Abstract
This study was conducted to examine the anti-inflammatory mechanisms of dexamethasone during leukocyte recruitment and expression of the CXC chemokines macrophage inflammatory protein 2 (MIP-2) (CXCL2) and cytokine-induced neutrophil chemoattractant (KC) (CXCL1) in staphylococcal enterotoxin B (SEB)-induced acute inflammation. To do this, SEB was injected into murine air pouches with or without dexamethasone pretreatment for 2 h. SEB induced infiltration of leukocytes in a dose- and time-dependent manner, with the maximal response observed after 4 h of treatment with 10 μg of SEB. The recruited leukocytes comprised more than 77% neutrophils. Moreover, SEB challenge (10 μg) provoked time-dependent secretion of CXC chemokines, which peaked after 1 h. Local administration of antibodies against MIP-2 and KC significantly reduced SEB-triggered neutrophil accumulation by 38 and 59%, respectively. Dexamethasone (10 mg kg−1) significantly decreased neutrophil recruitment by 82% and reduced secretion of MIP-2 and KC by 89 and 85%, respectively, in response to SEB challenge. Our data demonstrate that dexamethasone potently inhibits neutrophil recruitment in SEB-induced inflammation. Moreover, we provide evidence that MIP-2 and KC are key mediators in the neutrophil response to SEB. Furthermore, our findings demonstrate that dexamethasone attenuates SEB-induced expression of MIP-2 and KC. Thus, this study elucidates important signaling pathways of SEB-induced neutrophil recruitment and anti-inflammatory mechanisms of action of dexamethasone.
Bacterial toxemia is frequently associated with gram-positive infection and sepsis, leading to severe shock and multiple-organ failure. Staphylococcus aureus is a major human pathogen that produces a wide array of toxins, including staphylococcal enterotoxin A (SEA) and SEB, which are referred to as superantigens. SEA and SEB bind to the T-cell receptor, as well as the major histocompatibility complex class II molecule, and thus are capable of inducing excessive production of inflammatory mediators and leukocyte accumulation (6, 8, 44). Nevertheless, SEA and SEB exhibit a level of structural homology of only approximately 31% (2), and their biological effects are different. For example, a previous study showed that the leukocyte response to SEA is markedly stronger than that elicited by SEB (37).
Leukocyte recruitment from the microvasculature to sites of inflammation is a sequential process and includes rolling, activation, firm adhesion, and, finally, transmigration through the vessel wall (5, 17). There is an accumulating body of evidence demonstrating that leukocyte trafficking in inflammation is controlled by chemokines (4, 10). Due to structural differences in their amino acid sequences, the chemoattractive cytokines are classified into two main families (28). In mice, the best-known CXC chemokines are macrophage inflammatory protein 2 (MIP-2) (CXCL2) (35) and cytokine-induced neutrophil chemoattractant (KC) (CXCL1) (25, 28), both of which are potent stimulators of neutrophil activation and tissue infiltration (10, 28, 41). However, the detailed role of chemokines in the leukocyte response triggered by SEB remains to be clarified.
Glucocorticoids, including dexamethasone, are potent anti-inflammatory agents with a broad spectrum of mechanisms of action. We and other workers have previously demonstrated that treatment with dexamethasone reduces the expression and function of chemokines in response to pro-inflammatory cytokines and thus attenuates leukocyte adhesion and recruitment (16, 32, 34, 42). On the one hand, production of inflammatory mediators and recruitment of leukocytes in response to a gram-negative bacterial toxin, such as lipopolysaccharide, have been extensively studied and, in most reports, have been shown to be sensitive to glucocorticoid treatment (30, 40, 41). On the other hand, the anti-inflammatory mechanisms of action of glucocorticoids in SEB-induced acute inflammation remain to be defined.
Based on the considerations described above, the purpose of this study was to define the role of CXC chemokines in SEB-induced leukocyte recruitment. Moreover, we wanted to clarify the inhibitory mechanisms of action of glucocorticoids in the acute inflammatory response to SEB.
MATERIALS AND METHODS
Animals
Experiments were performed by using male C57BL6 mice weighing between 20 and 25 g. The animals were maintained by using a cycle consisting of 12 h of darkness and 12 h of light and had free access to standard pellet food and tap water ad libitum. Anesthesia was achieved by intraperitoneal injection of 7.5 mg of ketamine hydrochloride and 2.5 mg of xylazine per 100 g of body weight. Dexamethasone was given intraperitoneally (1 or 10 mg kg of body weight−1; Decadron; MSD, Haarlem, The Netherlands) 2 h before local treatment with SEB. After the experiment, a blood sample was taken from the tail artery of each mouse for subsequent leukocyte differential counting. The local ethics committee approved all experiments.
Experimental protocol
At zero time and on day 3, murine air pouches were raised by injecting 2.5 ml of sterile air subcutaneously under the dorsal skin as described previously (36). On day 6, different doses of SEB (0.1 to 10 μg; Sigma Chemical Co., St. Louis, Mo.) in 1 ml of phosphate-buffered saline (PBS) were injected into the air pouch cavities. The SEB had been tested for contamination with SEA and contained less than 0.3% SEA (Sigma). Exudates were harvested under anesthesia at different times after challenge by washing the subcutaneous cavities three times with ice-cold PBS (1, 2, and 2 ml) containing 3 mmol of EDTA. Harvested pouch fluids were centrifuged at 3,000 × g (4°C) for 10 min. The supernatants were stored at −20°C for subsequent enzyme-linked immunosorbent assay (ELISA) analysis, and the cell pellets were resuspended in 0.5 ml of PBS. The total and differential numbers of recruited leukocytes per air pouch were calculated after staining with Türk's solution (0.01% [wt/vol] crystal violet in 3% acetic acid) in a Bürker chamber. Leukocytes were identified as polymorphonuclear leukocytes and monomorphonuclear leukocytes (MNLs). The importance of CXC chemokines and tumor necrosis factor alpha (TNF-α) in SEB-induced subcutaneous leukocyte accumulation was evaluated in separate air pouch experiments by injecting monoclonal antibodies directed against murine MIP-2 (10 μg; rat immunoglobulin G [IgG]; clone 40605.111; R&D Systems Europe, Ltd., Abingdon, Oxon, United Kingdom), KC (10 μg; rat IgG; clone 48415.111; R&D Systems Europe), or TNF-α (20 μg; rat IgG clone MP6-XT22; Pharmingen, San Diego, Calif.) or an isotype control antibody (10 μg; rat IgG clone 9A2; BioExpress, West Lebanon, N.H.) concomitantly with SEB into the pouches.
ELISA
Pouch exudates were centrifuged, and the supernatants were analyzed for MIP-2 and KC by using double-antibody-specific Quantikine ELISA kits with recombinant murine MIP-2 and KC as the standards (R&D Systems Europe). Additionally, supernatants were analyzed for TNF-α by using two different ELISA kits with murine TNF-α as the standard (R&D Systems Europe and Endogen, Cambridge, Mass.).
Statistical analysis
Data are expressed below as means ± standard errors of the means, and n is the number of animals per experimental group. Statistical differences between the experimental groups were calculated by using a computer software package (SigmaStat 4.0; Jandel Scientific, Munich, Germany) with one-way analysis of variance followed by the Dunnet post hoc test. Probability values less than 0.05 were considered to indicate significant differences between groups.
RESULTS
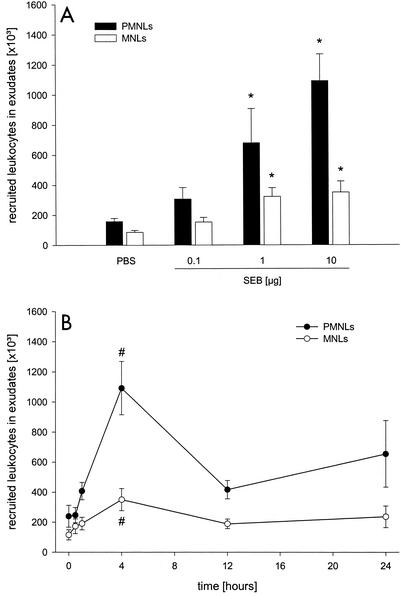
Local challenge with SEB provoked dermal infiltration of leukocytes in a time- and dose-dependent manner. The leukocyte response was maximal at 4 h after administration of 10 μg of SEB (Fig. 1) (P < 0.05 compared with the value for PBS [n = 6 to 10]; P < 0.05 compared with the value for the zero-time sample [n = 5 to 7]). Indeed, more than 77% of the leukocytes recruited after SEB treatment were polymorphonuclear leukocytes, indicating that there was a dominant neutrophilic response (Fig. 1). However, 1 or 10 μg of SEB also induced a significant, although small, increase in the number of extravascular MNLs (Fig. 1) (P < 0.05 compared with the value for PBS [n = 6 to 10]). Based on these findings, a dose of 10 μg of SEB was used for all subsequent experiments.
FIG. 1.
Dose-dependent (A) and time-dependent (B) leukocyte responses to SEB. The dose-dependent effect was studied 4 h after SEB challenge, and the time-dependent effect was evaluated by using 10 μg of SEB. Differential counts of leukocytes in harvested air pouch fluids were assessed after staining with Türk's solution by using a Bürker chamber. The data are means ± standard errors of the means. An asterisk indicates that the P value is <0.05 compared with the data obtained for PBS (n = 6 to 10); a number sign indicates that the P is <0.05 compared with the data obtained for the zero-time sample (n = 6 or 7). PMNLs, polymorphonuclear leukocytes.
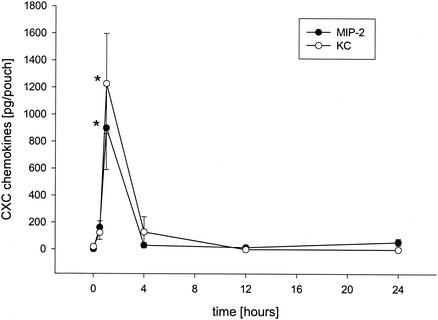
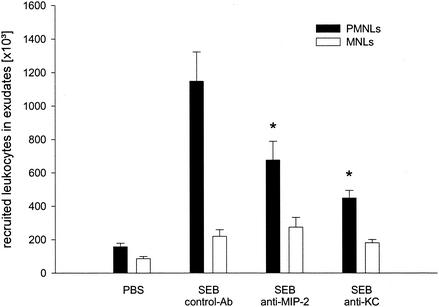
Next, we wanted to determine the role of CXC chemokines in SEB-induced inflammation. ELISA analysis of exudate supernatants revealed that the levels of MIP-2 and KC increased in a time-dependent manner in response to SEB challenge (10 μg). In fact, the maximal levels of MIP-2 and KC were observed 1 h after injection of SEB (Fig. 2) (P < 0.05 compared with the value for the zero-time sample [n = 5 to 7]), and after this the levels decreased rapidly to the baseline levels (Fig. 2). Moreover, neutralization of chemokine function by using monoclonal antibodies directed against MIP-2 and KC significantly reduced SEB-induced dermal neutrophil recruitment by 38 and 59%, respectively (Fig. 3) (P < 0.05 compared with the value for SEB alone [n = 7 to 9]). However, combined injection of monoclonal antibodies directed against MIP-2 and KC had no additional inhibitory effect on SEB-induced accumulation of neutrophils (data not shown). Importantly, administration of anti-MIP-2 and anti-KC did not decrease the number of circulating neutrophils (Table 1).
FIG. 2.
Time-dependent expression of CXC chemokines in air pouch exudates after challenge with 10 μg of SEB. Levels of MIP-2 and KC were determined by an ELISA. The data are means ± standard errors of the means. An asterisk indicates that the P value is <0.05 compared with the data obtained for the zero-time sample (n = 6 or 7).
FIG. 3.
Leukocyte response to SEB after immunoneutralization of MIP-2 and KC. Control animals received an isotype-matched control monoclonal antibody. Air pouch exudates were harvested 4 h after challenge with 10 μg of SEB, and differential leukocyte counts were assessed after staining with Türk's solution by using a Bürker chamber. The data are means ± standard errors of the means. An asterisk indicates that the P value is <0.05 compared with the data for control monoclonal antibody-treated animals (n = 8 to 10). PMNLs, polymorphonuclear leukocytes.
TABLE 1.
Peripheral blood leukocyte differential counts
| Group | n | No. of cells (106 ml−1)a
|
||
|---|---|---|---|---|
| PMNLs | MNLs | Total leukocytes | ||
| PBS | 10 | 1.2 ± 0.2 | 5.1 ± 0.4 | 6.4 ± 0.5 |
| PBS + SEB | 7 | 1.4 ± 0.2 | 4.3 ± 0.5 | 5.7 ± 0.7 |
| Anti-MIP-2 antibody + SEB | 9 | 1.4 ± 0.2 | 3.6 ± 0.3 | 5.0 ± 0.4 |
| Anti-KC antibody + SEB | 8 | 0.9 ± 0.1 | 3.6 ± 0.4 | 4.5 ± 0.5 |
| Dexamethasone (1 mg kg−1) + SEB | 7 | 1.3 ± 0.5 | 1.3 ± 0.2b | 2.1 ± 0.5b |
| Dexamethasone (10 mg kg−1) + SEB | 7 | 0.8 ± 0.3 | 0.3 ± 0.1b | 0.7 ± 0.1 |
Ten micrograms of SEB in 1 ml of PBS was injected into murine air pouches, and exudates were harvested and analyzed 4 h after challenge. Negative controls received 1 ml of PBS alone. In separate experiments, antibodies against MIP-2 and KC were locally injected concomitantly with SEB. Dexamethasone was given intraperitoneally 2 h before SEB challenge. Blood samples were stained with Türk's solution, and cells were identified as polymorphonuclear leukocytes (PMNLs) or MNLs by using a hematcytometer. The data are means ± standard errors of the means.
The P value is <0.05 compared with the value for 10 μg of SEB alone.
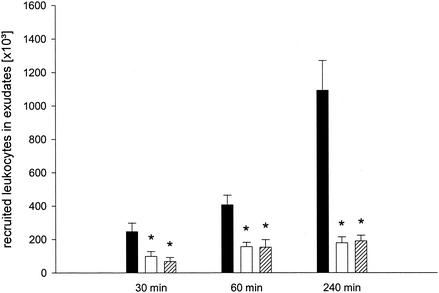
Pretreatment with 1 and 10 mg of dexamethasone kg−1 attenuated the SEB-induced neutrophil recruitment at different times (Fig. 4). Notably, at 4 h after SEB treatment, 1 and 10 mg of dexamethasone kg−1 reduced pouch infiltration of neutrophils by 84 and 82%, respectively (Fig. 4) (P < 0.05 compared with the values for PBS-treated controls [n = 6 or 7]). Treatment with dexamethasone did not reduce the systemic neutrophil counts (Table 1). However, it should be noted that both doses of dexamethasone reduced the numbers of circulating MNLs (Table 1). Accordingly, we found that 1 and 10 mg of dexamethasone kg−1 also significantly reduced SEB-induced MNL recruitment (data not shown).
FIG. 4.
Time-dependent leukocyte response to SEB. Animals received PBS (solid bars), 1 mg of dexamethasone kg−1 (open bars), or 10 mg of dexamethasone kg−1 (cross-hatched bars) 2 h prior to challenge with 10 μg of SEB. Differential counts of leukocytes were assessed in air pouch fluids harvested 30, 60, and 240 min after SEB challenge. The data are means ± standard errors of the means. An asterisk indicates that the P value is <0.05 compared with the data for the PBS-treated controls (n = 6 or 7).
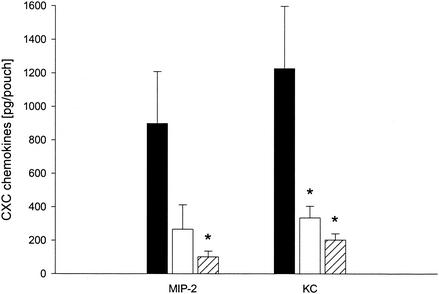
Interestingly, it was observed that SEB-induced MIP-2 and KC expression was reduced by dexamethasone; i.e., 1 and 10 mg of dexamethasone kg−1 significantly reduced the peak levels of SEB-elicited KC by more than 74 and 85%, respectively (Fig. 5) (P < 0.05 compared with the values for PBS-treated controls [n = 6]). However, SEB-triggered expression of MIP-2 was significantly inhibited (89% reduction) only by pretreatment with 10 mg of dexamethasone kg−1 (Fig. 5) (P < 0.05 compared with the values for PBS-treated controls [n = 6]).
FIG. 5.
Expression of CXC chemokines (MIP-2 and KC) in air pouch exudates 1 h after challenge with 10 μg of SEB. Animals were pretreated with PBS as controls (solid bars) or with 1 mg of dexamethasone kg−1 (open bars) or 10 mg of dexamethasone kg−1 (cross-hatched bars) 2 h prior to SEB challenge. Levels of MIP-2 and KC were determined by an ELISA. The data are means ± standard errors of the means. An asterisk indicates that the P value is <0.05 compared with the data for the PBS-treated controls (n = 7).
DISCUSSION
In this paper we describe the role of CXC chemokines and the potent anti-inflammatory mechanisms of dexamethasone in SEB-induced neutrophil recruitment. Our novel findings demonstrate that SEB provokes massive production of MIP-2 and KC and that tissue infiltration of neutrophils in response to SEB is critically dependent on the function of MIP-2 and KC. Moreover, our results demonstrate that dexamethasone is a potent inhibitor of SEB-triggered neutrophil recruitment, which may be attributable to the inhibitory effect of dexamethasone on CXC chemokine expression elicted by SEB.
Most experimental studies on sepsis have examined effector mechanisms induced by gram-negative bacteria (23, 30). However, it is important to note that gram-positive bacteria account for up to 50% of cases of severe sepsis and septic shock in clinics (33). Consequently, the molecular actions of toxins of gram-negative bacteria, such as lipopolysaccharide, are well known (39), whereas the pathogenesis of gram-positive septic shock is poorly understood. SEB is an S. aureus-derived enterotoxin, which belongs to a family of related proteins defined as superantigens due to their capacity to induce intensive T-cell activation and proliferation independent of classical antigen processing by macrophages and dendritic cells (20). Superantigens bind to major histocompatibility complex-encoded class II proteins outside the normal antigen-presenting groove and to variable regions of T-cell receptor β chains (20), causing massive release of proinflammatory mediators and, ultimately, tissue injury (15).
In order to study SEB-induced leukocyte recruitment, we utilized the murine subcutaneous air pouch model, which has been shown to be a suitable in vivo approach for defining the mechanisms and kinetics of inflammatory leukocyte recruitment, allowing not only quantification of leukocyte subtypes but also analyses of secreted substances, such as chemokines (23, 26, 31, 36). By using the air pouch model, Tessier et al. demonstrated that the leukocyte response to SEA was consistently greater than that to SEB, indicating that the neutrophil infiltration elicited by different superantigens may involve distinct and separate pathways (37). Although known to be less potent than SEA, SEB provoked marked accumulation of predominately neutrophils in the present study, which exhibited kinetics similar to those reported for SEA-induced leukocyte recruitment (37). Previous investigations have shown that neutrophil recruitment upon challenge with SEB is regulated by migration inhibitory factor and nitric oxide (3, 12). Here we show for the first time that MIP-2 and KC are key mediators of SEB-induced neutrophil accumulation in vivo, which suggests that CXC chemokines constitute an important component in superantigen-triggered acute inflammation. Notably, we observed that functional interference with MIP-2 and KC did not completely abolish neutrophil recruitment elicited by SEB, suggesting that other substances may also be involved in SEB-induced neutrophil accumulation. In fact, combined administration of anti-MIP-2 and anti-KC did not have an additional inhibitory effect on SEB-provoked infiltration of neutrophils (data not shown). One candidate molecule for mediating this residual neutrophil response is TNF-α, since it has been reported that SEA-induced neutrophil recruitment was partially reduced by treatment with an antibody directed against TNF-α (37). However, although we used two different ELISA kits, we did not detect TNF-α in air pouch exudates after challenge with SEB. Moreover, we found that SEB-induced neutrophil accumulation was intact after immunoneutralization of TNF-α (data not shown). These findings support the concept that different superantigens involve characteristic patterns of proinflammatory mediators. Thus, our data indicate that TNF-α is not expressed or does not have an important function in SEB-induced acute inflammation. This notion is in line with the findings of a previous study which showed that secretion of interleukin-8, a human homologue of murine MIP-2 and KC, is independent of TNF-α in monocytes incubated with SEB, and the author concluded that interleukin-8, but not TNF-α, may be a key mediator in SEB-induced neutrophil activation (18). Support for this concept was provided by Neumann et al., who demonstrated that SEB provokes similar levels of MIP-2 and KC in the lungs of wild-type and TNF-α receptor-deficient mice (24). It is also interesting that anti-TNF-α treatment has been suggested to be effective in gram-negative sepsis, while it is ineffective or even detrimental in gram-positive sepsis (13, 21, 25, 27). If the data are considered collectively, it may be speculated that TNF-α is not an important mediator in SEB-induced neutrophil recruitment. On the other hand, organ- and/or species-dependent differences in the inflammatory response to bacterial toxins (30) may help explain some reports of TNF-α expression in response to SEB challenge (3, 14).
Numerous studies have demonstrated that glucocorticoids are powerful tools for attenuating gram-negative bacterial toxin-induced mediator expression, leukocyte recruitment, and associated tissue injury during endotoxemia (1, 22, 41). In contrast, potential beneficial effects of corticoids on leukocyte recruitment in gram-positive bacterial toxin-induced inflammation have largely been neglected previously. Nonetheless, one study reported that SEA-provoked neutrophil migration is negatively regulated by dexamethasone treatment (9), and more recent data from an experimental study of staphylococcal endocarditis in rabbits demonstrated that there was reduced neutrophil infiltration or aortic valve destruction after combined treatment with vancomycin and dexamethasone (32). However, the anti-inflammatory mechanisms of action of dexamethasone in superantigen-induced inflammation remain elusive. In the present study, we demonstrated that dexamethasone is a powerful inhibitor of SEB-induced neutrophil recruitment via attenuated expression of the CXC chemokines MIP-2 and KC. In fact, the inhibitory effect of dexamethasone on neutrophil accumulation correlated very well with the reduction in CXC chemokine production. Thus, if the data are considered together with previous findings showing that CXC chemokine function also is sensitive to treatment with dexamethasone (30, 31, 34), it may be suggested that dexamethasone inhibits SEB-provoked neutrophil recruitment at least at two distinct levels (i.e., the expression and function of CXC chemokines). Leukocyte recruitment not only is regulated by chemokines but also is coordinated by specific expression of adhesion molecules orchestrating inflammatory cell navigation (5, 19). In this context, it is interesting that some previous studies have suggested that glucocorticoids may attenuate tissue neutrophilia via downregulated expression of adhesion molecules, such as CD18 (4, 7, 11). However, we and other workers could not confirm such findings and found that CD18 expression on neutrophils is not sensitive to dexamethasone treatment (29, 31, 38). Nevertheless, it is important to note that our data do not eliminate the possibility that adhesion molecules may also be targets of dexamethasone in SEB-provoked acute neutrophil recruitment. To clarify this, future studies will have to define adhesive pathways of neutrophil extravasation in SEB-induced inflammation.
In conclusion, in this study we documented important mechanisms of SEB-induced inflammation by elucidating a CXC chemokine-dependent pathway of neutrophil recruitment. Moreover, our data suggest that one fundamental anti-inflammatory mechanism of action of dexamethasone in attenuating SEB-induced acute inflammation is related to the inhibition of CXC chemokine expression. Thus, this study provided novel information supporting the concept that glucocorticoids may be a useful option to counter pathological inflammation during bacterial sepsis.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (grants K2000-04P-13411-01A and K2002-73-X-14273-01A), Cancerfonden (grant 4265-B99-01XAB), Crafoordska stiftelsen (grant 20010968), Blaceflors stiftelse, Einar och Inga Nilssons stiftelse, Harald och Greta Jaenssons stiftelse, Greta och Johan Kocks stiftelser, Fröken Agnes Nilssons stiftelse, Franke and Margareta Bergqvists stiftelse för främjande av cancerforskning, Nanna Svartz stiftelse, Ruth och Richard Julins stiftelse, Svenska Läkaresällskapet (grant 2001-907), Teggers stiftelse, Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, MAS fonder, Malmö University Hospital, and Lund University.
Editor: A. D. O'Brien
REFERENCES
- 1.Allcock, G. H., M. Allegra, R. J. Flower, and M. Perretti. 2001. Neutrophil accumulation induced by bacterial lipopolysaccharide: effects of dexamethasone and annexin 1. Clin. Exp. Immunol. 123:62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balaban, N., and A. Rasooly. 2000. Staphylococcal enterotoxins. Int. J. Food Microbiol. 61:1-10. [DOI] [PubMed] [Google Scholar]
- 3.Bozza, M., A. R. Satoskar, G. Lin, B. Lu, A. A. Humbles, C. Gerard, and J. R. David. 1999. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton, J. L., M. E. Kehrli, S. Kapil, and R. L. Horst. 1995. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J. Leukoc. Biol. 57:317-325. [DOI] [PubMed] [Google Scholar]
- 5.Carlos, T. M., and J. M. Harlan. 1994. Leukocyte-endothelial adhesion molecules. Blood 84:2068-2101. [PubMed] [Google Scholar]
- 6.Choi, Y. W., A. Herman, D. DiGiusto, T. Wade, P. Marrack, and J. Kappler. 1990. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature 346:471-473. [DOI] [PubMed] [Google Scholar]
- 7.Davenpeck, K. L., J. Zagorski, R. P. Schleimer, and B. S. Bochner. 1998. Lipopolysaccharide-induced leukocyte rolling and adhesion in the rat mesenteric microcirculation: regulation by glucocorticoids and role of cytokines. J. Immunol. 161:6861-6870. [PubMed] [Google Scholar]
- 8.Dellabona, P., J. Peccoud, J. Kappler, P. Marrack, C. Benoist, and D. Mathis. 1990. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell 62:1115-1121. [DOI] [PubMed] [Google Scholar]
- 9.Desouza, I. A., and G. Ribeiro-DaSilva. 1998. Neutrophil migration induced by staphylococcal enterotoxin type A in mice: a pharmacological analysis. Eur. J. Pharmacol. 363:189-195. [DOI] [PubMed] [Google Scholar]
- 10.Ebnet, K., and D. Vestweber. 1999. Molecular mechanisms that control leukocyte extravasation: the selectins and the chemokines. Histochem. Cell Biol. 112:1-23. [DOI] [PubMed] [Google Scholar]
- 11.Filep, J. G., A. Delalandre, Y. Payette, and E. Foldes-Filep. 1997. Glucocorticoid receptor regulates expression of L-selectin and CD11/CD18 on human neutrophils. Circulation 96:295-301. [DOI] [PubMed] [Google Scholar]
- 12.Franco-Penteado, C. F., I. Desouza, S. A. Teixeira, G. Ribeiro-DaSilva, G. De Nucci, and E. Antunes. 2001. Role of nitric oxide on the increased vascular permeability and neutrophil accumulation induced by staphylococcal enterotoxin B into the mouse paw. Biochem. Pharmacol. 61:1305-1311. [DOI] [PubMed] [Google Scholar]
- 13.Gawad, K. A., C. Schneider, B. Brinken, A. Huflander, B. Diederichs, D. Mack, K. Gutensohn, and J. R. Izbicki. 2001. Anti-TNF antibody treatment has no positive effect on survival in a model of pneumococcal sepsis in pigs. J. Investig. Surg. 14:291-297. [DOI] [PubMed] [Google Scholar]
- 14.Herz, U., R. Ruckert, K. Wollenhaupt, T. Tschernig, U. Neuhaus-Steinmetz, R. Pabst, and H. Renz. 1999. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness—a model for non-allergic asthma. Eur. J. Immunol. 29:1021-1031. [DOI] [PubMed] [Google Scholar]
- 15.Irwin, M. J., and N. R. Gascoigne. 1993. Interplay between superantigens and the immune system. J. Leukoc. Biol. 54:495-503. [DOI] [PubMed] [Google Scholar]
- 16.Joyce, D. A., G. Gimblett, and J. H. Steer. 2001. Targets of glucocorticoid action on TNF-alpha release by macrophages. Inflamm. Res. 50:337-340. [DOI] [PubMed] [Google Scholar]
- 17.Jung, U., K. E. Norman, K Scharffetter-Kochanek, A. L. Beaudet, and K. Ley. 1998. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J. Clin. Investig. 102:1526-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krakauer, T. 1998. Interleukin-8 production by human monocytic cells in response to staphylococcal exotoxins is direct and independent of interleukin-1 and tumor necrosis factor-alpha. J. Infect. Dis. 178:573-577. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence, M. B., and T. A. Springer. 1991. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65:859-873. [DOI] [PubMed] [Google Scholar]
- 20.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 21.Martin, R. A., A. T. Silva, and J. Cohen. 1993. Effect of anti-TNF-alpha treatment in an antibiotic treated murine model of shock due to Streptococcus pyogenes. FEMS Microbiol. Lett. 110:175-178. [DOI] [PubMed] [Google Scholar]
- 22.Messmer, U. K., G. Winkel, V. A. Briner, and J. Pfeilschifter. 1999. Glucocorticoids potently block tumour necrosis factor-alpha- and lipopolysaccharide-induced apoptotic cell death in bovine glomerular endothelial cells upstream of caspase 3 activation. Br. J. Pharmacol. 127:1633-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, A. J., G. N. Luheshi, N. J. Rothwell, and S. J. Hopkins. 1997. Local cytokine induction by LPS in the rat air pouch and its relationship to the febrile response. Am. J. Physiol. 272:R857-R861. [DOI] [PubMed]
- 24.Neumann, B., K. Emmanuilidis, M. Stadler, and B. Holzmann. 1998. Distinct functions of interferon-gamma for chemokine expression in models of acute lung inflammation. Immunology 95:512-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oquendo, P., J. Alberta, D. Z. Wen, J. L. Graycar, R. Derynck, and C. D. Stiles. 1989. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J. Biol. Chem. 264:4133-4137. [PubMed] [Google Scholar]
- 26.Perretti, M., and R. J. Flower. 1993. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J. Immunol. 150:992-999. [PubMed] [Google Scholar]
- 27.Reinhart, K., and W. Karzai. 2001. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit. Care Med. 29:121-125. [DOI] [PubMed] [Google Scholar]
- 28.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 29.Roth, J., M. Goebeler, U. Erpenstein, and C. Sorg. 1994. Differential regulation of the macrophage-specific surface antigen RM3/1 by cyclosporine, azathioprine, and dexamethasone. Transplantation 57:127-133. [DOI] [PubMed] [Google Scholar]
- 30.Rovai, L. E., H. R. Herschman, and J. B. Smith. 1998. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J. Leukoc. Biol. 64:494-502. [DOI] [PubMed] [Google Scholar]
- 31.Schramm, R., Q. Liu, and H. Thorlacius. 2000. Expression and function of MIP-2 are reduced by dexamethasone treatment in vivo. Br. J. Pharmacol. 131:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siaperas, P., A. Pefanis, D. Iliopoulos, I. Katsarolis, A. Kyroudi-Voulgari, I. Donta, P. Karayiannakos, and H. Giamarellou. 2001. Evidence of less severe aortic valve destruction after treatment of experimental staphylococcal endocarditis with vancomycin and dexamethasone. Antimicrob. Agents Chemother. 45:3531-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sriskandan, S., and J. Cohen. 1999. Gram-positive sepsis. Mechanisms and differences from gram-negative sepsis. Infect. Dis. Clin. North Am. 13:397-412. [DOI] [PubMed] [Google Scholar]
- 34.Tailor, A., A. Tomlinson, A. Salas, J. Panes, D. N. Granger, R. J. Flower, and M. Perretti. 1999. Dexamethasone inhibition of leucocyte adhesion to rat mesenteric postcapillary venules: role of intercellular adhesion molecule 1 and KC. Gut 45:705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tekamp-Olson, P., C. Gallegos, D. Bauer, J. McClain, B. Sherry, M. Fabre, S. van Deventer, and A. Cerami. 1990. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J. Exp. Med. 172:911-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tessier, P. A., P. H. Naccache, I. Clark-Lewis, R. P. Gladue, K. S. Neote, and S. R. McColl. 1997. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J. Immunol. 159:3595-3602. [PubMed] [Google Scholar]
- 37.Tessier, P. A., P. H. Naccache, K. R. Diener, R. P. Gladue, K. S. Neote, I. Clark-Lewis, and S. R. McColl. 1998. Induction of acute inflammation in vivo by staphylococcal superantigens. II. Critical role for chemokines, ICAM-1, and TNF-alpha. J. Immunol. 161:1204-1211. [PubMed] [Google Scholar]
- 38.Trowald-Wigh, G., L. Hakansson, A. Johannisson, and L. E. Edqvist. 1998. The effect of prednisolone on canine neutrophil function: in vivo and in vitro studies. Acta Vet. Scand. 39:201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner, J. G., and R. A. Roth. 1999. Neutrophil migration during endotoxemia. J. Leukoc. Biol. 66:10-24. [DOI] [PubMed] [Google Scholar]
- 40.Wu, C. C., J. D. Croxtall, M. Perretti, C. E. Bryant, C. Thiemermann, R. J. Flower, and J. R. Vane. 1995. Lipocortin 1 mediates the inhibition by dexamethasone of the induction by endotoxin of nitric oxide synthase in the rat. Proc. Natl. Acad. Sci. USA 92:3473-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi, E. S., D. G. Remick, Y. Lim, W. Tang, C. E. Nadzienko, A. Bedoya, S. Yin, and T. R. Ulich. 1996. The intratracheal administration of endotoxin. X. Dexamethasone downregulates neutrophil emigration and cytokine expression in vivo. Inflammation 20:165-175. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, X. W., G. Hedlund, P. Borgstrom, K. E. Arfors, and H. Thorlacius. 2000. Linomide abolishes leukocyte adhesion and extravascular recruitment induced by tumor necrosis factor alpha in vivo. J. Leukoc. Biol. 68:621-626. [PubMed] [Google Scholar]
- 43.Zlotnik, A., J. Morales, and J. A. Hedrick. 1999. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 19:1-47. [PubMed] [Google Scholar]
- 44.Zumla, A. 1992. Superantigens, T cells, and microbes. Clin. Infect. Dis. 15:313-320. [DOI] [PubMed] [Google Scholar]