Abstract
Vγ9Vδ2 T lymphocytes strongly respond to phosphoantigens from Plasmodium parasites. Thus, we analyzed the changes in Vγ9Vδ2 T-cell function and repertoire during the paroxysm phase of nonendemic malaria infection. During malaria paroxysm, Vγ9Vδ2 T cells were early activated but rapidly became anergic and finally loose Jγ1.2 Vγ9 complementarity-determining region 3 transcripts.
Malaria affects hundreds of millions of persons worldwide, and Plasmodium falciparum, the most common species responsible, causes a severe disease resulting in millions of deaths. The degree of malaria pathology depends on both parasite and host factors (27). T cells bearing the γδ T-cell receptor (TCR) represent a variable fraction (1 to 10%) of peripheral blood lymphocytes. The main subset expresses a skewed Vγ9Vδ2 TCR rearrangement. These cells are activated during malaria infection and recognize nonpeptidic phosphoantigens present in P. falciparum (1, 2) and in red blood cells that could be released by erythrocyte rupture (4). After stimulation, Vγ9Vδ2 T lymphocytes can produce significant amounts of gamma interferon and tumor necrosis factor alpha (TNF-α) (8, 14, 22) that can mediate killing of blood stage malaria parasites (11). However, the role of γδ T cells in malaria infection is still unclear.
In this study, we analyzed the longitudinal pattern of γδ T-cell activation during the early events of nonendemic malaria infection, both during the paroxysm stage and during recovery after successful therapy. Four patients with nonendemic P. falciparum malaria from the “L. Spallanzani” Institute were enrolled (mean age, 44 years; age range, 32 to 53 years; 3 males, 1 female). Informed consent was obtained from all persons prior to enrollment, and only residual blood samples from the diagnostic routine were used. Blood samples were collected before antimalarial treatment, when patients showed high fever, chills, and rigors (paroxysm), and after recovery from clinical symptoms (after 24 to 36 h of antimalarial treatment). Malaria diagnosis was made by microscopic examination of thick blood film and confirmed by species-specific PCR (26). The clinical state was evaluated by using a clinical score as previously described (12). Table 1 shows clinical data obtained during paroxysm and after successful antimalarial treatment. Six healthy donors were used as controls. Surface staining was performed by using anti-CD3 (immunoglobulin G1 [IgG1], clone UCHT1), anti-HLA-DR (IgG2a, clone I2), and anti-TCR Vδ2 (IgG1, clone IMMU389) monoclonal antibodies directly coupled to fluorochromes (fluorescein isothiocyanate, phycoerythrin, and Cy5; ImmunoTech-Coulter, Miami, Fla.). In vitro Vγ9Vδ2 expansion after stimulation with 120 μM isopentenyl pyrophosphate (IPP) was determined as previously described (20, 21). Supernatants from cultures stimulated for 24 h with IPP were analyzed for TNF-α production by enzyme-linked immunosorbent assay kits used in accordance with the manufacturer's (Endogen, Woburn, Mass.) instructions. Vγ9 TCR complementarity-determining region 3 (CDR3) length heterogeneity was determined as previously described (6, 7). Group comparison was performed with the Mann-Whitney U test, and values of P that were ≤0.05 were considered significant.
TABLE 1.
Clinical parameters of patients with nonendemic P. falciparum malariaa
| Period and patient | Thick blood filmb | PCRc | Clinical score |
|---|---|---|---|
| Paroxysm | |||
| 200299 | T | + | 13 |
| 120299 | T, G | + | 11 |
| 230399 | T | + | 7 |
| 190399 | T, G | + | 6 |
| Recovery | |||
| 200299 | + | 2 | |
| 120299 | − | 0 | |
| 230399 | T | + | 0 |
| 190399 | − | 0 |
The diagnosis was made by microscopic examination of thick blood film and confirmed by species-specific PCR (26). Clinical status was evaluated by a clinical scoring (12). Patients were studied at paroxysm (before therapy) and after successful antimalarial treatment (24 to 36 h after paroxysm).
T, trophozoites; G, gametocytes.
+, positive; −, negative.
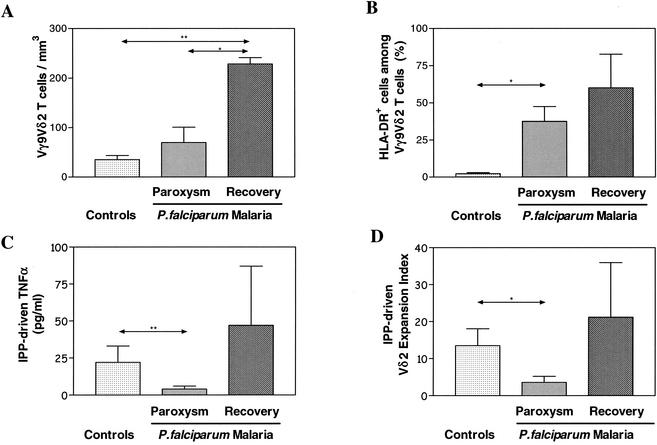
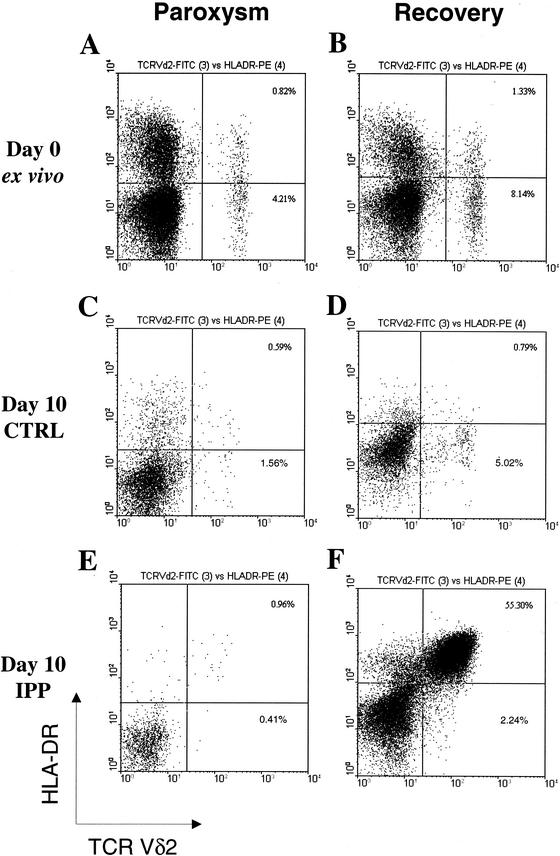
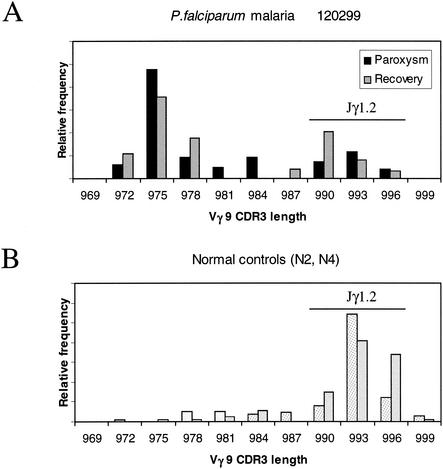
In Fig. 1A, a significant increase in the absolute Vδ2 T-cell subset number was found in malaria patients only after recovery. However, an increased percentage of activated HLA-DR+ Vδ2 T cells (Fig. 1B) was observed during paroxysm (1). Peripheral Vγ9Vδ2 T cells were stimulated in vitro with IPP ligand (20, 21), and 24-h supernatants were collected and studied for TNF-α production by enzyme-linked immunosorbent assay (Fig. 1C). Interestingly, Vγ9Vδ2 T cells were unable to produce TNF-α upon phosphoantigen stimulation during paroxysm. After 10 days, the CD3/Vδ2/HLA-DR phenotype was assessed by flow cytometry and a relative expansion index was determined as previously described (20, 21) (Fig. 1D). During paroxysm, the IPP reactivity was significantly reduced but it reverted after successful chemotherapy. This anergy to IPP stimulation was specific for Vγ9Vδ2 T cells, since the αβ T-cell response to phytohemagglutinin was unaffected (data not shown). Figure 2 shows flow cytometry panels from a representative patient. An increase in the Vδ2 T-cell number and an increased frequency of Vδ2+ HLA-DR+ activated cells were observed after recovery (Fig. 2A and B). When stimulated in vitro for 10 days with IPP, more than half of the lymphocytes were Vδ2+ HLA-DR+ after recovery (Fig. 2F), compared with less than 1% in the same culture performed during paroxysm (Fig. 2E). Since it has recently been shown that Vγ9Vδ2 T cells stimulated with nonpeptidic antigens mostly exhibit Jγ1.2 in the Vγ9 TCRγ chains (17), we used spectratyping analysis to assess Vγ9 TCR heterogeneity (18). In Fig. 3, Vγ9 CDR3 length profiles from a representative patient with P. falciparum infection are shown both during paroxysm and after recovery (panel A). Two normal controls are shown in Fig. 3B. In healthy donors, the Vγ9 CDR3 lengths were biased to favor Vγ9 mRNA open reading frames 990 to 996 bases in length (Fig. 3B), corresponding to Jγ1.2 fragment usage (6, 7). In contrast, a shift from longer to shorter Vγ9 lengths was shown during paroxysm in malaria patients (Fig. 3A). Indeed, Vγ9 transcripts of 990 to 996 bases (Jγ1.2) accounted for 75% ± 13% (standard deviation) in healthy controls, compared to only 11% ± 16% (P < 0.005) and 42% ± 14% (P < 0.05) in malaria patients during paroxysm and recovery, respectively. Interestingly, there were increases in the proportions of normally minor 972-, 975-, and 978-base Vγ9 transcripts for both the paroxysm samples and the recovery samples compared to uninfected controls (Fig. 3A and B).
FIG. 1.
Malaria-induced changes in ex vivo Vγ9Vδ2 T-cell subset absolute counts (A), ex vivo activation phenotype (HLA-DR+ percentage in the Vγ9Vδ2 subset of T cells) (B), in vitro phosphoantigen-driven TNF-α synthesis (C), and Vδ2 expansion index (D). Results are expressed as medians plus the upper quartiles. Four P. falciparum patients were studied in the paroxysm phase and after successful antimalarial treatment (24 to 36 h after paroxysm). As controls, six uninfected persons were studied. Differences between groups were evaluated with the Mann-Whitney U test. *, P ≤ 0.05; **, P ≤ 0.01.
FIG. 2.
Representative cytometric panel from a P. falciparum patient (120299) shown as Vδ2/HLA-DR plots during paroxysm and after successful antimalarial treatment (24 h after paroxysm). In panels A and B, ex vivo distributions are shown. In panels C and D, in vitro cultures stimulated with phosphoantigen (PPD) plus interleukin-2 for 10 days are shown. In panels E and F, in vitro cultures stimulated with phosphoantigen (interleukin-2 plus IPP) for 10 days are shown. Percentages refer to the indicated quadrant among total T cells. FITC, fluorescein isothiocyanate; PE, phycoerythrin; CTRL, control.
FIG. 3.
Vγ9 CDR3 length profile frequencies of a representative P. falciparum patient (120299) studied during the paroxysm and recovery phases (A) and those of two normal controls (B). CDR3 lengths 990 to 996 refer to phosphoantigen-reactive Vγ9/Jγ1.2 clones.
The possible role of the host immune response in determining the clinical outcome of malaria infection is controversial (27), with data supporting both a protective (9) and an immunopathogenetic (5, 13) role mediated by cytokines. In this context, Vγ9Vδ2 T lymphocytes have been shown to undergo deep changes during acute malaria infection: Vγ9Vδ2 expansion at onset was described by some authors (19, 23), while others found a delayed but persistent expansion occurring only after recovery (25). In this study, we observed a paroxysm-linked decrease in response to phosphoantigens, as both in vitro TNF-α synthesis and expansion, presumably as a consequence of specific hyperactivation. Moreover, γδ T-cell function recovered after successful antimalarial treatment, confirming that γδ T-cell anergy during infectious diseases is reversible (16). Interestingly, a study of experimental infection with P. falciparum (24) showed fast activation of Vγ9Vδ2 T cells, associated with a steep decline, linked to the erythrocyte cycle during which both Plasmodium phosphometabolites and red cell components are released. The overall outcome of Vγ9Vδ2 T-cell stimulation strongly depends on the activation level. At the early phases of infection, a massive antigenic exposure makes possible the induction of an abortive response (10, 15), as activated Vγ9Vδ2 T cells are highly susceptible to activation-induced cell death (4) triggered by Fas-FasL interaction (15). Moreover, the phosphoantigen 2,3-diphosphoglycerate, contained in red blood cells at millimolar concentrations, could be released by erythrocyte rupture and act as an antagonist (4), thus contributing to γδ T-cell anergy. Response to phosphoantigens is dependent on the Vγ9Vδ2 receptor (3). Vγ9Vδ2 T-cell populations maintain diversity in the CDR3s of Vγ9 mRNA after phosphoantigen stimulation, indicating that the response is polyclonal or oligoclonal, and are enriched for Vγ9 TCR chains containing the Jγ1.2 segment (7). During paroxysm, the changes in the Vγ9 repertoire indicated a preferential depletion of Jγ1.2 Vγ9 transcripts with higher affinity to phosphoantigens. Furthermore, human immunodeficiency virus-infected individuals were shown to have significant reductions in the frequency of Vγ9 containing the Jγ1.2 segment (6). Thus, the decrease in Vγ9Vδ2 response during active infectious diseases may reflect a specific depletion of Vγ9/Jγ1.2+ phosphoantigen-responding clones by a mechanism of hyperactivation-apoptosis. The recovery phase associated with successful chemotherapy is followed by fast reconstitution of the Vγ9Vδ2 T-cell number and response capability (16). This pattern of rapid activation, anergy depletion, and recovery after resolution of the infection may represent a common behavior of Vγ9Vδ2 T cells in response to acute-phase infections.
Acknowledgments
We thank Gennaro De Libero, Enrico Girardi, and Massimo Amicosante for critical reading of the manuscript.
This work was supported by a current-research grant from the Italian Ministry of Health. P.J.E. was supported by a Public Health Service viral oncology training grant (5T32 CA09075).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Behr, C., and P. Dubois. 1992. Preferential expansion of Vγ9 Vδ2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int. Immunol. 4:361-366. [DOI] [PubMed]
- 2.Behr, C., R. Poupot, M. A. Peyrat, Y. Poquet, P. Constant, P. Dubois, M. Bonneville, and J. J. Fournie. 1996. Plasmodium falciparum stimuli for human γδ T cells are related to phosphorylated antigens of mycobacteria. Infect. Immun. 64:2892-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukowski, J. F., C. T. Morita, H. Band, and M. B. Brenner. 1998. Crucial role of TCR γ chain junctional region in prenyl pyrophosphate antigen recognition by γδ T cells. J. Immunol. 161:286-293. [PubMed] [Google Scholar]
- 4.Burk, M. R., I. Carena, A. Donda, F. Mariani, L. Mori, and G. De Libero. 1997. Functional inactivation in the whole population of human Vγ9/Vδ2 T lymphocytes induced by a nonpeptidic antagonist. J. Exp. Med. 185:91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day, N. P., T. T. Hien, T. Schollaardt, P. P. Loc, L. V. Chuong, T. T. Chau, N. T. Mai, N. H. Phu, D. X. Sinh, N. J. White, and M. Ho. 1999. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J. Infect. Dis. 180:1288-1297. [DOI] [PubMed] [Google Scholar]
- 6.Enders, P. J., C. Yin, F. Martini, P. S. Evans, N. Propp, F. Poccia, and C. D. Pauza. 2003. HIV-mediated γδ T cell depletion is specific for Vγ2 cells expressing the Jγ1.2 segment. AIDS Res. Hum. Retrovir. 19:21-29. [DOI] [PubMed] [Google Scholar]
- 7.Evans, P. S., P. J. Enders, C. Yin, T. J. Ruckwardt, M. Malkovsky, and C. D. Pauza. 2001. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology 104:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Follows, G. A., M. E. Munk, A. J. Gatrill, P. Conradt, and S. H. E. Kaufmann. 1992. Gamma interferon and interleukin 2, but not interleukin 4, are detectable in γ/δ T-cell cultures after activation with bacteria. Infect. Immun. 60:1229-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 10.Janssen, O., S. Wesselborg, B. Heckl-Ostreicher, K. Pechhold, A. Bender, S. Schondelmaier, G. Moldenhauer, and D. Kabelitz. 1991. T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor γδ+ T cells. J. Immunol. 146:35-39. [PubMed] [Google Scholar]
- 11.Karunaweera, N. D., R. Carter, G. E. Grau, D. Kwiatkowski, G. Del Giudice, and K. N. Mendis. 1992. Tumour necrosis factor-dependent parasite-killing effects during paroxysms in non-immune Plasmodium vivax malaria patients. Clin. Exp. Immunol. 88:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karunaweera, N. D., R. Carter, G. E. Grau, and K. N. Mendis. 1998. Demonstration of anti-disease immunity to Plasmodium vivax malaria in Sri Lanka using a quantitative method to assess clinical disease. Am. J. Trop. Med. Hyg. 58:204-210. [DOI] [PubMed] [Google Scholar]
- 13.Karunaweera, N. D., G. E. Grau, P. Gamage, R. Carter, and K. N. Mendis. 1992. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl. Acad. Sci. USA 89:3200-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang, F., M. A. Peyrat, P. Constant, F. Davodeau, J. David-Ameline, Y. Poquet, H. Vie, J. J. Fournie, and M. Bonneville. 1995. Early activation of human Vγ9 Vδ2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J. Immunol. 154:5986-5994. [PubMed] [Google Scholar]
- 15.Li, B., H. Bassiri, M. D. Rossman, P. Kramer, A. F. Eyuboglu, M. Torres, E. Sada, T. Imir, and S. R. Carding. 1998. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human γδ T cells: a mechanism for the loss of γδ T cells in patients with pulmonary tuberculosis. J. Immunol. 161:1558-1567. [PubMed] [Google Scholar]
- 16.Martini, F., R. Urso, C. Gioia, A. De Felici, P. Narciso, A. Amendola, M. G. Paglia, V. Colizzi, and F. Poccia. 2000. γδ T-cell anergy in human immunodeficiency virus-infected persons with opportunistic infections and recovery after highly active antiretroviral therapy. Immunology 100:481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyagawa, F., Y. Tanaka, S. Yamashita, B. Mikami, K. Danno, M. Uehara, and N. Minato. 2001. Essential contribution of germ line-encoded lysine residues in Jγ1.2 segment to the recognition of nonpeptide antigens by human γδ T cells. J. Immunol. 167:6773-6779. [DOI] [PubMed] [Google Scholar]
- 18.Pannetiers, C., J. P. Levraud, A. Lim, J. Even, and P. Kourilsky. 1997. The immunoscopic analysis of T cell repertoires, p. 287-385. In J. Oskenberg (ed.), The antigen T cell receptor: selected protocols and applications. Chapman & Hall, Detroit, Mich.
- 19.Perera, M. K., R. Carter, R. Goonewardene, and K. N. Mendis. 1994. Transient increase in circulating γ/δ T cells during Plasmodium vivax malarial paroxysms. J. Exp. Med. 179:311-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poccia, F., S. Boullier, H. Lecoeur, M. Cochet, Y. Poquet, V. Colizzi, J. J. Fournie, and M. L. Gougeon. 1996. Peripheral Vγ9/Vδ2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J. Immunol. 157:449-461. [PubMed] [Google Scholar]
- 21.Poccia, F., B. Cipriani, S. Vendetti, V. Colizzi, Y. Poquet, L. Battistini, M. Lopez-Botet, J. J. Fournie, and M. L. Gougeon. 1997. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ9Vδ2 T lymphocytes. J. Immunol. 159:6009-6017. [PubMed] [Google Scholar]
- 22.Poccia, F., M. L. Gougeon, M. Bonneville, M. Lopez-Botet, A. Moretta, L. Battistini, M. Wallace, V. Colizzi, and M. Malkovsky. 1998. Innate T-cell immunity to nonpeptidic antigens. Immunol. Today 19:253-256. [DOI] [PubMed] [Google Scholar]
- 23.Roussilhon, C., M. Agrapart, P. Guglielmi, A. Bensussan, P. Brasseur, and J. J. Ballet. 1994. Human TcR γδ+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin. Exp. Immunol. 95:91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rzepczyk, C. M., S. Stamatiou, K. Anderson, A. Stowers, Q. Cheng, A. Saul, A. Allworth, J. McCormack, M. Whitby, C. Olive, and G. Lawrence. 1996. Experimental human Plasmodium falciparum infections: longitudinal analysis of lymphocyte responses with particular reference to γδ T cells. Scand. J. Immunol. 43:219-227. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz, E., R. Shapiro, S. Shina, and I. Bank. 1996. Delayed expansion of Vδ2+ and Vδ1+ γδ T cells after acute Plasmodium falciparum and Plasmodium vivax malaria. J. Allergy Clin. Immunol. 97:1387-1392. [DOI] [PubMed] [Google Scholar]
- 26.Singh, B., A. Bobogare, J. Cox-Singh, G. Snounou, M. S. Abdullah, and H. A. Rahman. 1999. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am. J. Trop. Med. Hyg. 60:687-692. [DOI] [PubMed] [Google Scholar]
- 27.White, N. J., and M. Ho. 1992. The pathophysiology of malaria. Adv. Parasitol. 31:83-173. [DOI] [PubMed]