Abstract
Monophosphoryl lipid A (MPL) is a nontoxic derivative of lipopolysaccharide (LPS) that exhibits adjuvant properties similar to those of the parent LPS molecule. However, the mechanism by which MPL initiates its immunostimulatory properties remains unclear. Due to the involvement of Toll-like receptors in recognizing and transducing intracellular signals in response to LPS, the aim of the present study was to determine the ability of MPL to utilize the Toll-like receptor 2 (TLR2) and TLR4. We provide evidence that MPL differentially utilizes TLR2 and TLR4 for the induction of tumor necrosis factor alpha, interleukin 10 (IL-10), and IL-12 by purified human monocytes as well as by human peripheral blood mononuclear cells. Assessment of NF-κB activity demonstrated that MPL utilized TLR2 and especially TLR4 for the activation of NF-κB p65 by human monocytes. In addition, stimulation of human monocytes by MPL led to an up-regulation of the costimulatory molecules CD80 and CD86, an effect that could be reduced by pretreatment of cells with a monoclonal antibody to TLR2 or TLR4. Analysis of MPL-induced activation of the extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein (MAP) kinases revealed that MPL utilized both TLR2 and TLR4 for the phosphorylation of ERK1/2, while TLR4 was the predominant receptor involved in the ability of MPL to phosphorylate p38. Moreover, using selective inhibitors for MAP kinase kinase (PD98059) and p38 (SB203580), we show that ERK1/2 exhibited differential effects on production of TNF-α and IL-12 p40 by human monocytes, whereas MPL-induced activation of p38 appeared to be predominantly involved in production of IL-10 and IL-12 p40 by MPL-stimulated monocytes. Taken together, these findings aid in understanding the cellular mechanisms by which MPL induces host cell activation and subsequent adjuvant properties.
The use of defined microbial products for vaccine development is believed to result in a higher level of safety than use of conventional microbial-based vaccines. However, a major problem encountered with this approach has been the lack of immunogenicity associated with subunit vaccines. Indeed, it has been reported that most antigens fail to induce a productive immune response unless they are administered along with an adjuvant (14). Thus, much attention has been focused on various molecules that possess adjuvant properties and can overcome the lack of immunogenicity associated with subunit vaccines. The lipopolysaccharide (LPS) component of gram-negative bacteria has been shown to act as a potent adjuvant; however, its extreme toxicity precludes its use as an adjuvant in humans. However, a chemically modified LPS derived from Salmonella minnesota R595, termed monophosphoryl lipid A (MPL), has been shown to exhibit potent adjuvanticity, while exhibiting essentially no toxicity (59). Assessment of the adjuvant characteristics of MPL has shown it to be an effective adjuvant for the induction of both humoral and cell-mediated immunity in which MPL can induce both Th1- and Th2-type immune responses in the systemic and mucosal compartments of the immune system (2, 7, 34, 44).
The ability of the innate immune system to recognize LPS or LPS-associated components of gram-negative microbes has recently been attributed to Toll-like receptors (TLRs) (33, 64). The TLRs represent a family of type I transmembrane proteins consisting of an extracellular leucine-rich repeat region and a cytoplasmic domain that is homologous to the signaling component of the type I interleukin 1 receptor (IL-1R) (43). Recognition of conserved microbial products by TLRs expressed on antigen-presenting cells leads to cell activation via a variety of signal transduction pathways, including the activation of NF-κB and mitogen-activated protein kinases (MAPK) and the subsequent induction of cytokines and costimulatory molecules (6, 8, 33, 47, 52, 64). Thus, the TLR-signaling pathway links both the innate and adaptive immune systems by inducing both cytokines and costimulatory molecules that can determine both qualitative and quantitative aspects of T-cell responses. Indeed, a deletion in certain components of the TLR pathway has been shown to affect the polarization of T-helper cells into Th1- or Th2-type cells (46).
Genetic analysis of the LPS-hyporesponsive C3H/HeJ and C57BL10/ScCr mouse strains indicates that these mice possess either a point mutation or a deletion in the tlr4 gene, respectively (37, 38). It has also been shown that TLR4 but not TLR2 knockout mice are unresponsive to enterobacterial LPS (51). Studies assessing the ability of commercial enterobacterial LPS preparations to utilize TLR2 and TLR4 have demonstrated that the TLR2 activity of these commercial preparations was associated with bioactive copurified products and not the parent LPS molecule (17, 53). However, it has also been reported that certain LPS molecules derived from Prevotella intermedia, Porphyromonas gingivalis, and Leptospira interrogans signal via TLR2 (18, 22, 62). While it is currently unclear how these various types of LPS can signal via TLR2 or TLR4, differences within the LPS structures are believed to contribute to their biological activity (18, 22, 62).
The mechanisms by which MPL initiates cellular activation and its resulting adjuvant properties remain unclear. A previous study assessing the adjuvant properties of MPL in the TLR4-deficient C3H/HeJ mouse strain demonstrated that MPL retained immunostimulatory properties (57), suggesting that MPL may be utilizing a TLR other than TLR4. However, no study to date has directly assessed the ability of MPL to utilize TLR2 or TLR4, nor has one assessed their relative contributions to its immunostimulatory properties. Thus, the purpose of the present study was to assess the role of TLR2 and TLR4 in the ability of MPL to induce cellular activation and to determine how these receptors are involved in the induction of cytokines and the up-regulation of costimulatory molecules.
MATERIALS AND METHODS
Reagents.
The MPL used in the present study was obtained from Corixa Corporation (Hamilton, Mont.) and contained <0.15% protein. Mouse anti-human TLR2 monoclonal antibody (MAb) (clone 2392; immunoglobulin G1 [IgG1]) was obtained from Genentech Corporation (San Francisco, Calif.). Mouse anti-human TLR4 MAb (clone HTA125; IgG2a) was purchased from eBioscience (San Diego, Calif.). These monoclonal antibodies have been characterized previously (15, 31, 48). Goat anti-mouse IgG1-FITC, IgG2a-PE, and all isotype-matched control antibodies (IgG1 and IgG2a) were purchased from eBioscience. Anti-human CD80-PE, CD86-FITC, and CD14-FITC were obtained from eBioscience. All cell culture reagents were purchased from Gibco Technologies (Grand Island, N.Y.). NF-κB activity was assessed using the Trans-AM NF-κB p65 kit purchased from Active Motif (Carlsbad, Calif.). Levels of tumor necrosis factor alpha (TNF-α), IL-10, IL-12 p40, and IL-12 p70 present in cell supernatants were determined using cytokine reagents purchased from eBioscience. PD98059 and SB203580 were purchased from Calbiochem (San Diego, Calif.). Antibodies used for the detection of total and phosphorylated, extracellular signal-regulated kinases (ERK1/2) and p38 MAPK were obtained from Cell Signaling (Beverly, Mass.).
Cell culture.
Human peripheral blood mononuclear cells (PBMC) were obtained from healthy donors and isolated from heparinized venous blood by collecting the buffy coat and eliminating red blood cell contamination by histopaque (SG-1.077) density gradients. After washing in phosphate-buffered saline (PBS) containing 1% fetal calf serum and 2 mM EDTA, PBMC were resuspended at a concentration of 2 × 106 cells/ml in RPMI 1640 supplemented with 10% fetal bovine serum, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 2 mM l-glutamine, 20 mM HEPES, 50 U of penicillin/ml, and 50 μg of streptomycin/ml (complete medium). Human monocytes were purified by negative selection using a monocyte isolation kit purchased from Miltenyi Biotech (Auburn, Calif.). Monocytes were isolated from PBMC by depletion of nonmonocyte cells. This was done with the aid of an indirect magnetic isolation kit using monoclonal hapten-conjugated CD3, CD7, CD19, CD45RA, CD56, and IgE antibodies (Miltenyi Biotec). This procedure routinely resulted in >90% pure CD14+ cells, as shown by flow cytometry. Human monocytes or PBMC, suspended in complete medium, were cultured in 24- or 96-well plates. To assess the functional role of TLR2 or TLR4, human monocytes or PBMC were incubated with 10 μg of anti-TLR2 MAb, anti-TLR4 MAb, or isotype-matched control (IC) antibodies/ml for 30 min before stimulation with MPL. To assess the involvement of ERK1/2 and p38 in MPL-induced cytokine production by monocytes, cells were pretreated for 45 min with the MAPK kinase (MEK) inhibitor PD98059 or the p38 inhibitor SB203580 at various concentrations. Cytokine levels were quantitated 24 h after stimulation using enzyme-linked immunosorbent assay (ELISA) kits according to the protocol suggested by the manufacturer (eBioscience).
Flow cytometry.
Human monocytes were cultured at a concentration of 4 × 105 cells/ml in polypropylene tubes and incubated for 24 h in medium alone or with MPL. Cells were collected by low-speed centrifugation and resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS containing 1% fetal bovine serum and 0.1% NaN3). Cells were then incubated with fluorochrome-labeled MAbs to CD80 or CD86 for 30 min on ice. Cells were washed three times in FACS buffer and resuspended in 2% paraformaldehyde before analysis by flow cytometry using a FACStar flow cytometer (Becton Dickinson, Mountain View, Calif.).
NF-κB activity.
Human monocytes were plated at a concentration of 106 cells/ml in a 24-well polystyrene tissue culture plate. Cells were pretreated for 30 min with various MAbs as indicated in the figure legends and then incubated with MPL or medium alone for 20 h. Cells were collected, washed two times in PBS, and then assayed for p65 NF-κB activity according to the manufacturer's instructions. The level of nuclear NF-κB p65 was expressed as the optical density emitted at 450 nm from 5 μg of whole-cell lysate.
Western blot analysis.
Human monocytes (3 × 106/ml) were pretreated with medium, MAbs to TLR2 or TLR4, SB203580, or PD98059 before the addition of MPL. After a 30-min incubation, cells were washed with PBS and then resuspended in 300 μl of lysis buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 1-mg/ml aprotonin) for 10 min on ice. The whole-cell lysate was passed through a 20-gauge needle and then incubated on ice for an additional 30 min. Cell debris was pelleted by centrifugation. Supernatants were collected and stored at −80°C until assayed for cytokine levels. Twenty micrograms of total cellular protein was suspended in Nu Page lithium dodecyl sulfate (LDS) sample buffer (Invitrogen, Carlsbad, Calif.), heated for 10 min at 70°C, resolved by LDS-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride membranes using the Novex system (Invitrogen). Probing and visualization of immunoreactive bands were done using the Western Breeze Chemiluminescents kit (Invitrogen) according to the manufacturer's protocol.
Statistical analysis.
Statistical significance between groups was evaluated by analysis of variance and the Tukey multiple-comparison test using the InStat program (GraphPad Software, San Diego, Calif.). Differences between groups were considered significant at P values of <0.05.
RESULTS
Involvement of TLR2 and TLR4 in MPL-induced TNF-α, IL-10, IL-12 p40, and IL-12 p70 production by human monocytes.
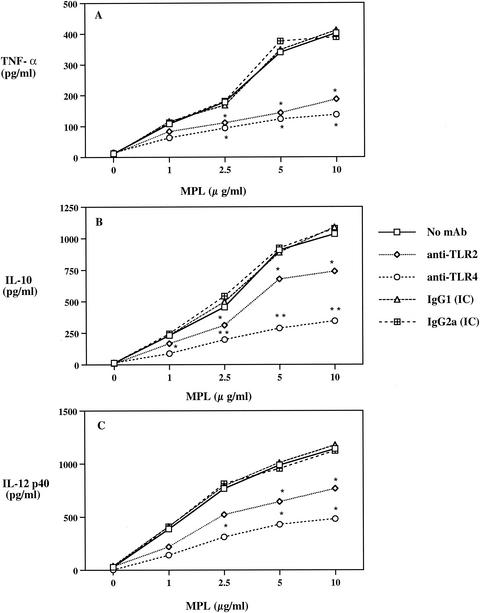
In our initial series of experiments, we assessed the ability of MPL to utilize the LPS-signaling components TLR2 and TLR4 and determined their relative contribution to MPL-induced cytokine responses. Human monocytes were preincubated with or without MAbs to TLR2 or TLR4 or IC antibody followed by stimulation with MPL. Analysis of TNF-α production by MPL-stimulated cells revealed that MPL induced a dose-dependent response that was significantly (P < 0.05) inhibited by both anti-TLR2 and anti-TLR4 MAbs (Fig. 1A). Furthermore, no significant difference was observed in the abilities of the anti-TLR2 and anti-TLR4 MAbs to inhibit TNF-α production (Fig. 1A). In contrast, the anti-TLR4 MAb was more effective (P < 0.01) than the anti-TLR2 MAb (P < 0.05) in inhibiting IL-10 production by MPL-stimulated human monocytes (Fig. 1B). Analysis of IL-12 p40 and p70 production from human monocytes treated with MPL resulted in enhanced IL-12 p40 production (Fig. 1C), but no IL-12 p70 was detected at all concentrations tested (data not shown). Moreover, coincubation with the anti-TLR2 MAb resulted in a reduction of more than 40% in the level of IL-12 p40 production, whereas a more than 60% reduction was observed with the anti-TLR4 MAb (Fig. 1C). No significant inhibitory effect was observed with the IgG1 or IgG2a IC antibody (Fig. 1). These data demonstrate that MPL utilizes both TLR2 and TLR4 for the induction of proinflammatory and Th1- and Th2-evoking cytokines by monocytes. Moreover, a difference exists in the relative contributions of TLR2 and TLR4 for MPL-induced cytokine production.
FIG. 1.
Involvement of TLR2 and TLR4 in MPL-induced TNF-α (A), IL-10 (B), and IL-12 p40 (C) production by human peripheral blood monocytes. Human monocytes (3 × 106/ml) were preincubated with anti-TLR2 or anti-TLR4 MAb or IC antibody for 30 min before the addition of MPL. Cell supernatants were collected 24 h after stimulation and analyzed by ELISA. Single and double asterisks indicate significant differences at P values of <0.05 and <0.01, respectively, compared to MPL-stimulated cultures. Data are expressed as means (n = 6). Standard deviations for each group were omitted for clarity.
Ability of MPL to induce activation of NF-κB p65 in the presence of MAbs to TLR2 or TLR4.
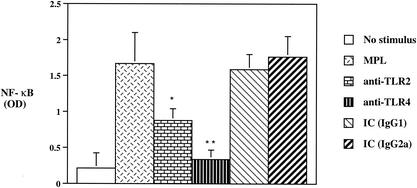
NF-κB is a major transcription factor activated by the TLR-signaling pathway and is involved in cellular activation as well as in linking the innate and adaptive immune systems (33). Thus, we next sought to determine the ability of MPL to activate NF-κB in the presence or absence of MAbs to TLR2 and TLR4. Preincubation of monocytes with a MAb to TLR2 resulted in almost a 50% reduction in the level of active NF-κB p65 compared to that for MPL-stimulated cultures (Fig. 2). In addition, a more than 70% reduction in NF-κB activity was observed when cells were preincubated with the anti-TLR4 MAb (Fig. 2). Moreover, the ability of the anti-TLR4 MAb to inhibit the activation of NF-κB was significantly (P < 0.05) greater than that of the anti-TLR2 MAb. The addition of either IC antibody to cultures stimulated with MPL had no significant effect on the ability of MPL to activate NF-κB (Fig. 2). These data demonstrate that TLR2 and especially TLR4 are involved in the ability of MPL to activate NF-κB p65.
FIG. 2.
Comparison of NF-κB activation induced by MPL in the presence or absence of anti-TLR2 or anti-TLR4 MAb. Human monocytes (106/ml) were preincubated for 30 min with the indicated anti-TLR2 or anti-TLR4 MAb or IC antibody and then incubated with MPL (10 μg/ml) for an additional 20 h. Nuclear extracts were analyzed for activation of NF-κB, and data are expressed as means optical density (OD) ± standard deviations (n = 6). Single and double asterisks indicate significant differences at P values of <0.05 and <0.01, respectively, compared to MPL-stimulated cultures.
Role of TLR2 and TLR4 in MPL regulation of CD80 and CD86 expression on human monocytes.
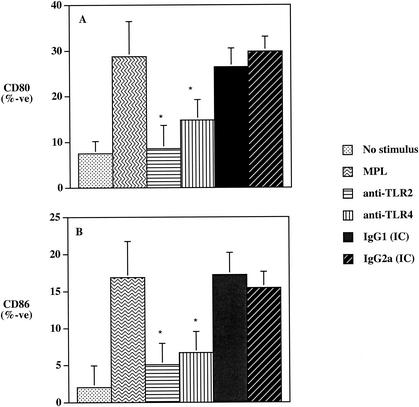
Previous studies assessing the role of the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) have shown that these molecules are critically involved in naive-T-cell activation, expansion, and differentiation into T-helper-cell subsets (1, 13, 23, 25, 28, 29). Furthermore, LPS-induced TLR signaling and subsequent NF-κB activation have been shown to be involved in the up-regulation of the costimulatory molecule CD80 (33). Recent work by our laboratory has demonstrated that MPL can up-regulate both CD80 and CD86 on antigen-presenting cells and induce a functional costimulatory signal to CD4+ T cells (63). Thus, due to the differential involvement of TLR2 and TLR4 observed in MPL-induced NF-κB activation (Fig. 2), we next wanted to determine how TLR2 and TLR4 affected the ability of MPL to up-regulate CD80 and CD86. Human monocytes incubated with MPL for 20 h exhibited a more than threefold increase in the number of cells expressing CD80 and CD86 compared to results for cells incubated in medium alone (Fig. 3). Furthermore, preincubation of monocytes with MAb to TLR2 or to TLR4 resulted in a significant reduction (P < 0.05) in the ability of MPL to upregulate both CD80 (Fig. 3A) and CD86 (Fig. 3B) expression. However, preincubation with the anti-TLR2 MAb rendered a slightly greater reduction in the levels of CD80 and CD86 expression than that observed with the anti-TLR4-treated cultures. Thus, the ability of MPL to up-regulate the costimulatory molecules CD80 and CD86 is dependent upon both TLR2 and TLR4 signal molecules.
FIG. 3.
The effects of MAbs to TLR2 or TLR4 on the ability of MPL to up-regulate the expression of the costimulatory molecules CD80 (A) and CD86 (B) on human monocytes. Human monocytes (4 × 105/ml) were preincubated for 30 min with the indicated MAb or IC antibody and then incubated with MPL for an additional 24 h. The levels of CD80 or CD86 were determined by flow cytometry. The asterisk indicates significant differences (P < 0.05) compared to the MPL-only treatment group. Data are expressed as the mean of percent positive cells ± standard deviation (n = 6).
Cytokine production by human PBMC stimulated with MPL.
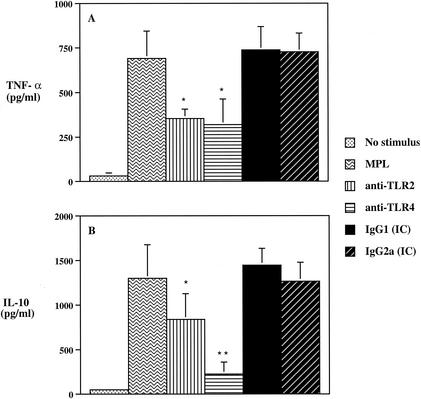
Previous studies have reported that the contribution of TLRs to cell activation may vary depending on the type of cells used and that such may reflect different TLR expression levels (53). Thus, in order to assess the effects of MPL in a setting that better resembles conditions following immunization, we investigated the involvement of TLR2 and TLR4 in the ability of MPL to induce cytokine production by PBMC. Human PBMC stimulated with MPL in the presence of either anti-TLR2 or anti-TLR4 MAb exhibited a more than 40% reduction in TNF-α production (Fig. 4A). The level of IL-10 induced by MPL-stimulated PBMC cultures was significantly (P < 0.05) reduced following pretreatment with an anti-TLR2 MAb (Fig. 4B); however, the predominant inhibitory effect was observed with the anti-TLR4 MAb (P < 0.01), with a more than 70% reduction in IL-10 production (Fig. 4B). No IL-12 p70 was detected in MPL-stimulated human PBMC cultures (data not shown). No significant differences were observed in the levels of TNF-α or IL-10 production in cultures stimulated with MPL in the presence of either IC antibody (Fig. 4). Taken together, these data demonstrate that MPL-induced cytokine production by human PBMC is differentially mediated by TLR2 and TLR4.
FIG. 4.
The ability of MPL to induce TNF-α (A) and IL-10 (B) by human PBMC is differentially dependent upon both TLR2 and TLR4. PBMC (2 × 106/ml) were preincubated with the different MAbs for 30 min and then incubated for 24 h in the presence of MPL (10 μg/ml). Cell supernatants were collected and analyzed for cytokine production by ELISA. The data are expressed as means ± standard deviations (n = 5). Single and double asterisks indicate significant differences at P values of <0.05 and <0.01, respectively, compared to MPL-treated group.
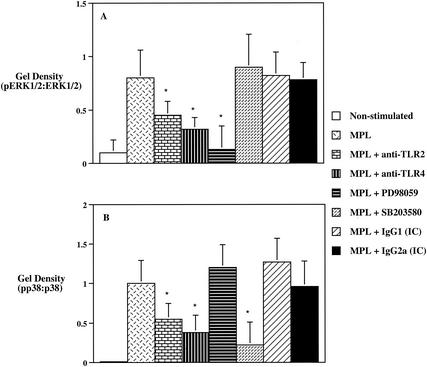
Roles of TLR2 and TLR4 in MPL-mediated phosphorylation of ERK1/2 and p38 and their involvement in MPL-induced cytokine production.
To investigate the ability of MPL to induce ERK1/2 and p38 phosphorylation, as well as the involvement of these MAPK in MPL-stimulated cytokine production, we next assessed how TLR2 and TLR4 were involved in the phosphorylation of these MAPK and how ERK1/2 and p38 affected the ability of MPL to induce production of TNF-α, IL-10, IL-12 p40, and IL-12 p70 by human monocytes. Preincubation of monocytes with anti-TLR2 or anti-TLR4 MAb significantly reduced (P < 0.05) the level of ERK1/2 phosphorylation from that for cultures stimulated with MPL alone (Fig. 5A). Moreover, pretreatment of human monocytes with the ERK inhibitor PD98059, which mediates its inhibitory properties by binding to the ERK-specific MAP kinase MEK, resulted in a significant (P < 0.05) reduction of the pERK1/2-to-ERK1/2 ratio to near nonstimulated control levels (Fig. 5A). In contrast, no significant affect was observed with either the IC antibodies or the p38 inhibitor SB203580 on pERK1/2-to-ERK1/2 ratio levels (Fig. 5A). Analysis of p38 activity induced by MPL showed that pretreatment of human monocytes with an anti-TLR2 MAb resulted in a significant (P < 0.05) decrease in the levels of p38 phosphorylation that was further reduced by preincubation with the anti-TLR4 MAb (Fig. 5B). In addition, the p38 inhibitor SB203580 significantly (P < 0.05) inhibited the levels of p38 phosphorylation, while PD98059 did not exhibit any discernible effect (Fig. 5B).
FIG. 5.
MPL-induced phosphorylation of ERK1/2 (A) and p38 (B) by human monocytes. Human monocytes (3 × 106/ml) were preincubated for 45 min with PD98059 (20 μM) or SB203580 (5 μM) or for 45 min with anti-TLR2, anti-TLR4, or IC antibodies prior to the addition of MPL to cell cultures. After a 30-min incubation with MPL (10 μg/ml), total cell lysates were resolved by LDS-polyacrylamide gel electrophoresis and immunoblotted with phospho-specific ERK1/2 and p38 or anti-total ERK1/2 or p38 antibodies. Densitometer scans of pp38, total p38, pERK1/2, and total ERK1/2 were performed and recorded as the ratio of pp38 to p38 or pERK1/2 to ERK1/2. The data are expressed as means ± standard deviations (n = 5). Single and double asterisks indicate significant differences at P values of <0.05 and <0.01, respectively, compared to MPL-treated group.
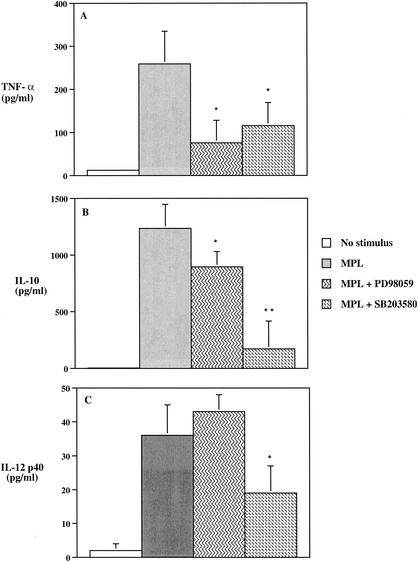
To assess the roles of ERK1/2 and p38 in the MPL-mediated cytokine production by human monocytes, we analyzed how PD98059 or SB203580 affected the ability of MPL to induce production of TNF-α, IL-10, IL-12 p40, and IL-12 p70. Pretreatment of monocytes with either PD98059 or SB203580 resulted in a significant (P < 0.05) decrease in the levels of TNF-α production by MPL-stimulated monocytes (Fig. 6A). However, the involvement of ERK1/2 and p38 in the ability of MPL to induce IL-10 production revealed different results. While PD98059 significantly (P < 0.05) inhibited the level of IL-10 production by MPL-treated monocytes, the inhibitory effect of SB203580 was more pronounced and resulted in a more than 70% reduction in the level of IL-10 produced (Fig. 6B). In contrast to the ability of PD98059 to inhibit both TNF-α and IL-10 production by MPL-stimulated monocytes, PD98059 enhanced the level of IL-12 p40 production by MPL-stimulated monocytes (Fig. 6C); however, no detectable IL-12 p70 was observed even in the presence of PD98059 (data not shown). SB203580 markedly (P < 0.05) inhibited the ability of MPL to induce IL-12 p40 (Fig. 6C). Taken together, these findings demonstrate that the abilities of MPL to induce TNF-α, IL-10, and IL-12 p40 are regulated differentially by MPL-induced activation of ERK and p38.
FIG. 6.
The involvement of MPL-induced phosphorylation of ERK1/2 and p38 in TNF-α (A), IL-10 (B), and IL-12 p40 (C) production by human monocytes. Human monocytes (3 × 106/ml) were preincubated for 45 min with PD98059 (20 μM) or SB203580 (5 μM) or for 45 min with anti-TLR2, anti-TLR4, or IC antibodies prior to the addition of MPL to cell cultures. Cell supernatants were collected 24 h after MPL stimulation and analyzed for cytokine production by ELISA. The data are expressed as the means ± standard deviations (n = 5). Single and double asterisks indicate significant differences at P values of <0.05 and <0.01, respectively, compared to MPL-treated group.
DISCUSSION
In recent years, it has been shown that the cell surface molecules responsible for the transduction of intracellular signals following LPS exposure are the TLRs (18, 22, 33, 58). Various types of LPS have been shown to signal via either TLR2 or TLR4, and the differences within the LPS structures, although not yet established, are believed to contribute to their biological activity (18, 22, 62).
MPL is a detoxified derivative of LPS that lacks the endotoxic properties but retains both the adjuvant and immunostimulatory activities of the parent LPS (4, 16, 35). Previous studies have shown that the removal of an acid-labile phosphate group and normal fatty acid groups from diphosphoryl lipid A markedly decreased the toxicity of the molecule without substantially affecting the adjuvant activity (59). In addition, it has also been shown that MPL differs from LPS in its ability to induce certain cytokines, such as IL-10, IL-12, and IFN-γ (19, 44). Therefore, MPL is distinct from enterobacterial LPS, differing in both structure and biological activity.
In the present study, we were interested in determining the relative contributions of TLR2 and TLR4 to MPL-induced cell activation and subsequent cytokine production. Evidence is provided that MPL uses both TLR2 and TLR4 for the induction of TNF-α, IL-10, and IL-12; however, a difference was shown in the relative contributions of TLR2 and TLR4 for the induction of these cytokines. In this regard, antibodies to TLR2 and to TLR4 were equally effective in inhibiting MPL-induced TNF-α production, whereas anti-TLR4 was more effective than anti-TLR2 in inhibiting IL-10 and IL-12 production.
Previous studies have demonstrated the induction of TNF-α, IL-10, and IL-12 production by TLR2 and TLR4 agonists (20, 26, 53, 54, 56, 60). Furthermore, it has been suggested that different mechanisms may control TNF-α production (32) and that the different contributions of TLR2 and TLR4 may be contingent upon the levels of cell expression of these receptors (53). It has also been suggested that a difference in the contribution of TLR2 and TLR4 may be explained by the requirement of TLR2 to form heterodimeric complexes with either TLR1 or TLR6, whereas TLR4 forms homodimers (36). Although many molecules may act as TLR2 and/or TLR4 agonists, it has become more evident that they can exert different effects (19, 53, 56). Stimulation of TLRs has been shown to lead to the activation of NF-κB, suggesting that TLRs have or share a common signaling pathway (6, 19, 20, 36, 41, 52). However, signaling through TLR2 or TLR4 can result in activation of different pathways involving the MAPK ERK1/2 and p38 (3, 39, 49, 61). Thus, it was also of interest to us to determine the involvement of TLR2 and TLR4 in the activation of the MAPK pathways and in mediating cytokine production following stimulation of human monocytes with MPL.
Our results provide evidence that MPL stimulation of human monocytes via TLR2 and especially TLR4 resulted in the activation of NF-κB. Furthermore, an assessment of effects of MAP kinase inhibitors on cytokine production revealed the involvement of the MAPK ERK1/2 and p38 in TNF-α and IL-10 production. Both PD98059 and SB203580 were shown to significantly reduce MPL-induced production of TNF-α by human monocytes. However, it appears that MPL-induced IL-10 production was primarily dependent on p38 activity. These results were in agreement with the findings of others showing that both ERK1/2 and p38 mediate TNF-α production, whereas IL-10 production was mainly mediated by p38 (9, 12, 26, 30, 42).
In terms of MPL-induced IL-12 production, we were able to detect IL-12 p40 but not bioactive IL-12 p70. Ismaili et al. (19) also observed the production of IL-12 p40 following MPL stimulation of human dendritic cells; however, they also indicated the presence of IL-12 p70 by assessing the production of IFN-γ by PBMC incubated with culture supernatants from dendritic cells previously stimulated with MPL. Perhaps this assay is more sensitive for detecting bioactive IL-12 p70 than the method used in our study. In our study, levels of IFN-γ were not assessed. Although the mechanisms involved in IL-12 production are still largely unknown, it has been shown that IL-12 production can be negatively regulated by cytokines, including prostaglandin E2 and IL-10 (50). Furthermore, Kalinski et al. (21) have shown that prostaglandin E2 can selectively induce IL-12 p40 production while inhibiting IL-12 p70. It is also possible that a lack of IL-12 p70 could be due to an overproduction of IL-12 p40 compared to IL-12 p35 and the formation of p40 homodimers, which can act as an antagonist for the IL-12 p70 molecule (27).
In the present study, we have shown that MPL-induced IL-12 p40 production was inhibited by SB203580 but not by PD98059. Salmon et al. (45) reported that LPS activation of p38 MAPK is required for the production of IL-10 and proinflammatory cytokines including IL-12. Their findings suggested that activation of p38 can negatively influence IL-12 production due to an enhancing effect on IL-10 production, which limits IL-12 production. In our study, we showed that SB203580 inhibited MPL-induced production of IL-12 p40 and especially IL-10. These data are in agreement with the findings of others showing that p38 plays an important role for both IL-10 and IL-12 production (9, 11, 12, 30). Since in our studies PD98059 did not have an effect on IL-12 p40 production, and since SB203580 did not completely inhibit the production of IL-12 p40, it is possible that another pathway, such as phosphatidylinositol-3′-kinase, may be involved. In this regard, it has been shown that the induction of transcripts for several cytokines, including IL-12 p40, by TLR2 and TLR4 agonists was effectively blocked by inhibitors of p38 and phosphatidylinositol-3′-kinase, while inhibitors of the ERK1/2 MAPK pathway were less effective (42). We are currently investigating this possibility.
Since the original work by Medzhitov et al. (33) demonstrating a link between TLRs and cytokines and costimulatory molecules, considerable effort has been devoted to understanding these interactions and the resulting host immune responses. Previous studies from our laboratory (63) and those of others (10, 19, 34) have shown that MPL induces up-regulation of the expression of the costimulatory molecules CD80 and/or CD86 in the mouse and human systems. In the present study, we demonstrated the involvement of TLR2 and TLR4 in the up-regulation of CD80 and CD86 expression following stimulation of human monocytes with MPL. The ability of the anti-TLR2 antibody to inhibit MPL-induced expression of CD80 was slightly greater than that seen with the anti-TLR4 antibody, suggesting a greater dependency of TLR2 in this process. Interestingly, it has been suggested that TLR2 agonists induce conditions that favor Th2 development, whereas TLR4 agonists promote the induction of cytokines associated with Th1 responses (42). Studies have shown an association between CD80 and a Th1 phenotype and have suggested a role of CD80 and CD86 in the polarization of T-helper cells in immune responses (23, 25, 55). However, other studies have failed to observe any differential effects on T-helper cell differentiation by these costimulatory molecules (5, 24). Cytokines have also been implicated in the stimulation of CD80 and CD86 expression. Ranheim and Kipps (40) demonstrated the induction of CD80 by TNF-α, while Lim et al. (26) showed the down-regulation of CD86 by IL-10. In the present study, we observed an up-regulation of both CD80 and CD86 expression, as well as in IL-10 production, which was similar to findings by Todate et al. (56). Furthermore, we have shown that MPL-induced IL-10 production was primarily mediated via TLR4 and the activation of p38 MAPK.
The discovery of mammalian TLRs has provided us with more information regarding the interactions between the innate and adaptive immune systems. However, it is becoming clear that TLRs are not functionally equivalent, since findings in one system may differ from those in another depending on the specific elements involved in each event. Further studies are needed to determine the precise interactions between TLRs, costimulatory molecules, and T-cell subsets and their cytokines.
In the present study, we have demonstrated that MPL differentially uses TLR2 and TLR4 for the induction of TNF-α, IL-10, and IL-12 by human monocytes and PBMC, thus indicating that MPL can induce Th1- and Th2-type cytokines via TLRs. The differential use of TLR2 and TLR4 by MPL was reflected in the activation of different MAPKs. TLR2 and TLR4 were used for ERK1/2 phosphorylation, whereas TLR4 was the main receptor involved in the phosphorylation of p38 and subsequent cytokine production. The ERK1/2 pathway was shown to exhibit differential effects on TNF-α and IL-12 p40 production, whereas activation of p38 was involved in IL-10 and IL-12 p40 production. Finally, TLR2 and TLR4 were shown to be involved in the up-regulation of CD86 and especially CD80 expression on human monocytes stimulated with MPL. These findings help define the cellular mechanisms by which MPL induces host cell activation and adjuvant activity and should be helpful in the design of vaccines.
Acknowledgments
We thank J. Terry Ulrich and Jory Baldridge for their helpful discussions on MPL. We also acknowledge Corixa Corporation for providing the MPL and Genentech Corporation for supplying the mouse anti-human TLR2 MAb used in this study.
This work was supported by Public Health Service grants DE 14215, DE 09081, DE 08182, and HL 07553 from the National Institutes of Health.
Editor: J. D. Clements
REFERENCES
- 1.Azuma, M., H. Yssel, J. H. Phillips, H. Spits, and L. L. Lanier. 1993. Functional expression of B7/BB1 on activated lymphocytes. J. Exp. Med. 177:845-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldridge, J. R., Y. Yorgensen, J. R. Ward, and J. T. Ulrich. 2000. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine 18:2416-2425. [DOI] [PubMed] [Google Scholar]
- 3.Carl, V. S., K. Brown-Steinke, M. J. Nicklin, and M. F. Smith, Jr. 2002. Toll-like receptor 2 and 4 (TLR2 and TLR4) agonists differentially regulate secretory interleukin-1 receptor antagonist gene expression in macrophages. J. Biol. Chem. 277:17448-17456. [DOI] [PubMed] [Google Scholar]
- 4.Carozzi, S., M. Salit, A. Cantaluppi, M. G. Nasini, S. Barocci, S. Canterella, and S. Lamperi. 1989. Effect of monophosphoryl lipid A on the in vitro function of peritoneal leukocytes from uremic patients on continuous ambulatory peritoneal dialysis. J. Clin. Microbiol. 27:1748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, T. T., C. Jabs, R. A. Sobel, V. K. Kuchroo, and A. H. Sharpe. 1999. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both the induction and effector phases of experimental autoimmune encephalomyelitis. J. Exp. Med. 190:733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary, P. M., C. Ferguson, V. Nguyen, O. Nguyen, H. F. Massa, M. Eby, A. Jasmin, B. J. Trask, L. Hood, and P. S. Nelson. 1998. Cloning and characterization of two Toll/interleukin-1 receptor-like genes TIL3 and TIL4: evidence for a multi-gene receptor family in humans. Blood 91:4020-4027. [PubMed] [Google Scholar]
- 7.Childers, N. K., K. L. Miller, G. Tong, J. C. Llarena, T. Greenway, J. T. Ulrich, and S. M. Michalek. 2000. Adjuvant activity of monophosphoryl lipid A for nasal and oral immunization with soluble or liposome-associated antigen. Infect. Immun. 68:5509-5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang, T. H., and R. J. Ulevitch. 2000. Cloning and characterization of a subfamily of human Toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur. Cytokine Netw. 11:372-378. [PubMed] [Google Scholar]
- 9.De, A. K., K. M. Kodys, B. S. Yeh, and C. Miller-Graziano. 2000. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J. Immunol. 165:3951-3958. [DOI] [PubMed] [Google Scholar]
- 10.De Becker, G., V. Moulin, B. Pajak, C. Bruck, M. Francotte, C. Thiriart, J. Urbain, and M. Moser. 2000. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int. Immunol. 12:807-815. [DOI] [PubMed] [Google Scholar]
- 11.Feng, G. J., H. S. Goodridge, M. M. Harnett, X. Q. Wei, A. V. Nikolaev, A. P. Higson, and F. Y. Liew. 1999. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 163:6403-6412. [PubMed] [Google Scholar]
- 12.Foey, A. D., S. L. Parry, L. M. Williams, M. Feldmann, B. M. Foxwell, and F. M. Brennan. 1998. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J. Immunol. 160:920-928. [PubMed] [Google Scholar]
- 13.Freeman, G. J., V. A. Boussiotis, A. Anumanthan, G. M. Bernstein, X. Y. Ke, P. D. Rennert, G. S. Gray, J. G. Gribbon, and L. M. Nadler. 1995. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity 2:523-532. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, R. K., and G. R. Siber. 1995. Adjuvants for human vaccines—current status, problems, and future prospects. Vaccine 13:1263-1276. [DOI] [PubMed] [Google Scholar]
- 15.Hajishengallis, G., M. Martin, H. T. Sojar, A. Sharma, R. E. Schifferle, E. DeNardin, M. W. Russell, and R. J. Genco. 2002. Dependence of bacterial protein adhesins on Toll-like receptors for proinflammatory cytokine induction. Clin. Diagn. Lab. Immunol. 9:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henricson, B. E., C. L. Manthey, P. Y. Perera, T. A. Hamilton, and S. N. Vogel. 1993. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect. Immun. 61:2325-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165:18-22. [DOI] [PubMed] [Google Scholar]
- 18.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2001. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ismaili, J., J. Rannesson, E. Aksoy, J. Vekemans, B. Vincart, Z. Amraoui, F. V. Laethem, M. Goldman, and P. M. Dubois. 2002. Monophosphoryl lipid A activates both human dendritic cells and T cells. J. Immunol. 168:926-932. [DOI] [PubMed] [Google Scholar]
- 20.Jones, B. W., K. A. Heldwein, T. K. Means, J. J. Saukkonen, and M. J. Fenton. 2001. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann. Rheum. Dis. 60:iii6-iii12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalinski, P., P. L. Vieira, J. H. N. Schuitemaker, E. C de Jong, and M. L. Kapsenberg. 2001. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97:3466-3469. [DOI] [PubMed] [Google Scholar]
- 22.Kirikae, T., T. Nitta, F. Kirikae, Y. Suda, S. Kusumoto, N. Qureshi, and M. Nakano. 1999. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect. Immun. 67:1736-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavi, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 development pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 24.Lanier, L. L., S. O'Fallon, C. Somoza, J. H. Phillips, P. S. Linsley, K. Okumura, D. Ito, and M. Azuma. 1995. CD80 (B7) and CD86 (70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 154:97-105. [PubMed] [Google Scholar]
- 25.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 26.Lim, W., W. Ma, K. Gee, S. Aucoin, D. Nandan, F. Diaz-Mitoma, M. Kozlowski, and A. Kumar. 2002. Distinct role of p38 and c-Jun N-terminal kinases in IL-10 dependent and IL-10 independent regulation of the costimulatory molecule B7.2 in lipopolysaccharide-stimulated human monocytic cells. J. Immunol. 168:1759-1769. [DOI] [PubMed] [Google Scholar]
- 27.Ling, P., M. K. Gately, U. Gubler, A. S. Stern, P. Lin, K. Hollfelder, C. Su, E. P. Yu-Ching, and J. Hakimi. 1995. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 154:116-127. [PubMed] [Google Scholar]
- 28.Linsley, P. S., J. L. Greene, P. Tan, J. Bradshaw, J. A. Ledbetter, C. Anasetti, and N. K. Damle. 1992. Coexpression and functional cooperation of CTLA-4 and CD28 on activated lymphocytes. J. Exp. Med. 176:1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linsley, P. S., and J. A. Ledbetter. 1993. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 11:191-212. [DOI] [PubMed] [Google Scholar]
- 30.Ma, W., W. Lim, K. Gee, S. Aucoin, D. Nandan, M. Kozlowski, F. Diaz-Mitoma, and A. Kumar. 2001. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 276:13664-13674. [DOI] [PubMed] [Google Scholar]
- 31.Martin, M., J. Katz, S. N. Vogel, and S. M. Michalek. 2001. Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. J. Immunol. 167:5278-5285. [DOI] [PubMed] [Google Scholar]
- 32.Means, T. K., R. P. Pavlovich, D. Roca, M. W. Vermeulen, and M. J. Fenton. 2000. Activation of TNF-α transcription utilizes distinct MAP kinase pathways in different macrophages populations. J. Leukoc. Biol. 67:885-893. [DOI] [PubMed] [Google Scholar]
- 33.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 34.Moore, A., L. McCarthy, and K. H. G. Mills. 1999. The adjuvant combination of monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine 17:2517-2527. [DOI] [PubMed] [Google Scholar]
- 35.Myers, K., P. Beining, M. Betts, H. Snippe, J. Inman, and B. Golding. 1995. Monophosphoryl lipid A behaves as a T-cell-independent type I carrier for hapten-specific antibody responses in mice. Infect. Immun. 63:168-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabehi, L., T. Irinopoulou, B. Cholley, N. Haeffner-Cavaillon, and M.-P. Carreno. 2001. Gram-positive and Gram-negative bacteria do not trigger monocytic cytokine production through similar intracellular pathways. Infect. Immun. 69:4590-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranheim, E. A., and T. J. Kipps. 1995. Tumor necrosis factor-α facilitates induction of CD80 (B7-1) and CD54 on human B cells by activated T cells: complex regulation by IL-4, IL-10 and CD40L. Cell. Immunol. 161:226-235. [DOI] [PubMed] [Google Scholar]
- 41.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 42.Reiling, N., A. Blumenthal, H. D. Flad, M. Ernst, and S. Ehlers. 2001. Mycobacteria-induced TNF-alpha and IL-10 formation by human macrophages is differentially regulated at the level of mitogen-activated protein kinase activity. J. Immunol. 167:3339-3345. [DOI] [PubMed] [Google Scholar]
- 43.Rock, F. L., G. Hardiman, J. C. Timans, R. Kastelein, and F. J. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salkowski, C. A., G. R. Detore, and S. N. Vogel. 1997. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and IL-10 mRNA production in mouse macrophages. Infect. Immun. 65:3239-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmon, R. A., X. Guo, H.-S. Teh, and J. W. Schrader. 2001. The p38 mitogen-activated protein kinase can have opposing roles in the antigen-dependent or endotoxin-stimulated production of IL-12 and IFN-γ. Eur. J. Immunol. 31:3218-3227. [DOI] [PubMed] [Google Scholar]
- 46.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 47.Sebastiani, G., G. Leveque, L. Lariviere, L. Laroche, E. Skamene, P. Gros, and D. Malo. 2000. Cloning and characterization of the murine Toll-like receptor 5 (Tlr5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics 64:230-240. [DOI] [PubMed] [Google Scholar]
- 48.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 50.Snijders, A., C. M. Hilkens, T. C. van der Pouw Kraan, M. Engel, L. A. Aarden, and M. L. Kapsenberg. 1996. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J. Immunol. 156:1207-1212. [PubMed] [Google Scholar]
- 51.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi, O., T. Kawai, H. Sanjo, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, K. Takeda, and S. Akira. 1999. TLR6: a novel member of an expanding toll-like receptor family. Gene 231:59-65. [DOI] [PubMed] [Google Scholar]
- 53.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165:5780-5787. [DOI] [PubMed] [Google Scholar]
- 54.Thoma-Uszynski, S., S. M. Kiertscher, M. T. Ochoa, D. A. Bouis, M. V. Norgard, K. Miyake, P. J. Godowski, M. D. Roth, and R. L. Modlin. 2000. Activation of Toll-like receptor 2 on human dendritic cells triggers induction of IL-12 but not IL-10. J. Immunol. 165:3804-3810. [DOI] [PubMed] [Google Scholar]
- 55.Thompson, C. B. 1995. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell 81:979-982. [DOI] [PubMed] [Google Scholar]
- 56.Todate, A., T. Suda, H. Kuwata, K. Chida, and H. Nakamura. 2001. Muramyl dipeptide-Lys stimulates the function of human dendritic cells. J. Leukoc. Biol. 70:723-729. [PubMed] [Google Scholar]
- 57.Tomai, M. A., L. E. Solem, A. G. Johnson, and E. Ribi. 1987. The adjuvant properties of monophosphoryl lipid A in hyporesponsive and aging mice. J. Biol. Response Mod. 6:99-107. [PubMed] [Google Scholar]
- 58.Ulevitch, R., and P. Tobias. 1999. Recognition of Gram-negative bacteria and endotoxin by the innate immune system. Curr. Opinion Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 59.Ulrich, J. T., and K. R. Myers. 1995. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions, p. 465-524. In M. F. Powell and M. J. Newman (ed.), Vaccine design: the subunit and adjuvant approach. Plenum Press, New York, N.Y. [PubMed]
- 60.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vabulas, R. M., P. Ahmad-Nejad, C. da Costa, T. Miethke, C. J. Kirschning, H. Hacker, and H. Wagner. 2001. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 276:31332-31339. [DOI] [PubMed] [Google Scholar]
- 62.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. S. Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 63.Yang, Q.-B., M. Martin, S. M. Michalek, and J. Katz. 2002. Mechanisms of monophosphoryl lipid A augmentation of host responses to recombinant HagB from Porphyromonas gingivalis. Infect. Immun. 70:3557-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]