Abstract
Helicobacter pylori is able to utilize several lectin-like, protein-carbohydrate interactions for binding to mucins, cell surfaces, and extracellular matrix proteins. As determined by hemagglutination assays and binding of radiolabeled bacteria to glycosphingolipids on thin-layer chromatograms, strains of gastric helicobacters and enterohepatic helicobacters, including Helicobacter canis, Helicobacter hepaticus, and Helicobacter bilis, also demonstrated evidence for the presence of lectin-hemagglutinin adhesins. In addition, in H. hepaticus and H. bilis, binding may be sialic acid dependent. The presence or absence and differences in the levels of activity of lectin adhesins may reflect the species' ecological niche.
The helicobacter genus includes more than 20 adequately characterized species to date (23). These species have been isolated from a number of hosts, including primates, pigs, cats, dogs, poultry, and rodents (23; http://www.infek.lu.se/bakt /english/helicobacter.html). Moreover, within their hosts, Helicobacter spp. have been identified from both the gastric and enterohepatic niches of the gastrointestinal tract, where they have been associated with a wide spectrum of clinical outcomes (5, 21). Helicobacter pylori is the most widely studied species of the genus and is associated with gastric pathology (9). The majority of H. pylori cells remain free swimming within the gastric mucosa. However, a minority of the population attach to the gastric epithelial surface and these play a vital role in the maintenance of infection (13). In addition, the population of H. pylori cells which attach may sporadically enter and survive within gastric epithelial cells and play a role in therapeutic failure (1, 15). Studies of many mucosal-surface-associated pathogens, including H. pylori, have shown that they utilize lectin-like adhesins on their surfaces to bind to glycoconjugate receptors in the mucin layer and on the epithelial surface (17, 27). Such lectins have been studied exhaustively in the case of H. pylori (16-18, 26). In particular, the bacterium has the noted ability to attach to both Lewis (Le)b and sialyl-dimeric-Lex antigens, which may be extremely relevant in the maintenance of a chronic infection (10, 20). However, little or no data exist regarding the potential adhesion strategies of other gastric and enterohepatic helicobacters. The aim of our study was to define lectin interactions for a range of gastric and enterohepatic helicobacters.
H. pylori strains (all human isolates) from the Culture Collection of the University of Gothenberg (CCUG), namely, CCUG 17874 and CCUG 17875 as well as the clinical isolate 119/95 from Lund University Hospital, were used. Gastric species examined in the present study included Helicobacter mustelae ferret isolates from the National Collection of Type Cultures and the CCUG, including NCTC 12198/CCUG 25175 (equivalent strains), CCUG 23950, and CCUG 23951. “Helicobacter suncus” was originally isolated from the Japanese mouse shrew (6), and Helicobacter felis CCUG 28539 was isolated from a cat. Also, a human gastric clinical isolate initially identified as “Helicobacter heilmannii” was used (2). “H. heilmannii” organisms are a morphometry-based group and may contain a number of taxa, including Helicobacter bizzozeronii. Subsequent to the performance of our study, it was reported that the isolate used in the present study has been more definitively identified as H. bizzozeronii R-53 (11), and this designation is used here. A similar situation arises with the enterohepatic grouping of strains designated “Helicobacter rappini”/“Flexispira rappini,” which can include many taxa. The strain in the present study is a canine isolate from the University of Helsinki and is referred to as Helicobacter sp. flexispira taxon KT0201 here. Other enterohepatic helicobacters from various hosts were purchased from the CCUG, including Helicobacter canis CCUG 33835 (dog isolate), Helicobacter bilis CCUG 38995 (mouse isolate), Helicobacter hepaticus CCUG 33637 (mouse isolate), Helicobacter fennelliae CCUG 18820 (human isolate), and Helicobacter pullorum strains CCUG 33837 (chicken isolate), CCUG 33839 (human isolate), and NCTC 12827 (human isolate).
All helicobacters were tested in a hemagglutination (HA) assay by mixing equal volumes (30 μl) of a bacterial suspension (109 CFU ml−1) with a 0.75% (vol/vol) solution of washed erythrocytes in phosphate-buffered saline (PBS; pH 7.2). We used erythrocytes from horses, sheep, goats, rabbits, guinea pigs, hens (State Veterinary Institute, Uppsala, Sweden), and humans of blood types O and AB (blood bank, University Hospital Lund) (18). Alternatively, erythrocytes were mixed with PBS as a negative control. In cases of positive binding, the HA titer was determined, with 1 HA unit being defined as the minimum amount of bacterial cells required to cause complete HA.
An HA inhibition assay utilized glycoproteins (10 mg ml−1), including fetuin, α1-acid glycoprotein (orosomucoid), and asialofetuin (Sigma Chemical Co., St. Louis, Mo.), with the first two expressing terminal sialic acids which are important in the lectin activities of certain strains of H. pylori. Other terminal monosaccharide residues predominate in the case of asialofetuin, including galactose, mannose, and N-acetylgalactosamine. In addition, gastric mucin fractions of human and porcine origins were also used in HA inhibition as described previously (18). Gastric mucins are rich in fucose, galactose, N-acetylgalactosamine, N-acetylglucosamine, and terminal sialic acid. All assays were carried out at least in triplicate.
Two broad HA profiles were observed (Table 1). H. pylori 119/95, H. mustelae NCTC 12198, H. bizzozeronii, “H. suncus,” and H. bilis CCUG 38995 caused HA for all or the majority of erythrocytes tested (five to seven of seven kinds). Variable HA activity was noted for H. hepaticus, although in the majority of tests, no binding was observed. In contrast, most enterohepatic helicobacters and H. felis CCUG 28539 did not cause the HA of any erythrocytes tested, with the exception of rabbit blood cells. H. mustelae, H. pylori, H. bizzozeronii, and H. bilis were analyzed in more detail for their precise HA titers (Table 2). The HA of rabbit erythrocytes could be largely inhibited by orosomucoid. However, human and porcine mucin preparations proved to be poor inhibitors for HA. In most cases, asialofetuin proved to be more effective than fetuin in inhibiting HA, except with H. bilis CCUG 38995, for which the converse was true (data not shown). Subsequently, both fetuin and asialofetuin were covalently coupled to carboxylate-modified latex particles (diameter, 0.8 μm; Seradyn, Indianapolis, Ind.) as described previously (17). Bacterial suspensions (109 CFU ml−1) were mixed with equal volumes (10 μl) of coupled latex beads on a glass slide and tilted for 30 s, and results were assessed visually. Both gastric and enterohepatic helicobacters demonstrated reactivity with fetuin- and asialofetuin-coated beads. However, H. felis CCUG 28539 failed to react with fetuin or asialofetuin and reactions with all H. pullorum isolates were weak. Furthermore, H. bilis CCUG 38995 agglutinated more strongly with these beads than any other enterohepatic helicobacter.
TABLE 1.
HA patterns of gastric and enterohepatic helciobacters with native erythrocytes
| Bacterial type and species | Reactiona with native erythrocytes from:
|
||||||
|---|---|---|---|---|---|---|---|
| Humanb | Horse | Sheep | Goat | Guinea pig | Hen | Rabbit | |
| Gastric | |||||||
| H. mustelae NCTC12198 | + | + | + | + | + | + | + |
| H. pylori CCUG 17874c | + | + | + | ND | + | ND | + |
| H. pylori 119/95 | + | + | + | + | + | + | + |
| H. bizzozeronii R53 | + | + | + | + | + | + | + |
| “H. suncus” | + | + | − | + | + | + | + |
| H. felis CCUG 28539 | − | − | − | − | − | − | + |
| Enterohepatic | |||||||
| H. canis CCUG 33835 | − | − | − | − | − | − | + |
| H. bilis CCUG 38995 | + | + | + | − | + | − | + |
| H. hepaticus CCUG 33637 | ± | ± | ± | − | ± | − | + |
| H. fennelliae CCUG 18820 | − | − | − | − | − | − | + |
| Helicobacter sp. flexispira taxon KT0201 | − | − | − | − | − | − | + |
| H. pullorum NCTC 12827 | − | − | − | − | − | − | + |
| H. pullorum CCUG 33837 | − | − | − | − | − | − | + |
| H. pullorum CCUG 33839 | − | − | − | − | − | − | + |
HA reaction in U-shaped wells, determined visually. +, a carpet of erythrocytes; −, a dot of cells; ±, occasional positive results for binding; nd, not determined.
Results for AB and O blood groups were similar.
See reference 18.
TABLE 2.
HA titers for HA-positive species in native and neuraminidase-treated goat and rabbit erythrocytes
| Bacterial type and species | HA titera
|
|||
|---|---|---|---|---|
| Goat erythrocytes
|
Rabbit erythrocytes
|
|||
| Native | Neuraminidase treated | Native | Neuraminidase treated | |
| Gastric | ||||
| H. mustelae NCTC 12198 | 2 | 2 | 8 | >1,024 |
| H. pylori 119/95 | 8 | 4 | 8 | 1 |
| H. bizzozeronii R-53 | 1 | 1 | >1,024 | >1,024 |
| Enterohepatic H. bilis CCUG 38995 | 8 | 2 | 1 | 2 |
HA titers were determined with 1 HA unit being defined as the minimum amount of bacterial cells required to cause complete HA.
Erythrocytes were treated with neuraminidase in order to remove terminal N-acetylneuraminic (sialic) acid and expose other potential carbohydrate binding sites (8, 16). The principal effect of neuraminidase treatment of goat erythrocytes was a decrease in the titers of H. pylori 119/95 and H. bilis CCUG 38995 (Table 2). Neuraminidase treatment of rabbit erythrocytes had a strain-specific effect on titer.
HA was also decreased or eliminated following proteolytic treatments of the bacteria using either proteinase K (1 mg ml−1, 65°C, 1 h), pronase E (1 mg ml−1, 37°C, 1 h), or heat treatment (100°C for 5 min). In order to determine the localization of the factors involved in positive HA results, an acid glycine extract from the bacteria was tested. Acid-glycine extracts have been routinely used to characterize the cell surface of H. pylori, and this fraction contains predominantly surface-associated proteins with minor amounts of cytoplasmic proteins and lipopolysaccharide. Proteins were mildly extracted using a 0.2 M acidic glycine (pH 2.2) wash for 15 min at 20°C (19). The use of acid-glycine extracts in both the HA and the particle agglutination assay yielded results identical to those for whole bacterial cells.
The binding of Helicobacter spp. to both acidic and nonacidic fractions of glycosphingolipids was subsequently assessed. Glycosphingolipids were isolated by standard procedures (12). The identities of the purified glycosphingolipids were confirmed by mass spectrometry (22), proton nuclear magnetic resonance spectroscopy (14), and degradation studies (24, 29).
Mixtures of glycosphingolipids (40 μg/lane) or pure compounds (2 μg/lane) were subsequently separated by thin-layer chromatography (TLC) on glass- or aluminum-backed Silica Gel 60 high-performance TLC plates (Merck, Darmstadt, Germany), with chloroform-methanol-water (60:35:8, by volume) as the solvent system. Chemical detection was accomplished with anisaldehyde (28). For labeling, colonies were inoculated on Brucella agar plates, and 50 mCi of [35S]methionine (Amersham, Little Chalfont, United Kingdom) diluted in 0.5 ml of PBS, pH 7.3, was sprinkled over the plates. Alternatively, colonies were inoculated (105 CFU ml−1) in Ham's F-12 medium (Gibco BRL, Paisley, United Kingdom), with 10% heat-inactivated fetal calf serum and 50 mCi of [35S]methionine per 10 ml of medium. After incubation under standard conditions, the cells were harvested, washed, resuspended to 108 CFU ml−1 in PBS, and incubated with shaking under microaerophilic conditions at 37°C for 12 to 24 h. Both labeling procedures resulted in suspensions with specific activities of approximately 1 cpm per 100 H. pylori organisms (3). Binding of the labeled bacteria to glycosphingolipids separated by TLC was achieved using a bacterial-overlay technique coupled with autoradiography detection using XAR-5 X-ray films (Eastman Kodak, Rochester, N.Y.) as described elsewhere (7).
It has been established previously that both H. pylori and H. mustelae bind gangliotetraosylceramide (5a), which was confirmed in this study; a similar capacity was also demonstrated for H. felis CCUG 28539, H. canis CCUG 33835, and H. hepaticus CCUG 33637 (Table 3). Furthermore, in common with H. pylori, we found that both the gastric and enterohepatic Helicobacter spp. tested were capable of binding to lactotetraosylceramide, lactosylceramide with phytosphingosine, and/or hydroxy fatty acids and isoglobotriaosylceramide. In contrast, within the strains and species tested, binding to Leb glycosphingolipid was solely observed for H. pylori CCUG 17875 (Table 3).
TABLE 3.
Binding of 35S-labeled Helicobacter spp. to glycosphingolipids on thin-layer chromatogramsa
| Trivial name | Structureb | Bindingc of glycosphingolipids to:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Gastric species
|
Enterohepatic species
|
||||||||
| H. mustelae 25715 | H. mustelae 23950/1 | H. pylori 17874 | H. pylori 17875 | H. felis 28539 | H. canis 33835 | H. bilis 38995 | H. hepaticus 33637 | ||
| Lactosylceramide | Galβ4Glcβ1Cer | + | + | + | + | + | + | + | + |
| Isoglobotri | Galα3Galβ4Glcβ1Cer | + | + | + | + | + | + | + | + |
| Gangliotetra | Galβ3GalNAcβ4Galβ4Glcβ1Cer | + | + | + | + | + | + | + | + |
| Lea-5 | Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | − | − | − | − | − |
| Leb-6 | Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | + | − | − | − | − |
| Globotetra | GalNAcβ3Galα4Galβ4Glcβ1Cer | − | − | − | − | − | − | − | − |
| Lactotetra | Galβ3GlcNAcβ3Galβ4Glcβ1Cer | + | + | + | + | + | + | + | + |
| Acid glycosphingolipids of human granulocytes | ± | − | + | − | − | − | − | + | |
Identical results were noted for agar- and broth-grown bacteria.
“Cer”, ceramide composition; Fuc, fucose composition.
Binding is defined as follows: + denotes a significant darkening on the autoradiogram when 2 μg (or 40 μg in the case of the last sample) was applied to TLC plates, − denotes no binding, and ± denotes occasional positive results for binding.
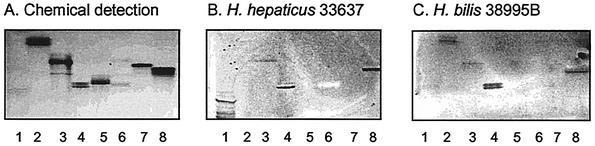
The binding of certain H. pylori strains to slow-migrating gangliosides in the acid glycosphingolipid fraction of human granulocytes is sialic acid dependent, and this fraction was therefore used as an indicator of sialic acid recognition. Binding to this fraction was noted for H. hepaticus CCUG 33637 (exemplified in Fig. 1B, lane 1) and H. pylori CCUG 17874 and occasionally for H. mustelae CCUG 25715 (Table 3). It should be noted that no differences were observed in the levels of binding to glycosphingolipids of the agar- and broth-grown bacteria used in this study, in contrast to what occurred with the polyglycosylceramide binding of H. pylori.
FIG. 1.
(A to C) Chemical detection of glycosphingolipids (A) and chromatogram assay detection of their binding to Helicobacter hepaticus (B) and Helicobacter bilis (C). Lanes (where Cer is ceramide and Fuc is fucose): 1, acid glycosphingolipid fraction of human granulocytes, 40 μg; 2, Galβ4Glcβ1Cer (lactosylceramide) of dog intestine, 2 μg; 3, Galα3Galβ4Glcβ1Cer (isoglobotriaosylceramide) of dog intestine, 2 μg; 4, Galβ3GalNAcβ4Galβ4Glcβ1Cer (gangliotetraosylceramide) of mouse feces, 2 μg; 5, Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer (Lea-5 glycosphingolipid) of human meconium, 2 μg; 6, Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer (Leb-6 glycosphingolipid) of human meconium, 2 μg; 7, GalNAcβ3Galα4Galβ4Glcβ1Cer (globotetraosylceramide) of human erythrocytes, 2 μg; 8, lactotetraosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer) of human meconium, 2 μg.
The ability of predominantly gastric and also certain enterohepatic Helicobacter spp. to both hemagglutinate erythrocytes and bind to glycosphingolipids is indicative of the use of host-carbohydrate binding by these species in their adhesion strategies. The elimination of HA following proteolytic and heat treatment characterizes these interactions with erythrocytes as protein-carbohydrate or lectin based. In addition, the ability of cell surface acid-glycine extracts and whole cells to yield identical levels of HA implies that this lectin-like activity is not dependent on viable bacteria and, not surprisingly for putative adhesins, that it is likely to be present on the bacterial surface. Such adhesins have been described previously for H. pylori and have been classified as either sialic acid dependent or independent (8, 18). The decrease in HA titer following neuraminidase treatment of goat erythrocytes suggests a role for sialic acid in the binding of strains of H. bilis and H. pylori. It has been established that horse and rabbit erythrocytes have comparatively low levels of sialic acid compared with those of the other erythrocytes tested (4). In addition, the structures and composition of sialic acid may differ among the types of erythrocytes (4). The lower HA titer for H. bilis noted for native rabbit erythrocytes is consistent with these data. However, the identical HA titers for H. pylori 119/95 for horse and rabbit erythrocytes suggests the ability of this strain to also use sialic acid-independent binding. In addition, the presence of a sialic acid-dependent adhesin on the surface of H. bilis was further illustrated by the ability of the bacterium to strongly bind fetuin, which was also a more potent inhibitor of its HA activity than asialofetuin.
However, sialic acid recognition as evidenced by binding to the acid glycosphingolipid fraction of human granulocytes was observed for strains of H. hepaticus (Fig. 1B, lane 1) and H. pylori but was not observed for H. bilis (Fig. 1C, lane 1). Differences noted in the two methods used to characterize the protein-carbohydrate interactions in particular for strains of H. hepaticus and H. bilis may have been due to phase or strain variation in the levels of expression of these adhesins or the presence of a mixed culture of strains. Both hypotheses are supported by our observation of occasional binding for H. hepaticus CCUG 33637 and H. mustelae CCUG 25715 in the HA and overlay assays, respectively. More-sensitive techniques to detect binding, including surface plasmon resonance, may improve the detection of such lectin-like activity.
A further possibility is that the putative bacterial lectins have different fine specificities. It is known that human erythrocytes contain a number of gangliosides which may be involved in lectin interactions. Their HA profiles and their ability to bind to various nonacid glycosphingolipids from human erythrocytes suggest that gastric helicobacter strains are capable of recognizing heterogenous glycoconjugate receptors but that the majority of enterohepatic helicobacters and H. felis may have a more defined set of adhesins which do not seem to rely on sialic acid. Alternatively, it has been previously established that different gastric helicobacter isolates from dogs, including H. felis and H. bizzozeronii, differ in morphology and perhaps also motility, which may in turn have an impact on adhesion (25). A similar situation may arise in relation to the enterohepatic species. However, as demonstrated with H. pylori, the expression of sialic acid-dependent binding may be strain specific or due to phase variation as noted by Mahdavi and colleagues (20). Thus, the low numbers of strains of different helicobacter species, in particular for the enterohepatic species, represent a limitation in the present study that awaits better methods of isolation, culture, transport, and resuscitation for Helicobacter spp.
The differential levels of expression of lectins on the surfaces of helicobacters discerned in our study may reflect the bacterium's niche and/or ability to strongly adhere to the host's cells. As was suggested recently, a varied lectin-based adhesion strategy may be useful at specific instances during the course of a chronic infection, as changes occur in the levels of receptors expressed in inflamed tissue (20). From the present study it is apparent that certain hepatobiliary helicobacters, namely, H. hepaticus and H. bilis may share common adhesion strategies with H. pylori. The pathogenic potential of similar adhesins and other common pathogenic factors, in particular, urease, for these hepatic helicobacters is as yet unknown. Further characterization of the effects of these putative adhesins in the variations of particular strains would be important to gain insights into the different adherence strategies utilized by these emerging pathogens but requires a greater availability of fresh clinical isolates of these species.
Acknowledgments
This study was supported by the Swedish Medical Research Council (grants 16X04723 [T.W.] and 12628 [S.T.]) and the Swedish Cancer Foundation. N.R. is supported by a grant from the program Glycoconjugates in Biological Systems, sponsored by the Swedish Foundation for Strategic Research.
We gratefully acknowledge receipt of strains used in this study from the following sources: Leif Andersen, Rigshospitalet, Copenhagen, Denmark; Margeruite Clyne, Our Lady's Hospital for Sick Children, Dublin, Ireland; Marja-Liisa Hanninen, University of Helsinki, Finland; Kazuo Goto, Central Institute for Experimental Animals, Kanagawa, Japan; and Manfred Kist, University of Freiburg, Freiburg, Germany. We thank Kyoko Hotta, Kitasato University, Tokyo, Japan, for providing mucin fractions for study.
Editor: J. D. Clements
REFERENCES
- 1.Amieva, M. R., N. R. Salama, L. S. Tompkins, and S. Falkow. 2002. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell. Microbiol. 4:677-690. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, L. P., A. Norgaard, S. Holck, J. Blom, and L. Elsborg. 1996. Isolation of a “Helicobacter heilmanii”-like organism from the human stomach. Eur. J. Clin. Microbiol. Infect. Dis. 15:95-96. [DOI] [PubMed] [Google Scholar]
- 3.Angstrom, J., S. Teneberg, M. A. Milh, T. Larsson, I. Leonardsson, B. M. Olsson, M. O. Halvarsson, D. Danielsson, I. Naslund, A. Ljungh, T. Wadstrom, and K. A. Karlsson. 1998. The lactosylceramide binding specificity of Helicobacter pylori. Glycobiology 8:297-309. [DOI] [PubMed] [Google Scholar]
- 4.Eylar, E. H., M. A. Madoff, O. V. Brody, and J. L. Oncley. 1962. The contribution of sialic acid to the surface charge of the erythrocytes. J. Biol. Chem. 237:1992-2000. [PubMed] [Google Scholar]
- 5.Fox, J. G. 2002. The non-H. pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut 50:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Gold, B. D., M. Huesca, P. M. Sherman, and C. A. Lingwood. 1993. Helicobacter mustelae and Helicobacter pylori bind to common lipid receptors in vitro. Infect. Immun. 61:2632-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goto, K., H. Ohashi, S. Ebukuro, K. Itoh, Y. Tohma, A. Takakura, S. Wakana, M. Ito, and T. Itoh. 1998. Isolation and characterization of Helicobacter species from the stomach of the house musk shrew (Suncus murinus) with chronic gastritis. Curr. Microbiol. 37:44-51. [DOI] [PubMed] [Google Scholar]
- 7.Hansson, G. C., K. A. Karlsson, G. Larson, N. Stromberg, and J. Thurin. 1985. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms—a rationalized approach to the study of host-cell glycolipid receptors. Anal. Biochem. 146:158-163. [DOI] [PubMed] [Google Scholar]
- 8.Hirmo, S., S. Kelm, R. Schauer, B. Nilsson, and T. Wadstrom. 1996. Adhesion of Helicobacter pylori strains to alpha-2,3-linked sialic acids. Glycoconj. J. 13:1005-1011. [DOI] [PubMed] [Google Scholar]
- 9.Hunt, R. H. 1996. The role of Helicobacter pylori in pathogenesis: the spectrum of clinical outcomes. Scand. J. Gastroenterol. Suppl. 220:3-9. [PubMed] [Google Scholar]
- 10.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 11.Jalava, K., S. L. On, C. S. Harrington, L. P. Andersen, M. L. Hanninen, and P. Vandamme. 2001. A cultured strain of “Helicobacter heilmannii,” a human gastric pathogen, identified as H. bizzozeronii: evidence for zoonotic potential of Helicobacter. Emerg. Infect. Dis. 7:1036-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson, K. A. 1987. Preparation of total non-acid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 138:212-220. [DOI] [PubMed] [Google Scholar]
- 13.Kirschner, D. E., and M. J. Blaser. 1995. The dynamics of Helicobacter pylori infection of the human stomach. J. Theor. Biol. 176:281-290. [DOI] [PubMed] [Google Scholar]
- 14.Koerner, T. A. W., Jr., J. H. Prestegard, P. C. Demou, and R. K. Yu. 1983. High-resolution proton NMR-studies of gangliosides. Use of two-dimensional nuclear Overhauser effect spectroscopy and sialylation shifts for determination of oligosaccharide sequence and linkage sites. Biochemistry 22:2687-2690. [DOI] [PubMed] [Google Scholar]
- 15.Kwok, T., S. Backert, H. Schwarz, J. Berger, and T. F. Meyer. 2002. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect. Immun. 70:2108-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lelwala-Guruge, J., F. Ascencio, A. S. Kreger, A. Ljungh, and T. Wadstrom. 1993. Isolation of a sialic acid-specific surface haemagglutinin of Helicobacter pylori strain NCTC 11637. Zentralbl. Bakteriol. 280:93-106. [DOI] [PubMed] [Google Scholar]
- 17.Lelwala-Guruge, J., F. Ascencio, A. Ljungh, and T. Wadstrom. 1993. Rapid detection and characterization of sialic acid-specific lectins of Helicobacter pylori. APMIS 101:695-702. [DOI] [PubMed] [Google Scholar]
- 18.Lelwala-Guruge, J., A. Ljungh, and T. Wadstrom. 1992. Haemagglutination patterns of Helicobacter pylori. Frequency of sialic acid-specific and non-sialic acid-specific haemagglutinins. APMIS 100:908-913. [PubMed] [Google Scholar]
- 19.Logan, S. M., and T. J. Trust. 1983. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect. Immun. 42:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahdavi, J., B. Sonden, M. Hurtig, F. O. Olfat, L. Forsberg, N. Roche, J. Angstrom, T. Larsson, S. Teneberg, K. A. Karlsson, S. Altraja, T. Wadstrom, D. Kersulyte, D. E. Berg, A. Dubois, C. Petersson, K. E. Magnusson, T. Norberg, F. Lindh, B. B. Lundskog, A. Arnqvist, L. Hammarstrom, and T. Boren. 2002. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.On, S. L., S. Hynes, and T. Wadstrom. 2002. Extragastric Helicobacter species. Helicobacter 7(Suppl. 1):63-67. [DOI] [PubMed] [Google Scholar]
- 22.Samuelsson, B. E., W. Pimlott, and K. A. Karlsson. 1990. Mass spectrometry of mixtures of intact glycosphingolipids. Methods Enzymol. 193:623-646. [DOI] [PubMed] [Google Scholar]
- 23.Solnick, J. V., and D. B. Schauer. 2001. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin. Microbiol. Rev. 14:59-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stellner, K., H. Saito, and S. I. Hakomori. 1973. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch. Biochem. Biophys. 155:464-472. [DOI] [PubMed] [Google Scholar]
- 25.Utriainen, M., K. Jalava, A. Sukura, and M. L. Hanninen. 1997. Morphological diversity of cultured canine gastric Helicobacter spp. Comp. Immunol. Microbiol. Infect. Dis. 20:285-297. [DOI] [PubMed] [Google Scholar]
- 26.Valkonen, K. H., M. Ringner, A. Ljungh, and T. Wadstrom. 1993. High-affinity binding of laminin by Helicobacter pylori: evidence for a lectin-like interaction. FEMS Immunol. Med. Microbiol. 7:29-37. [DOI] [PubMed] [Google Scholar]
- 27.Wadstrom, T., S. Hirmo, and B. Nilsson. 1997. Biochemical aspects of H. pylori adhesion. J. Physiol. Pharmacol. 48:325-331. [PubMed] [Google Scholar]
- 28.Waldi, D. 1962. Spruhreagentien für die Dunnschnicht-Chromatographie. In E. Stahl (ed.), Dunnschicht-Chromatographie. Springer-Verlag, Berlin, Germany.
- 29.Yang, H. J., and S. I. Hakomori. 1971. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose. J. Biol. Chem. 246:1192-1200. [PubMed] [Google Scholar]