Abstract
In vitro and in vivo studies from various groups have suggested that Helicobacter pylori lipopolysaccharide (LPS) Lewis x (Lex) antigens mediate bacterial adhesion. We have now reevaluated this hypothesis by studying the adherence in situ of H. pylori strain 11637 and its corresponding Lex-negative rfbM mutant to human gastric mucosa from patients (n = 22) with various gastric pathologies. Significant binding of the parent strain was observed in only 8 out of 22 sections; in four out of eight patients, the Lex-negative mutant bound less well. One of these four patients displayed no gastric abnormalities, and the other three showed dysplasia, metaplasia, and adenocarcinoma, respectively; hence, we are unable to define the circumstances under which LPS-mediated adhesion takes place. We conclude that H. pylori LPS plays a distinct but minor role in adhesion.
More than 80% of Helicobacter pylori strain express Lewis blood group antigens on their lipopolysaccharide (LPS); most often, Lewis x (Lex) and/or Ley is expressed (2, 13). Thus, in contrast to the case for many other pathogens (for example, Escherichia coli), the H. pylori LPS O antigen is strongly conserved. This suggests that it may play a role in pathogenesis other than its role as a (weak) endotoxin. Despite intense efforts, no such role has been assigned with certainty, but the following three options have been investigated in some detail (2): (i) H. pylori evades the host immune response by molecular mimicry of its Lex antigen with similar blood group antigens in the gastric mucosa, (ii) H. pylori induces a gastric autoimmune response by inducing anti-Lex antibodies that bind to its LPS and also to gastric epithelium, or (iii) H. pylori LPS could demonstrate adhesive properties.
The data to support a role of H. pylori LPS in adhesion are as follows. Inactivation of H. pylori genes which encode glycosyltransferases of importance for LPS glycosylation patterns yields mutants that do not express certain Lewis antigens. In a series of studies, such Lewis antigen-negative mutants colonized less well in experimental mouse infection studies (9, 7, 11). In a recent study it was shown that Lewis antigen-negative LPS mutants did not adhere to the gastric mucosa; that study was based on adherence of bacterial cells in vitro, i.e., to histological tissue sections (5). Moreover, synthetic Lex applied to the surface of fluorescent latex beads bound to the gastric sections in patterns similar to the adherence “blueprint” displayed by H. pylori bacterial cells. This series of results suggests that the Lex LPS antigens might confer adhesive properties to H. pylori (5). However, the results were based on the characterization of adherence properties of a single H. pylori strain and its isogenic mutants, and for the binding studies histological sections from a single patient with gastric carcinoma were used (G. Faller, personal communication). In contrast, in several studies the H. pylori BabA adhesin has consistently been demonstrated to mediate binding of bacterial cells to gastric epithelial cells, specifically the mucosal Leb blood group epitope (4, 6).
The results described above raise the question of when H. pylori would use LPS-carbohydrate-based interactions for adherence, as alternatives to regular adhesin proteins. Could LPS binding possibly optimize targeting of the microbe to unique microniches, or could it possibly increase binding strength by the use of multiple binding sites simultaneously (multivalent binding)? Alternatively, would the mechanisms of phase variation as described for expression of LPS antigen variation (1, 3) endow the microbe with adhesive properties that would lead to escape to the immune response? One obvious answer for an LPS-dependent binding activity would be to complement BabA adhesin-mediated adherence, such as in individuals who do not express Leb antigen (nonsecretor individuals), or for multivalent glycan-glycan interactions with highly glycosylated structures such as gastric mucins.
Thus, we decided to reevaluate the functional role of LPS antigens in adhesion of H. pylori. In this work we studied the influence of LPS Lex antigen expression among H. pylori strains that express (positive) or do not express (negative) the BabA adhesin for its role in adherence to gastric sections from many different patients, including an individual of the nonsecretor phenotype.
The strains used are shown in Table 1. Most strains have been described before (1, 3, 6, 4), apart from strains ATCC 45304 KO babA and K4.1 KO babA, which were derived from their respective parent strains by insertional activation of the babA gene as described previously (4, 6). Serotyping with anti-Lewis monoclonal antibodies was done as described previously (13). Expression of a functional BabA adhesin was investigated through binding studies with Leb coupled to human serum albumin (Isosep, Tullinge, Sweden) as described previously (4, 6). Briefly, the neoglycoprotein was labeled with 125I by the chloramine T method, and approximately 20,000 cpm of labeled material was incubated with bacterial cells Binding of Leb to bacterial cells was expressed as the percentage of counts per minute added. Binding studies were performed on three or four independent occasions. In situ binding of bacteria to gastric tissue was done essentially as described previously (6, 4). Briefly, bacteria were grown on agar plates, washed, and labeled with fluorescein isothiocyanate, and 200 μl of bacterial suspension with an optical density at 600 nm of 0.2 was incubated with deparaffinated gastric sections from the corpus or antrum. After FITC labeling, the bacteria are dead. Bacterial adherence was evaluated by visually counting the number of adherent bacteria per gastric pit for 10 pits, each in a different microscopic field (magnification, ×200). In situ binding studies were done on two to four independent occasions, and hence in total 20 to 40 pits (fields) were evaluated per strain tested. The average number of adherent bacteria per pit was calculated, as well as the standard error of the mean. Significant binding was defined as >50 bacteria per gastric pit. Differences in binding were calculated with the Student's t test. In initial studies (Table 1), three zones of binding were discriminated: surface mucus cells, the parietal cell region, and the glandular region. In later studies (see Table 2), only the most relevant zone, i.e., the surface mucus epithelium, was evaluated.
TABLE 1.
Strains used in this study and their LPS phenotypes, expression of functional BabA, and ability to bind to healthy gastric mucosa
| Strain testeda | LPS phenotype | Leb bindingb | In situ adhesion to gastric epitheliumb (mean ± SEM)
|
Reference(s) | ||
|---|---|---|---|---|---|---|
| Surface mucus cells | Parietal cells | Deep glands | ||||
| 17875 | No Lex or Ley | 44 ± 2.06 | 647 ± 24 | 0 | 0 | 4, 6 |
| 17875 babA KO | No Lex or Ley | <1 ± 0.03 | 36 ± 5 | 0 | 0 | 4, 6 |
| ATCC 43504 | Lex, Ley | 25 ± 1.98 | 545 ± 13 | 0 | 0 | 1, 3 |
| ATCC 43504 variant 1b | Ley | 29.5 ± 0.97 | 596 ± 22 | 21 ± 4 | 0 | 1, 3 |
| ATCC 43504 variant 1c | Lex, Ley | 32.8 ± 1.0 | 607 ± 9 | 0 | 28 ± 2 | 1, 3 |
| ATCC 43504 variant D1.1 | No Lex or Ley | 29.8 ± 1.22 | 537 ± 11 | 25 ± 5 | 79 ± 7 | 1, 3 |
| ATCC 43504 variant K4.1 | i-antigen, H type I | 24.8 ± 1.5 | 512 ± 7 | 35 ± 3 | 113 ± 9 | 1, 3 |
| ATCC 43504 babA KO | Lex, Ley | <1 ± 0.08 | 298 ± 19 | 0 | 0 | This study |
| K4.1 babA KO | i-antigen, H type I | <1 ± 0.06 | 259 ± 28 | 34 ± 4 | 108 ± 13 | This study |
| 4187 | Lex, Ley | 3.3 ± 0.25 | 391 ± 8 | 0 | 18 ± 2 | 3 |
| 4187 KO 379/651 | i-antigen, H type I | 1.3 ± 0.12 | 538 ± 14 | 0 | 84 ± 9 | 3 |
| NCTC 11637 | Lex, Ley | <1 ± 0.03 | 0 | 0 | 0 | 5 |
| NCTC 11637 galE mutant | No Lex or Ley | <1 ± 0.07 | 0 | 0 | 0 | 5 |
| NCTC 11637 rfbM mutantc | No Lex or Ley | <1 ± 0.03 | 0 | 0 | 0 | 5 |
Compared to the NCTC 11637 parent, variant 1b phase varies in an as-yet-unidentified N-acetylglusosaminyltransferase, 1c varies in α3-fucosyltransferase HP0651, K4.1 varies in α3-fucosyltransferase HP0379, and D1.1 varies both in N-acetylglusosaminyltransferase and HP0379. In strain 4187 KO 379/651, both HP0379 and HP0379 are inactivated. The Lewis phenotypes of all strains were tested repeatedly and found to be stable.
Based on two to four independent experiments. Bacterial adherence was defined as the number of bacteria per gastric pit.
rfbM codes for GDP mannose pyrophosphorylase, an enzyme specific for LPS biosynthesis.
TABLE 2.
In situ binding of H. pylori strain NCTC 11637 and its Lewis x-negative rfbM mutant to gastric surface mucus epithelia from patients of diverse histopathological status
| Patient | Binding (mean ± SEM)a of strain
|
Patient characteristics
|
|||
|---|---|---|---|---|---|
| NCTC 11637 | rfbM KO | Gastric histopathologyb | ABO blood group | Lewis blood group | |
| 1 | 0 | 0 | Normal | A | Leb |
| 2 | 0 | 0 | Gastritis, atrophy | B | Leb |
| 3 | 57 ± 6 | 26 ± 8 | Normal | A | Leb |
| 4 | 0 | 0 | Normal | NDc | Leb |
| 5 | 0 | 0 | Gastritis, atrophy | ND | Leb |
| 6 | 134 ± 8 | 38 ± 7** | Dyplasia | A | Leb |
| 7 | 0 | 0 | Gastritis | ND | Leb |
| 8 | 140 ± 6 | 156 ± 8 | Normal | A | Leb |
| 9 | 0 | 0 | Normal | ND | Leb |
| 10 | 6 ± 2 | 0 | Normal | ND | Leb |
| 11 | 10 ± 3 | 7 ± 4 | Normal | ND | Leb |
| 12 | 0 | 20 ± 3* | Normal | A | Leb |
| 13 | 306 ± 21 | 32 ± 6** | Gastritis, atrophy, metaplasia | ND | Leb |
| 14 | 0 | 0 | Dysplasia | ND | Leb |
| 15 | 0 | 0 | Dysplasia | ND | Leb |
| 16 | 119 ± 18 | 59 ± 13 | Gastritis | O | Leb |
| 17 | 336 ± 8 | 283 ± 66 | Hyperplasia | ND | Leb |
| 18 | 329 ± 12 | 30 ± 8** | Normal | ND | Leb negative |
| 19 | 0 | 0 | Adenocarcinoma | ND | Leb |
| 20 | 331 ± 12 | 25 ± 11** | Adenocarcinoma | ND | Leb |
| 21 | 0 | 0 | Adenocarcinoma | ND | ND |
Based on two to four independent experiments. Significant differences in binding between parent and rfbM mutant: *, P < 0.05, **, P < 0.01.
For an overview of gastric histopathology, see reference 10.
ND, not done.
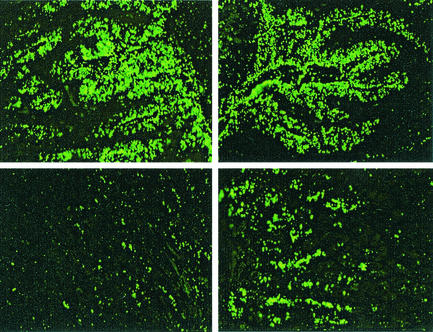
Table 1 shows that LPS structure had no influence on in situ adherence to surface mucus cells. The isogenic strains not expressing Lex and or Ley adhere most similarly to the parental strain. LPS phase variants D1.1 and K4.1 were compared with the parental strain ATCC 43504, and the α3-fucosytransferase double-knockout strain 4187 KO 379/651 was compared with the parental strain 4187. Interestingly in comparison to the wild-type strains, D1.1, K4.1, and 4187 KO 379/651 actually demonstrated some adherence to the deeper glandular region also (binding of K4.1 is shown in Fig. 1), which suggests the unmasking of additional binding properties. Strains K4.1 and 4187 KO 379/651 strongly express the H type I and i antigen epitopes, and hence adhesion might be H type I (or i-antigen) mediated.
FIG. 1.
Binding of H. pylori 43504 (left panels) and its H type I-expressing LPS phase variant K4.1 (right panels) to superficial mucosa (upper panels) or deeper glandular region (lower panels). Both strains bind well to superficial mucosa, but variant K4.1 displays enhanced binding to the deeper region
In contrast, inactivation of babA in strain 17875 strongly affected adherence. We reasoned that the observed lack of effect of LPS structure on adhesion might be due to a BabA-Leb high-affinity-mediated interaction, dominant over LPS-mediated binding, with the gastric mucosa for strains NCTC 43504 and 4187 (and their phase variants and mutants, respectively). We therefore decided to investigate the expression of a functional BabA adhesin activity through Leb-neoglycoprotein binding studies for a variety of strains. Table 1 shows that strain 17875 bound Leb very well, while its corresponding babA knockout strain was devoid of Leb binding activity. Likewise, strains ATCC 43504 and its phase variants, as well as strains 4187and 4187 KO 379/651, were found to bind Leb, although the latter two strains were less active. Hence, a potential effect of LPS structure on in situ adhesion might be concealed by BabA-dependent binding. For this reason, we constructed babA knockout mutations in strain ATCC 43504 and its Lex-negative phase variant K4.1. Inactivation indeed led to a complete loss of Leb binding. However, again no effect of LPS structure on adhesion was observed.
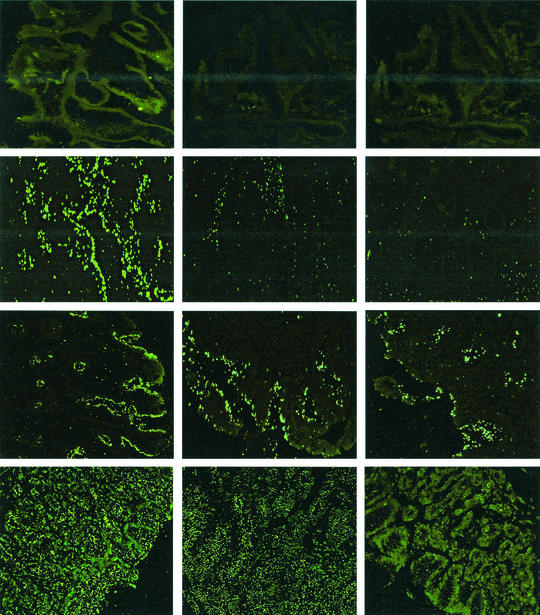
The original observation (5) of the role of LPS in adhesion was made with strain NCTC 11637 and its Lex-negative mutants (galE and rfbM knockout strains), with the rfbM knockout strain showing the largest of decrease in adhesion. Furthermore, the binding data presented in Table 1 were obtained with serial sections from a single patient without gastric abnormalities. Strikingly, strain 11637 does not bind Leb and also does not bind to gastric tissue of this patient; the reason for this lack of binding to Leb is not known. Recently we showed that gastric inflammation affects H. pylori adhesion (8). We therefore decided to test NCTC 11637 and its rfbM knockout mutant for in situ binding to a large series of gastric tissue sections obtained from patients with various gastric histopathological abnormalities (Table 2). As this strain is negative for Leb binding, its binding is not mediated through an active BabA. Significant binding (>50 bacteria per pit) was observed in 8 out of 21 patient sections tested. In 4 out of 21 patients, namely, patients 6, 13, 18, and 20, a statistically significant effect of LPS structure was found, where the rfbM mutant bound less well; an example is shown in Fig. 2. The series of sections tested were obtained from patients with widely varying gastric inflammatory status, ranging from no inflammation at all through gastritis and atrophy, to dys-, hyper-, and metaplasia and gastric adenocarcinoma. The sections on which the rfbM knockout strain showed decreased binding were obtained from one patient with dysplasia (patient 6), one patient with atrophy and intestinal metaplasia (patient 13), a nonsecretor patient without gastric inflammation (patient 18), and one patient with gastric adenocarcinoma and metaplasia (patient 20), and hence we are unable to define the circumstances under which LPS-mediated adhesion, within the context of this adherence model, takes place.
FIG. 2.
Binding of H. pylori strains to gastric mucosa in situ (histological sections) of diverse pathological conditions. The strains tested were NCTC 11637 (left column) and its galE and rfbM knockout mutants (middle and right columns, respectively). Upper row, healthy tissue, with no binding of parent and knockout strains. Second row, metaplastic tissue; the parent strain binds well, while LPS mutants show strongly decreased binding. Third row, hyperplastic tissue; LPS structure has almost no effect on binding. Lower row, noninflamed gastric tissue of a nonsecretor patient, with strongly decreased binding of the rfbM mutant.
Up to now a role of LPS in in situ adhesion of H. pylori has been reported for tissue sections of five patients only, i.e., the four described above in this study and the one described before by Edwards et al. (5). Therefore, NCTC 11637 and its rfbM mutant were tested for adhesion to sections obtained from the same gastric carcinoma patient from the previous study (5), although we used corpus sections (patient 21), while in the other study (5) antral sections were tested. To our surprise, the parent strain did not bind, which demonstrates that the putative gastric lectin is unevenly distributed over the stomach. In a very recent study no effect of LPS structure on in situ adherence of H. pylori to gastric tissue sections was observed (12).
We conclude that H. pylori LPS has a limited but distinct role in adhesion. However, the data presented here were obtained from in vitro adhesion experiments, and hence we dot not yet know how necessary H. pylori Lewis antigens are for in vivo colonization. Several studies have shown that they are crucial for colonization of mice. However, one recent study demonstrated LPS structure also to be irrelevant to mouse colonization, and α3-fucosyltransferase knockout mutants not expressing Lex or Ley colonized mice well (14). In addition, the knockout strain adhered well to human gastric celline cells. To what degree the H. pylori Lewis antigens are required for colonization of humans remains an unanswered question, as does the nature of Lex-binding gastric receptors.
Acknowledgments
We thank N. High (Manchester, United Kingdom) for providing strain 11637 and its galE and rfbM knockout mutants. We thank G. Faller for providing corpus sections from a patient with gastric adenocarcinoma.
We thank the Dutch Organization for Scientific Research (NWO) and Swedish Medical Research Council for a Research Visit Grant.
Editor: J. D. Clements
REFERENCES
- 1.Appelmelk, B. J., S. L. Martin, M. A. Monteiro, C. A. Clayton, A. A. McColm, P. Y. Zheng, T. Verboom, J. J. Maaskant, D. H. van den Eijnden, C. H. Hokke, M. B. Perry, C. M. J. E. Vandenbroucke-Grauls, and J. G. Kusters. 1999. Phase variation in Helicobacter pylori lipopolysaccharide due to changes in the lengths of poly(C) tracts in α3-fucosyltransferase genes. Infect. Immun. 67:5361-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelmelk, B. J., M. A. Monteiro, S. L. Martin, A. P. Moran, and C. M. Vandenbroucke-Grauls. 2000. Why Helicobacter pylori has Lewis antigens. Trends Microbiol. 8:565-570. [DOI] [PubMed] [Google Scholar]
- 3.Appelmelk, B. J., B. Shiberu, C. Trinks, N. Tapsi, P. Y. Zheng, T. Verboom, J. Maaskant, C. H. Hokke, W. E. Schiphorst, D. Blanchard, I. M. Simoons-Smit, D. H. van den Eijnden, and C. M. Vandenbroucke-Grauls. 1998. Phase variation in Helicobacter pylori lipopolysaccharide. Infect. Immun. 66:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boren, T., P. Falk, K. A. Roth, G. Larson, and S. Normark. 1993. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262:1892-1895. [DOI] [PubMed] [Google Scholar]
- 5.Edwards, N. J., M. A. Monteiro, G. Faller, E. J. Walsh, A. P. Moran, I. S. Roberts, and N. J. High. 2000. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Mol. Microbiol. 35:1530-1539. [DOI] [PubMed] [Google Scholar]
- 6.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 7.Logan, S. M., J. W. Conlan, M. A. Monteiro, W. W. Wakarchuk, and E. Altman. 2000. Functional genomics of Helicobacter pylori: identification of a beta-1,4 galactosyltransferase and generation of mutants with altered lipopolysaccharide. Mol. Microbiol. 35:1156-1167. [DOI] [PubMed] [Google Scholar]
- 8.Mahdavi, J., B. Sonden, M. Hurtig, F. O. Olfat, L. Forsberg, N. Roche, J. Angstrom, T. Larsson, S. Teneberg, K. A. Karlsson, S. Altraja, T. Wadstrom, D. Kersulyte, D. E. Berg, A. Dubois, C. Petersson, K. E. Magnusson, T. Norberg, F. Lindh, B. B. Lundskog, A. Arnqvist, L. Hammarstrom, and T. Boren. 2002. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin, S. L., A. A. McColm, and B. J. Appelmelk. 2000. Helicobacter pylori adhesion and Lex. Gastroenterology 119:1414-1416. [DOI] [PubMed] [Google Scholar]
- 10.Meining, A., A. Morgner, S. Miehlke, E. Bayerdorffer, and M. Stolte. 2001. Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis? Best Pract. Res. Clin. Gastroenterol. 15:983-998. [DOI] [PubMed] [Google Scholar]
- 11.Moran, A. P., E. Sturegard, H. Sjunnesson, T. Wadstrom, and S. O. Hynes. 2000. The relationship between O-chain expression and colonization ability of Helicobacter pylori in a mouse model. FEMS Immunol. Med. Microbiol. 29:263-270. [DOI] [PubMed] [Google Scholar]
- 12.Odenbreit, S., G. Faller, and R. Haas. 2002. Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int. J. Med. Microbiol. 292:247-256. [DOI] [PubMed] [Google Scholar]
- 13.Simoons-Smit, I. M., B. J. Appelmelk, T. Verboom, R. Negrini, J. L. Penner, G. O. Aspinall, A. P. Moran, S. F. Fei, B. S. Shi, W. Rudnica, A. Savio, and J. de Graaff. 1996. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. J. Clin. Microbiol. 34:2196-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takata, T., E. El Omar, M. Camorlinga, S. A. Thompson, Y. Minohara, P. B. Ernst, and M. J. Blaser. 2002. Helicobacter pylori does not require Lewis X or Lewis Y expression to colonize C3H/HeJ mice. Infect. Immun. 70:3073-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]