Xu, E. O. Esfandiari, W. Sands, X. Q. Wei, and F. Y. Liew. 1998. Mice defective in Fas are highly susceptible to Leishmania major infection despite elevated IL-12 synthesis, strong Th1 responses, and enhanced nitric oxide production. J. Immunol. 160:4143-4147. [PubMed] [Google Scholar]
- 21.Jenkins, M. K., A. Khoruts, E. Ingulli, D. L. Mueller, S. J. McSorley, R. L. Reinhardt, A. Itano, and K. A. Pape. 2001. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19:23-45. [DOI] [PubMed] [Google Scholar]
- 22.Kamath, A. T., J. Pooley, M. A. O'Keeffe, D. Vremec, Y. Zhan, A. M. Lew, A. D'Amico, L. Wu, D. F. Tough, and K. Shortman. 2000. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J. Immunol. 165:6762-6770. [DOI] [PubMed] [Google Scholar]
- 23.Kremer, I. B., M. P. Gould, K. D. Cooper, and F. P. Heinzel. 2001. Pretreatment with recombinant Flt3 ligand partially protects against progressive cutaneous leishmaniasis in susceptible BALB/c mice. Infect. Immun. 69:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laufs, H., K. Muller, J. Fleischer, N. Reiling, N. Jahnke, J. C. Jensenius, W. Solbach, and T. Laskay. 2002. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect. Immun. 70:826-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moll, H., H. Fuchs, C. Blank, and M. Röllinghoff. 1993. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur. J. Immunol. 23:1595-1601. [DOI] [PubMed] [Google Scholar]
- 26.Noben-Trauth, N., W. E. Paul, and D. L. Sacks. 1999. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 162:6132-6140. [PubMed] [Google Scholar]
- 27.Pajak, B., T. De Smedt, V. Moulin, C. De Trez, R. Maldonado-Lopez, G. Vansanten, E. Briend, J. Urbain, O. Leo, and M. Moser. 2000. Immunohistowax processing, a new fixation and embedding method for light microscopy, which preserves antigen immunoreactivity and morphological structures: visualization of dendritic cells in peripheral organs. J. Clin. Pathol. 53:518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, A. Y., B. D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896-902. [DOI] [PubMed] [Google Scholar]
- 29.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 30.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 31.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 32.Scott, P., E. Pearce, A. W. Cheever, R. L. Coffman, and A. Sher. 1989. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol. Rev. 112:161-182. [DOI] [PubMed] [Google Scholar]
- 33.Steinman, R. M., M. Pack, and K. Inaba. 1997. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156:25-37. [DOI] [PubMed] [Google Scholar]
- 34.Stenger, S., N. Donhauser, H. Thuring, M. Rollinghoff, and C. Bogdan. 1996. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 183:1501-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stobie, L., S. Gurunathan, C. Prussin, D. L. Sacks, N. Glaichenhaus, C. Y. Wu, and R. A. Seder. 2000. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA 97:8427-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallada, N., A. Raventos, S. Martinez, C. Compano, and B. Almirante. 1993. Leishmania lymphadenitis diagnosed by fine-needle aspiration biopsy. Diagn. Cytopathol. 9:673-676. [DOI] [PubMed] [Google Scholar]
- 37.von Stebut, E., Y. Belkaid, T. Jakob, D. L. Sacks, and M. C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm, P., U. Ritter, S. Labbow, N. Donhauser, M. Rollinghoff, C. Bogdan, and H. Kornern. 2001. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J. Immunol. 166:4012-4019. [DOI] [PubMed] [Google Scholar]
- 39.Wu, S.-J. L., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovitch, H. K. Wong, A. Blauvelt, G. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]