Abstract
Staphylococcus aureus has been shown to invade and induce the death of various cell types. Here we investigate whether the cytotoxicity of intracellular S. aureus is a general feature or rather characteristic of individual S. aureus strains. The majority of 23 randomly collected clinical S. aureus isolates were killed inside keratinocytes and fibroblasts, indicating that the uptake of S. aureus represents an important mechanism of cell-autonomous host defense. However, seven independent S. aureus isolates survived intracellularly and induced significant cytotoxicity for their host cells. Subcloning analysis revealed that the ability or inability to kill host cells is a stable, apparently genetically determined trait of a given S. aureus isolate. We show that noncytotoxic strains but not cytotoxic strains colocalize with the lysosomal marker LAMP-1, suggesting that only cytotoxic strains escape degradation by the endolysosomal pathway. In a mouse septicemic model, cytotoxic S. aureus isolates produce significantly greater lethality (96%) compared to noncytotoxic strains (41%), which corresponds to 23-, 63-, and 30,000-fold increases of bacterial loads in the liver, spleen, and kidney, respectively. Finally, cytotoxic S. aureus strains produce clinically apparent arthritis in mice at a greater frequency than compared to noncytotoxic S. aureus strains. The results of our study unravel a previously unrecognized dichotomy of cytotoxic and noncytotoxic S. aureus isolates, which may play an important role in the dissemination of, and mortality induced by, S. aureus infection.
Staphylococcus aureus is one of the most important human pathogens, which causes both community-acquired and nosocomial infections (12). The diseases caused by this bacterium range from wound and soft tissue infections to endocarditis and septic shock. To defeat the innate and the adaptive immune system of the host, S. aureus employs both single-gene-encoded virulence factors such as alpha-toxin, coagulase, and protein A, as well as complex mechanisms such as adhesion or slime production. Although S. aureus is generally classified as an extracellular pathogen, recent data revealed its ability to infect various types of host cells: both professional phagocytes and nonphagocytes, including endothelial cells, fibroblasts, and others (1, 15, 25). This invasion is initiated by the adherence of S. aureus to the cellular surface, a process in which staphylococcal fibronectin-binding proteins play a prominent role (11, 14, 26, 30). Phagocytosed S. aureus can either induce apoptosis of the host cell (4) or survive for several days intracellularly in the cytoplasm, which is thought to be devoid of antistaphylococcal effector mechanisms (2, 28). Variants of S. aureus, termed small-colony variants (SCV) due to their colony morphology, have been reported to be especially able to persist intracellularly (35, 36). Although S. aureus has been shown to escape from the phagosome (4), neither the kinetics of escape nor the underlying mechanisms employed by S. aureus have yet been determined. Moreover, it is still unclear whether invasion and cytotoxicity are a common feature of clinical S. aureus isolates and whether these factors contribute to pathogenicity.
We show here that each of 23 randomly selected clinical S. aureus isolates infects nonphagocytic host cells. However, after uptake into the cell, the fate of both the bacteria and the host cells are shown to depend on the individual S. aureus strain. Specifically, a significant fraction of the clinical isolates killed more than 50% of the host cells within 24 h. We finally demonstrate that this in vitro cytotoxicity strongly correlates with the pathogenicity in mice, suggesting that the trait of cytotoxicity has to be considered as an important individual virulence factor of a given S. aureus strain.
MATERIALS AND METHODS
Antibodies.
Lysosomes were stained with anti-LAMP-1 monoclonal antibody (BD Pharmingen, San Diego, Calif.), followed by Cy3 (Amersham Biosciences)-coupled anti-rat immunoglobulin G secondary antibody (Dianova, Hamburg, Germany). Nonlabeled intracellular S. aureus were stained with anti-protein A antibody (Sigma, Taufkirchen, Germany) followed by Cy3-coupled anti-mouse immunoglobulin G secondary antibody (Dianova).
Bacteria.
A total of 23 randomly selected S. aureus clinical isolates, as well as the ATCC 29213 reference strain, were used throughout the present study. All S. aureus isolates were typed by RAPD [random(ly) amplified polymorphic DNA] PCR to ascertain unrelatedness of origin. S. aureus grown overnight at 37°C in Luria-Bertani broth were diluted with fresh broth and cultured until mid-logarithmic phase of growth (optical density at 600 nm = 0.3). Bacteria were harvested, washed with PBSE (phosphate-buffered saline [PBS], 5 mM EDTA), and adjusted to 109 CFU/ml. The pair of S. aureus DU1090 strains stably expressing wild-type or mutant H35R amino acid substitution alpha-toxin was generously provided by S. Bhakdi (18).
RAPD PCR analysis.
Genomic DNA was prepared by using DNeasy tissue kit (Qiagen, Hilden, Germany). PCR was performed by using Ready-To-Go RAPD PCR kit with Primer1 (GGTGCGGGGAA) according to the manufacturer's instructions (Amersham Biosciences, Freiburg, Germany).
FITC staining of S. aureus.
Bacterial pellets of 1010 CFU were resuspended in carbonate buffer (pH 9.0) containing 100 μg of fluorescein isothiocyanate (FITC) isomer I (Sigma, Taufkirchen, Germany)/ml for 1 h at room temperature. Staphylococci were extensively washed with PBSE, adjusted with 10% glycerol to 109 CFU/ml, and kept at −70°C until use. No significant loss of viability or fluorescence intensity during the freeze-thaw procedure was observed.
Eukaryotic cell culture.
Mouse keratinocyte PAM212 and fibroblast mKSA cell lines were cultured, respectively, in RPMI 1640 and Dulbecco modified Eagle medium (both from Biochrom, Berlin, Germany). Both media were supplemented with 10% heat-inactivated fetal calf serum (Biochrom), 100 U of penicillin G/ml, and 100 μg of streptomycin sulfate (Biochrom)/ml. At 18 h prior to infection, 5 × 105 cells were seeded in six-well plates (total volume = 3 ml) (Nunc, Wiesbaden, Germany). Viability was monitored by using trypan blue (Biochrom) exclusion (7).
Invasion assay.
Prior to infection, cells were washed with growth medium without antibiotics and kept for 1 h at 37°C. A total of 106 to 108 FITC-stained staphylococci were added per well. After a 1 h of incubation, cells were detached from the culture plate and washed with growth medium. Extracellular staphylococci were killed by incubation of cells with 100 μg of lysostaphin (Sigma)/ml for 7 min at 37°C. Cells were washed with growth medium supplemented with antibiotics and reseeded in a new six-well plate (total volume = 3 ml). After 24 h of incubation, cells were harvested and analyzed by flow cytometry and microscopy. Host cell viability was measured by using trypan blue exclusion (7). For fluorescence microscopy, cells were grown on coverslips. After the invasion procedure, cells were rinsed with PBS and fixed for 15 min with 4% paraformaldehyde. Cells were permeabilized with 0.1% saponin prior to staining with antibodies specific for organelles or intracellular bacteria. Specimens were mounted on microscopy slides in 10% glycerol supplemented with 100 mg of DABCO (diazabicyclo[2.2.2]octane; Sigma)/ml. Images were acquired by using an Axioscop 2 microscope (Zeiss, Oberkochen, Germany) equipped with an Axiocam charge-coupled device camera and then analyzed by using Axiovision software (Zeiss).
Flow cytometry.
The supernatants of the infected cultures were removed to preserve detached cells. Subsequently, the adherent cells were harvested by standard trypsin treatment and combined with cells from the supernatants. Cell samples were washed once with PBS and submitted to analysis by flow cytometry. Noninfected cells served as negative control to set the cutoff marker for the discrimination of FITC-negative and positive cells.
CFU determination.
Infected cells were lysed in PBSE containing 0.05% Triton X-100. Lysates were sonicated for 5 min at 4°C. This procedure was found to be most effective to resolve the bacterial clumps. Then, lysates were diluted with PBS and plated on Mueller-Hinton agar by using a spiral plater (EDDY-Jet; IUL Instruments, Königswinter, Germany). Colony counting and CFU determination were performed according to the instructions of the manufacturer.
Mouse infection model.
C57BL/6N Crl BR mice were purchased from Charles River Laboratories (Sulzfeld, Germany) and kept under barrier conditions. Mice of 6 to 10 weeks age were infected by intravenous (i.v.) injection of graded doses of S. aureus in 0.3 ml of PBS. Mice were monitored daily for clinical signs of S. aureus infection (ruffled fur, reduced activity, hunched posture) and mortality. Arthritis induced by S. aureus infection was defined as the impairment of the mobility in major joints leading to alterations of the gait (8). In order to determine the bacterial load in various organs, mice were sacrificed 24 h after infection. Livers, spleens, and kidneys were removed under aseptic conditions and then homogenized in 0.05% Triton X-100, sonicated for 5 min at 4°C, and plated on blood agar by using a spiral plater.
Statistical analysis.
Experimental data were assessed with a two-tailed unpaired Student t test for comparison between means. Arthritis frequencies and survival data were compared by using the Mann-Whitney U test. P values of <0.01 were considered to be statistically significant.
RESULTS
Invasiveness and cytotoxicity of S. aureus.
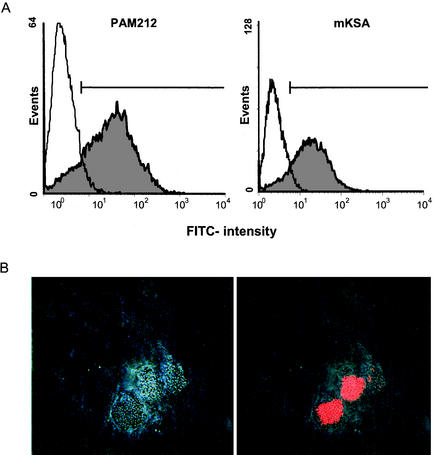
Up to the present, the ability of S. aureus to invade and induce the death of eukaryotic cells has been investigated by employing select host cell types and specific laboratory strains S. aureus such as NCTC 8325-4. To investigate whether invasion and cytotoxicity are general phenomena of S. aureus, 23 randomly selected clinical isolates were tested for their ability to infect either keratinocytes (PAM212) or fibroblasts (mKSA). RAPD PCR analysis revealed unrelatedness of all S. aureus isolates investigated (data not shown). To monitor the invasiveness of S. aureus, all 23 clinical isolates were FITC labeled and incubated with either PAM212 or mKSA cells at a multiplicity of infection (MOI) of 200. After 1 h, extracellular bacteria were destroyed by the addition of lysostaphin. The outgrowth of potentially surviving extracellular S. aureus was inhibited by penicillin. As shown in Fig. 1A, intracellular S. aureus were readily detected in PAM212 and mKSA cells by flow cytometry. To rule out that invasion was facilitated by FITC, nonlabeled S. aureus were incubated with PAM212 cells and stained with anti-protein A antibody. As shown in Fig. 1B, S. aureus was detected inside host cells by indirect immunofluorescence, indicating that the invasion of S. aureus occurs independently of FITC.
FIG. 1.
(A) S. aureus infection of mouse keratinocytes (PAM212) and fibroblasts (mKSA) cells. Cells were infected with clinical S. aureus isolate K42. Invasion of the cells by FITC-labeled staphylococci was measured 1 h after infection by flow cytometry. The marker indicates the cutoff used for the determination of the percentage of infected cells. (B) PAM212 cells were infected with nonlabeled S. aureus (ATCC 29213), fixed, and stained with Cy3-labeled anti-protein A antibody. The bright light differential interference contrast image (left) was superimposed with Cy3 fluorescence (right).
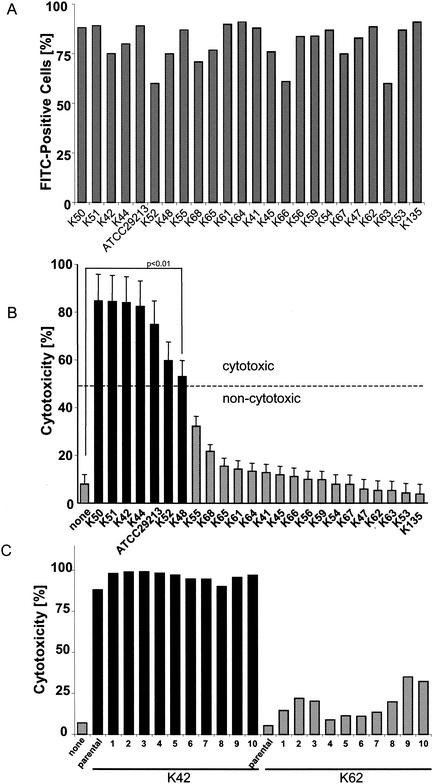
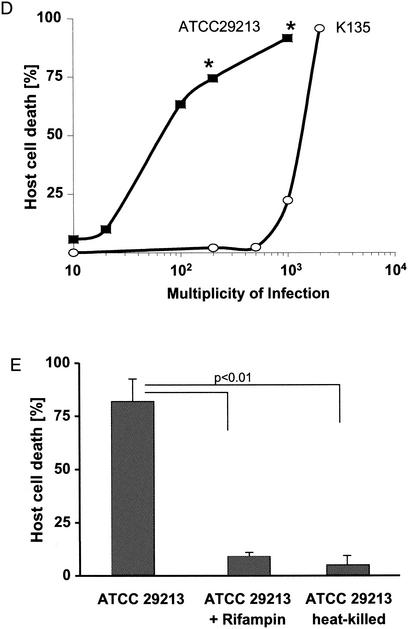
Each of the tested S. aureus clinical isolates proved able to invade both cell types with similar efficiency (Fig. 2A). However, only a fraction of these clinical isolates (7 of 23 [30%]) produced significant (>50% of host cells killed; P < 0.01) cytotoxicity 24 h after infection (Fig. 2B). It should be noted that invasion occurs rapidly upon incubation of the host cells with bacteria. In contrast, the cytotoxicity for host cells manifests more slowly to become visible only >4 h after infection (data not shown). The well-characterized reference strain ATCC 29213 belongs to the cytotoxic fraction of S. aureus. It is important to note that the cytotoxic potential of a given S. aureus strain appears to be a genetically determined trait. Subclones of any of these strains exerted the same degree of host cell cytotoxicity compared to the parental strain (Fig. 2C). The dichotomy of cytotoxic versus noncytotoxic strains of S. aureus also becomes apparent in dose-response studies. Although host cell killing by cytotoxic strains started at an MOI of 20, no signs of cytotoxicity were detected with noncytotoxic strains at MOIs of <500 (Fig. 2D). To prove that cell death was not induced during the 1-h incubation-invasion phase, we infected mKSA cells with heat-killed cytotoxic S. aureus. As shown in Fig. 2E, heat-killed bacteria were unable to induce cytotoxicity. Additionally, infected cells were treated with the plasma membrane-permeable antibiotic rifampin. As shown in Fig. 2E, rifampin added after the infection phase rescued the host cells from S. aureus-induced cell death. Thus, induction of host cell death requires death signals from metabolically active, intracellular S. aureus.
FIG. 2.
Cytotoxicity of individual S. aureus clinical isolates. mKSA cells were infected as described in Materials and Methods. (A) Percentage of FITC-positive cells was determined by flow cytometry 1 h after infection. (B) At 24 h after infection, host cell viability was assessed by trypan blue exclusion. Statistically significant differences are indicated (P < 0.01). (C) Strains K42 and K62 of cytotoxic or noncytotoxic phenotypes, respectively, were subcloned and analyzed for host cell cytotoxicity. (D) mKSA cells were infected with ATCC 29213 (cytotoxic strain) or K135 isolate (noncytotoxic strain) at different MOIs. Host cell death was measured 24 h after infection by trypan blue exclusion. Significant differences are denoted with asterisks (✽, P < 0.01). (E) Influence of bacterial viability or rifampin on S. aureus induced cytotoxicity. mKSA cells were infected with ATCC 29213 isolate or heat-killed bacteria. 10 μg of rifampin/ml was added to the culture medium 1 h after infection. The percentage of cell death was measured 24 h after infection. Statistically significant differences (P < 0.01) are indicated.
Intracellular fate of S. aureus.
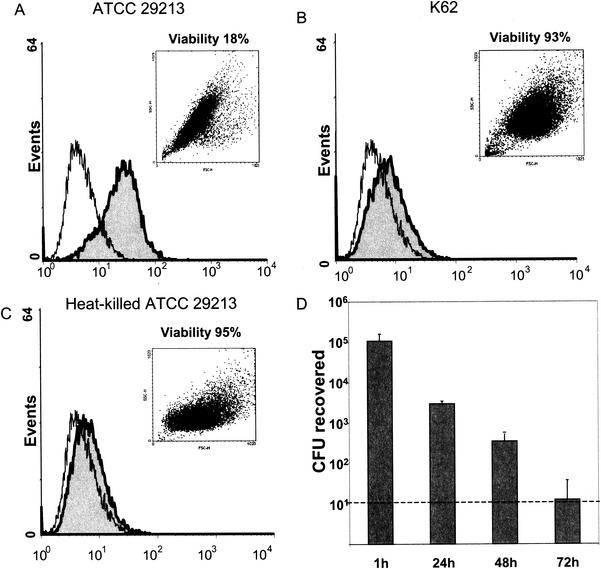
In infected host cells, only FITC-labeled cytotoxic S. aureus strains could be detected by flow cytometry after 24 h (Fig. 3A). In contrast, the fluorescence of cells infected with noncytotoxic S. aureus strains or with heat-killed cytotoxic S. aureus isolates rapidly declined to become undetectable within 24 h (Fig. 3B and C). Indeed, the recovery of viable bacteria rapidly declined in the case of noncytotoxic S. aureus without killing of the host cells (Fig. 3D). By 72 h postinfection, only minute numbers of CFU of noncytotoxic S. aureus could be detected, confirming the results obtained by flow cytometry. These findings indicate a different rate of elimination and suggested a different intracellular fate of cytotoxic versus noncytotoxic S. aureus strains.
FIG. 3.
Detection of intracellular S. aureus 24 h after infection. mKSA cells were infected with FITC-labeled S. aureus and FITC fluorescence distribution of infected cells (in gray) was analyzed by flow cytometry. Host cell viability was determined by trypan blue exclusion. Forward- to side-scatter dot plots indicate changes in cell size and granularity associated with infection. (A) ATCC 29213 (cytotoxic); (B) K62 clinical isolate (noncytotoxic); (C) heat-killed ATCC 29213. (D) Cells infected with the noncytotoxic S. aureus isolate K62 were harvested at the indicated times, and the CFU numbers of intracellular S. aureus were determined. Means and standard deviations were determined by four independent experiments. The dashed line indicates the limit of detection.
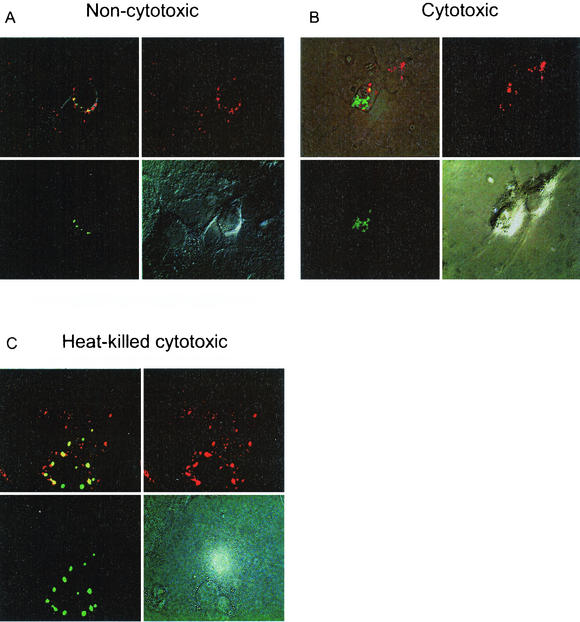
Host cells kill intracellular bacteria by means of phagolysosomal fusion and subsequent degradation by oxygen radicals, NO, and proteases. Obligate and facultative intracellular bacteria, however, have developed effective mechanisms to avoid killing by host cells that include evasion of the phagosome and inhibition of phagolysosomal fusion. We thus addressed the question of whether cytotoxic and noncytotoxic S. aureus strains localize to the same or different subcellular compartments. As shown in Fig. 4A, after 3 h of infection noncytotoxic S. aureus colocalizes with the lysosomal marker antigen LAMP-1, suggesting that noncytotoxic S. aureus enters the lysosomal degradation pathway. Indeed, by 24 h the signal of FITC-labeled S. aureus remained associated with LAMP-1 and yet was significantly decreased, which may be explained by either quenching of FITC fluorescence at acidic pH, degradation of bacteria, or both. In contrast, cytotoxic S. aureus strains do not colocalize with LAMP-1 (Fig. 4B), that is, do not enter the lysosomal compartment. Like noncytotoxic S. aureus, heat-killed cytotoxic S. aureus also colocalized with LAMP-1 (Fig. 4C), indicating that escape from the lysosomal pathway and killing of host cells is an achievement of viable, metabolically active staphylococci rather than due to a preformed cytotoxic component of S. aureus. Specifically, no differences in intracellular cytotoxicity were observed with a pair of congenic wild-type alpha-toxin-producing and H35R alpha-toxin mutant S. aureus strains (data not shown), indicating that alpha-toxin is not responsible for the killing of host cells by intracellular S. aureus.
FIG. 4.
Intracellular localization of noncytotoxic (A), cytotoxic (B), and heat-killed (C) S. aureus strains. PAM212 were infected with FITC-labeled bacteria. At 3 h after infection, cells were fixed and stained with anti-LAMP-1 antibody and visualized by using Cy3-labeled secondary antibody. Images captured by using an FITC filter (lower left), by using rhodamine (upper right), in bright light (lower right), or by superimposition of fluorescence images (upper right) are shown.
Enhanced virulence of cytotoxic S. aureus.
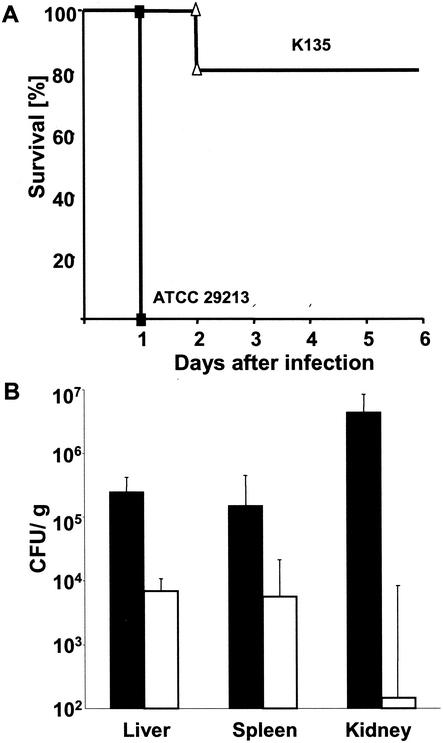
In order to address the question of whether the distinction between cytotoxic and noncytotoxic S. aureus is a mere in vitro phenomenon or instead relates to in vivo virulence, mice were challenged i.v. with five representative cytotoxic or seven noncytotoxic S. aureus strains. Injection of 108 CFU of either cytotoxic or noncytotoxic S. aureus resulted within 24 h in the death of 21 of 23 mice and 4 of 27 mice, respectively (Fig. 5A and Table 1). The bacterial loads in the liver, spleen, and kidney of mice challenged with cytotoxic S. aureus were approximately 36-, 26-, and 30,000-fold greater (P < 0.01), respectively, than those observed with noncytotoxic S. aureus (Fig. 5B).
FIG. 5.
In vivo virulence of cytotoxic S. aureus. (A) Mice were infected i.v. with 108 CFU of either cytotoxic (ATCC 29213; n = 7) or noncytotoxic (K135; n = 5) S. aureus isolates, respectively. The percentages of surviving animals in the cytotoxic (▪) and noncytotoxic (▵) groups are shown. (B) Two groups of three mice were infected i.v. with 107 of either cytotoxic (ATCC 29213, black bars) or noncytotoxic (K135, white bars) S. aureus isolates. After 18 h, mice were sacrificed and livers, spleens, and kidneys were removed to determine the numbers of CFU per gram of organ. The results are representative for two independent experiments. Means and standard deviations are calculated from individual values of the two experiments.
TABLE 1.
Comparison of in vivo virulence of cytotoxic and noncytotoxic S. aureus
| S. aureus strain | Time until death (days)a | No. dead/total no. at an ID of 108 CFUb (%) | No. with arthritis/total no. at an ID of 106 CFUc (%) |
|---|---|---|---|
| Cytotoxic isolates | |||
| ATCC 29213 | 1, 1, 1, 1, 1, 1, 1 | 7/7 | 6/7 |
| K42 | 1, 1, 1, 1 | 4/4 | 4/4 |
| K44 | 1, 1, 1, 1 | 4/4 | 2/4 |
| K50 | 1, 1, 1, 1 | 4/4 | 2/4 |
| K51 | 1, 1, 2, >14 | 3/4 | 3/4 |
| Total | 22/23 (96)*d | 17/23 (73)** | |
| Noncytotoxic isolates | |||
| K135 | 2, >14, >14, >14, >14 | 1/5 | 0/5 |
| K62 | 2, 3, 4 | 3/3 | 1/3 |
| K63 | 1, >14, >14 | 1/3 | 0/3 |
| K53 | 1, 3, 4 | 3/3 | 1/3 |
| K64 | 1, 2, >14 | 2/3 | 1/3 |
| DU1090 WT | 1, >14, >14, >14, >14 | 1/5 | 1/5 |
| DU1090 H35R | >14, >14, >14, >14, >14 | 0/5 | 1/5 |
| Total | 11/27 (41)* | 5/27 (19)** |
Results represent the time until death of each individual mice infected i.v. with 108 CFU of either cytotoxic or noncytotoxic S. aureus isolates.
Frequency of lethality in groups of mice challenged with cytotoxic and noncytotoxic S. aureus isolates. ID, infectious dose.
Results represent arthritis frequencies and the total number of mice challenged with 106 CFU of either cytotoxic or noncytotoxic S. aureus isolates. ID, infectious dose.
For values indicated by * and **, P < 0.01 for the overall difference between the indicated groups.
The enhanced virulence of cytotoxic S. aureus became also apparent when mice were infected with sublethal doses of either five cytotoxic or seven noncytotoxic S. aureus strains. As shown in Table 1, infection of mice with 106 CFU of cytotoxic S. aureus resulted in clinically apparent arthritis in the limbs of 73% of the animals, whereas the same infectious dose of noncytotoxic S. aureus produced arthritis in only 19% of the mice (P < 0.01). Together, these results indicate that the ability of S. aureus to kill host cells corresponds well with their replication, systemic dissemination, and organ infiltration in vivo.
DISCUSSION
S. aureus is one of the most prevalent bacterial pathogens isolated from patients with bloodstream infections. An important feature of S. aureus bacteremia is the frequency with which it penetrates from the bloodstream into other tissues such as bones and joints, the lungs, and the central nervous system (21). The rate of metastatic infection following S. aureus bacteremia varies from 1 to 53% depending on the authors and study with a median rate of 23.3% (16). The data of our present study show that 7 of 23 clinical S. aureus isolates after infection of keratinocytes and fibroblasts are able to escape phagolysosomal degradation, survive intracellularly, and produce significant cytotoxicity. The respective ability or inability to survive in and kill host cells turned out to be a stable trait of a given clinical S. aureus isolate. This suggests that the probability of metastatic infection in S. aureus bacteremia might be determined, at least in part, by the cytotoxicity of S. aureus. In fact, we observed significantly greater lethality, organ infiltration, and arthritis in mice challenged with cytotoxic S. aureus strains. We therefore propose that the ability to survive in and kill host cells may be a virulence factor that determines the propensity of S. aureus to negotiate cellular barriers and to penetrate from the bloodstream into tissues.
Using select pathogenic and nonpathogenic laboratory strains of S. aureus, it has been shown previously by many investigators that S. aureus can invade, survive in, and kill nonprofessional phagocytes such as endothelial cells, fibroblasts, keratinocytes, osteoblasts, and macrophages (4, 19, 23, 24, 33). This invasion is initiated by the adherence of S. aureus to the cellular surface, a process in which staphylococcal fibronectin-binding proteins play a prominent role (1, 11, 14, 15, 25, 26, 30). The adherent bacteria induce polymerization of cellular actin, leading to the engulfment by the plasma membrane, internalization via the “zipper mechanism” (13), and finally their transport within phagosomes into the cell (22). The uptake of S. aureus, for example, by endothelial cells, leads to the expression of adhesion molecules (5, 6, 10) and the secretion of proinflammatory cytokines (37). Phagocytosis of extracellular bacteria by professional phagocytes and internalization by nonprofessional phagocytes is generally viewed as a cell-autonomous defense mechanism. Several important human pathogens have developed effective antiphagocytic mechanisms, including encapsulation. The antiphagocytic activity of capsular polysaccharides and increased virulence of encapsulated strains were shown for Haemophilus influenzae, Pseudomonas aeruginosa, and Streptococcus pyogenes (9, 29, 31). Particularly, group A streptococci can be internalized by epithelial cells and seem to be trapped without producing any cytopathic effects (9). With regard to S. aureus, 90% of S. aureus strains express a capsule that is antiphagocytic (32), and capsule expression has been reported to negatively correlate with adherence to endothelial cells (27). However, all clinical isolates investigated are obviously able to invade nonprofessional phagocytes in equal quantities and with similar kinetics, indicating that possible differences in encapsulation do not impact on invasiveness.
In our study, we provide evidence that S. aureus strains can be distinguished by their survival abilities after internalization. For the majority of clinical S. aureus isolates (>70%), phagocytosis and subsequent lysosomal degradation seems to be a powerful means for host cells to eliminate these bacteria. This holds also true for SCV of S. aureus with reduced energy metabolism that have been reported to be especially adapted to survive intracellularly (35, 36). We have analyzed three clinical isolates of SCV. Although any of these SCV readily infected host cells, they were eliminated within 48 h and did not produce cytotoxicity (unpublished observations). These data indicate an obviously mutually exclusive relationship between S. aureus and host cells: either S. aureus is killed by the host cells and the host cell survives, or S. aureus remains viable and the host cells are killed.
By lysing their host cells, intracellular S. aureus once again become extracellular and can invade the adjacent tissue. Our mouse models demonstrate that, compared to noncytotoxic strains, cytotoxic S. aureus produce greater lethality; dramatically increased bacterial loads in liver, spleen, as well as kidney; and induced arthritis at greater frequency. In order to spread into various organs, circulating S. aureus organisms have to pass the endothelial barrier. We therefore conclude that cytotoxic S. aureus may disrupt the integrity of the endothelial barrier, facilitating bacterial metastasis and seeding. With regard to S. aureus-induced arthritis formation, Tarkowsky and coworkers recently implied that peptidoglycan was a driving force (20). According to the results of our study this might be secondary to cytolysis of cellular barriers and bacterial invasion of joints.
Up to the present, the molecular mechanisms of S. aureus-mediated killing of host cells remained controversial. Staphylococcal alpha-toxin has been implicated as the main factor in lysing endothelial cells (23). This soluble, pore-forming protein of S. aureus reported to induce apoptosis via the caspase death pathway (3). Bantel et al. noted that alpha-toxin present in the supernatant of S. aureus cultures induced cytotoxicity that does not require bacterial internalization (3). This finding is in striking contrast to previous reports by many investigators who proposed that S. aureus-induced apoptosis requires internalization of bacteria (4, 19, 23, 24, 33). These contradictory findings may reflect a cell-type-specific phenomenon, because compared to other cell types lymphocytes have been shown to be especially sensible to killing by alpha-toxin (17, 34). In addition, the observations by Bantel et al. may be secondary to unphysiological alpha-toxin concentrations used for cell death studies. In their experimental approach, supernatants of 14-h S. aureus cultures were used, which are stationary, well above quorum sensing, and which, albeit filtered, probably contain significant amounts of toxic molecules derived from dead bacteria. Whether circulating S. aureus can accumulate to such large numbers and produce comparable amounts of alpha-toxin is questionable. In fact, we did not observe a correlation between the expression levels of alpha-toxin and the cytotoxic activity of the various clinical S. aureus isolates. Specifically, a laboratory strain selected for high production of alpha-toxin did not cause host cell death, indicating that alpha-toxin production inside host cells does not suffice to initiate apoptotic cell death. The fact that rifampin rescued host cells previously infected with cytotoxic S. aureus provides strong evidence that S. aureus can kill their host cells from inside, that is, after internalization.
Acknowledgments
We thank Herdis Sommer for excellent technical assistance. We thank S. Bhakdi for providing isogenic alpha-toxin-producing and mutant S. aureus strains and for helpful discussion.
This work was partially supported by the DFG (SFB 589) and Maria Pesch Stiftung, Cologne, Germany.
Editor: A. D. O'Brien
REFERENCES
- 1.Almeida, R. A., K. R. Matthews, E. Cifrian, A. J. Guidry, and S. P. Oliver. 1996. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 79:1021-1026. [DOI] [PubMed] [Google Scholar]
- 2.Balwit, J. M., P. van Langevelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033-1037. [DOI] [PubMed] [Google Scholar]
- 3.Bantel, H., B. Sinha, W. Domschke, G. Peters, K. Schulze-Osthoff, and R. U. Janicke. 2001. α-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155:637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beekhuizen, H., J. S. van de Gevel, B. Olsson, I. J. van Benten, and R. van Furth. 1997. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J. Immunol. 158:774-782. [PubMed] [Google Scholar]
- 6.Bengualid, V., V. B. Hatcher, B. Diamond, E. A. Blumberg, and F. D. Lowy. 1990. Staphylococcus aureus infection of human endothelial cells potentiates Fc receptor expression. J. Immunol. 145:4279-4283. [PubMed] [Google Scholar]
- 7.Bonifacio, J. S. (ed.). 2000. Current protocols in cell biology. John Wiley & Sons, Inc., New York, N.Y.
- 8.Bremell, T., S. Lange, A. Yacoub, C. Ryden, and A. Tarkowski. 1991. Experimental Staphylococcus aureus arthritis in mice. Infect. Immun. 59:2615-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cywes, C., and M. R. Wessels. 2001. Group A streptococcus tissue invasion by CD44-mediated cell signalling. Nature 414:648-652. [DOI] [PubMed] [Google Scholar]
- 10.Drake, T. A., and M. Pang. 1988. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J. Infect. Dis. 157:749-756. [DOI] [PubMed] [Google Scholar]
- 11.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emori, T. G., and R. P. Gaynes. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 14.Fowler, T., E. R. Wann, D. Joh, S. Johansson, T. J. Foster, and M. Hook. 2000. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell β1 integrins. Eur. J. Cell Biol. 79:672-679. [DOI] [PubMed] [Google Scholar]
- 15.Hudson, M. C., W. K. Ramp, N. C. Nicholson, A. S. Williams, and M. T. Nousiainen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 19:409-419. [DOI] [PubMed] [Google Scholar]
- 16.Ing, M. B., L. M. Baddour, and A. S. Bayer. 1997. Bacteremia and infective endocarditis: pathogenesis, diagnosis, and complications, p. 331-354. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone, New York, N.Y.
- 17.Jonas, D., I. Walev, T. Berger, M. Liebetrau, M. Palmer, and S. Bhakdi. 1994. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect. Immun. 62:1304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jursch, R., A. Hildebrand, G. Hobom, J. Tranum-Jensen, R. Ward, M. Kehoe, and S. Bhakdi. 1994. Histidine residues near the N terminus of staphylococcal alpha-toxin as reporters of regions that are critical for oligomerization and pore formation. Infect. Immun. 62:2249-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahl, B. C., M. Goulian, W. van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Z. Q., G. M. Deng, S. Foster, and A. Tarkowski. 2001. Staphylococcal peptidoglycans induce arthritis. Arthritis Res. 3:375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 22.Lowy, F. D., J. Fant, L. L. Higgins, S. K. Ogawa, and V. B. Hatcher. 1988. Staphylococcus aureus-human endothelial cell interactions. J. Ultrastruct. Mol. Struct. Res. 98:137-146. [DOI] [PubMed] [Google Scholar]
- 23.Menzies, B. E., and I. Kourteva. 1998. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 66:5994-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuzzo, I., M. R. Sanges, A. Folgore, and C. R. Carratelli. 2000. Apoptosis of human keratinocytes after bacterial invasion. FEMS Immunol. Med. Microbiol. 27:235-240. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa, S. K., E. R. Yurberg, V. B. Hatcher, M. A. Levitt, and F. D. Lowy. 1985. Bacterial adherence to human endothelial cells in vitro. Infect. Immun. 50:218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 27.Pohlmann-Dietze, P., M. Ulrich, K. B. Kiser, G. Doring, J. C. Lee, J. M. Fournier, K. Botzenhart, and C. Wolz. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect. Immun. 68:4865-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proctor, R. A., J. M. Balwit, and O. Vesga. 1994. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect. Agents Dis. 3:302-312. [PubMed] [Google Scholar]
- 29.Ruhen, R. W., P. G. Holt, and J. M. Papadimitriou. 1980. Antiphagocytic effect of Pseudomonas aeruginosa exopolysaccharide. J. Clin. Pathol. 33:1221-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha, B., P. P. Francois, O. Nusse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 31.St Geme, J. W., III, and S. Falkow. 1992. Capsule loss by Haemophilus influenzae type b results in enhanced adherence to and entry into human cells. J. Infect. Dis. 165(Suppl. 1):S117-S118. [DOI] [PubMed] [Google Scholar]
- 32.Thakker, M., J. S. Park, V. Carey, and J. C. Lee. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tucker, K. A., S. S. Reilly, C. S. Leslie, and M. C. Hudson. 2000. Intracellular Staphylococcus aureus induces apoptosis in mouse osteoblasts. FEMS Microbiol. Lett. 186:151-156. [DOI] [PubMed] [Google Scholar]
- 34.Valeva, A., I. Walev, M. Pinkernell, B. Walker, H. Bayley, M. Palmer, and S. Bhakdi. 1997. Transmembrane beta-barrel of staphylococcal alpha-toxin forms in sensitive but not in resistant cells. Proc. Natl. Acad. Sci. USA 94:11607-11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesga, O., M. C. Groeschel, M. F. Otten, D. W. Brar, J. M. Vann, and R. A. Proctor. 1996. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J. Infect. Dis. 173:739-742. [DOI] [PubMed] [Google Scholar]
- 36.von Eiff, C., K. Becker, D. Metze, G. Lubritz, J. Hockmann, T. Schwarz, and G. Peters. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with darier's disease. Clin. Infect. Dis. 32:1643-1647. [DOI] [PubMed] [Google Scholar]
- 37.Yao, L., V. Bengualid, F. D. Lowy, J. J. Gibbons, V. B. Hatcher, and J. W. Berman. 1995. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect. Immun. 63:1835-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]