Abstract
Genetic analysis of two Helicobacter pylori strains isolated from a single gastric biopsy showed evidence of extensive horizontal gene transfer. Several large recombinations were identified in the rdxA gene, which is involved in metronidazole resistance.
The gastric pathogen Helicobacter pylori displays considerable genetic diversity (2). This diversity can be attributed to genetic drift, which was observed in clinical isolates obtained at intervals of several years from one patient (7, 10, 14), and to horizontal gene transfer. Several studies have demonstrated that recombination between H. pylori strains occurs frequently enough to virtually eliminate the effect of clonal descent on the population structure of H. pylori and to generate a linkage equilibrium between alleles at different loci (1, 8, 17, 19). Furthermore, some H. pylori strains contain a 37-kb genomic region, the cag pathogenicity island (PAI), which is located in the glutamate racemase gene between two short direct repeats. The cag PAI is not a stable genomic region, since it can be readily lost from the chromosomes of cag+ strains by recombination with strains lacking the cag gene (13, 22). The variability between strains can be significant for the outcome of H. pylori infection. For instance, the cag PAI and certain alleles of the vacA and iceA genes are more frequent in strains that cause peptic ulceration or gastric cancer (4, 24).
In H. pylori, random mutations that inactivate the rdxA gene cause metronidazole resistance (9). rdxA mutations that have been described for resistant isolates include frameshifts, premature stop codons, codon changes resulting in amino acid changes, and promoter alterations (9, 11, 12, 15, 20). In a previous study, we reported that in stomach biopsies with both metronidazole-sensitive (Mtzs) and metronidazole-resistant (Mtzr) bacteria, usually a single H. pylori strain is present, which suggested that a subpopulation of the original, susceptible strain had become resistant by mutation (3). However, in one biopsy, random amplified polymorphic DNA and restriction fragment length polymorphism genotyping showed that two different strains were present. Interestingly, both strains contained Mtzs as well as Mtzr subpopulations. In the present study we determined whether horizontal gene transfer has occurred between these two strains. The results show that extensive recombination has taken place in the rdxA locus.
Strain identification.
Twelve isolates were subcultured from biopsy BH9809-109 and named L1 to L12 according to increasing Mtz resistance (Table 1). Seven isolates (L1 to L7) were Mtzs (MIC, <8 mg/liter) (Table 1), and five (L8 to L12) were Mtzr (MIC, ≥8 mg/liter). Six Mtzs isolates (L1 to L4, L6, and L8) and three Mtzr isolates (L9, L11, and L12) belonged to the first genotype (strain 1). The second genotype (strain 2) included two Mtzs isolates (L5 and L7) and one Mtzr isolate (L10). cag and vacA statuses were assessed by line probe assay. The line probe assay types two variable regions of the vacA gene, the S region and M region. Furthermore, it determines the presence or absence of the cag PAI (23). Strain 1 isolates contained vacA type S1a/M2 and were cag+; strain 2 isolates had vacA type S2/M2 and lacked cag (Table 1). Neither strain showed variation in vacA or cagA status among individual isolates; this indicated that no recombination that affected the S/M type of the vacA gene and the presence of the cag PAI had taken place.
TABLE 1.
Metronidazole susceptibilities, genotypes, rdxA alleles, presence of the cag PAI, and vacA type of isolates L1 to L12
| Isolate | Metronidazole MIC (mg/liter)a | Genotypeb | rdxA allele | cagA gene present | VacA type |
|---|---|---|---|---|---|
| L1 | 0.5 | 1 | B | + | S1a/M2 |
| L2 | 0.5 | 1 | B | + | S1a/M2 |
| L3 | 0.38 | 1 | A | + | S1a/M2 |
| L4 | 0.75 | 1 | A | + | S1a/M2 |
| L5 | 1.5 | 2 | A | − | S2/M2 |
| L6 | 1.5 | 1 | A | + | S1a/M2 |
| L7 | 1.5 | 2 | A/B | − | S2/M2 |
| L8 | 48 | 1 | A | + | S1a/M2 |
| L9 | >256 | 1 | A | + | S1a/M2 |
| L10 | >256 | 2 | A | − | S2/M2 |
| L11 | >256 | 1 | A | + | S1a/M2 |
| L12 | >256 | 1 | A | + | S1a/M2 |
Sensitive, <8 mg/liter; resistant, ≥8 mg/liter.
According to random amplified polymorphic DNA typing and restriction fragment length polymorphism of the ureC gene.
The rdxA alleles.
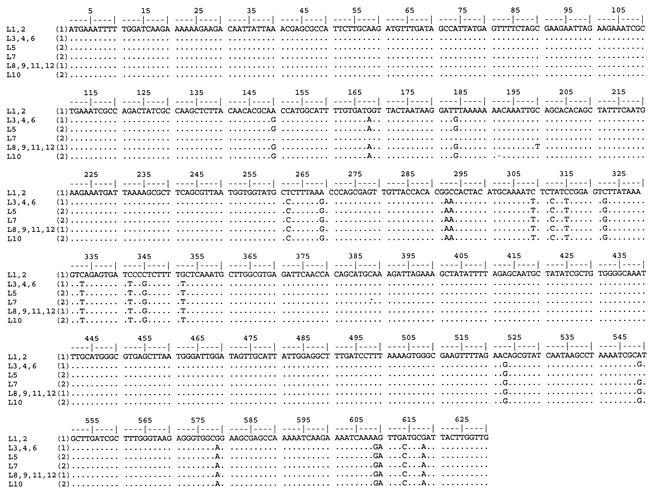
Because both strains included Mtzs as well as Mtzr isolates and reversion of nonsense mutations is unlikely, resistant subpopulations appeared during colonization from the Mtzs ancestors of either strain (with intact rdxA alleles). To demonstrate a possible transfer of a resistant rdxA allele from one strain to the other, we sequenced the rdxA genes in all 12 isolates. The results are shown in Fig. 1. Two different alleles of the rdxA gene (allele A and allele B), which differed from each other at 22 base positions in the 630-bp open reading frame (ORF), were present in the set of isolates. However, the distribution of the rdxA alleles did not correspond to the genotypes of the isolates. Strain 1 included isolates with rdxA allele A (L3, L4, L6, L8, L9, L11, and L12) as well as isolates with rdxA allele B (L1 and L2), which indicates that one of the alleles must have been acquired by horizontal DNA transfer. Of the isolates with the genetic background of strain 2, L10 had allele A but the other two (L5 and L7) possessed a mixed allele. L5 had an allele that was predominantly type A, with a single polymorphism at base position 549. This change was consistent with allele B, which suggests the transfer of a very small DNA fragment. However, this change could also result from a single point mutation which happened to match the sequence of allele B.
FIG. 1.
Alignment of the rdxA ORFs of isolates L1 to L12. Numbers in parentheses indicate the strain type (1 or 2).
In L7, of the 22 polymorphic base pairs that distinguish allele A from allele B, the 3 polymorphic sites at the 5′-end region of the ORF were identical to those in the allele B group, whereas the other 19 in the middle and at the 3′ end were concordant with the allele A group. Between the allele A and allele B regions of L7, there was a gene fragment of 77 bases with perfect homology between allele A and allele B. These data indicate that a recombination event introduced part of the rdxA locus of strain 1 into the L7 genome.
Origin of metronidazole resistance.
All resistant isolates contained rdxA allele A, which suggests transfer of the resistance mutation. However, there was a difference between the resistant isolate of strain 2 (L10) and the resistant isolates of strain 1 (L8, L9, L11, and L12). In L10, a single-base-pair deletion was found at base position 192 of the ORF, causing a frameshift that interrupts the reading frame. This frameshift occurred in a homopolymeric stretch of 10 adenine residues interrupted by one cytosine. An identical deletion was observed earlier in an unrelated strain (11). Apparently, this locus is vulnerable to slipped-strand mispairing. The resistant isolates of strain 1 (L8, L9, L11, and L12) did not possess this frameshift but showed a C-to-T mutation at position 200 of the ORF, which causes an amino acid change from alanine to valine. To confirm that this minor difference causes metronidazole resistance, we used a PCR amplimer of the L8 rdxA allele for natural transformation (25) to susceptible H. pylori strains. This yielded Mtzr transformants with a frequency of 10−3 per recipient cell, which indicated that this amino acid change is sufficient to cause metronidazole resistance. We conclude that resistance in both strains arose by an independent mutation of the rdxA gene after transfer of an intact rdxA allele A had taken place. Despite the high frequency of horizontal gene transfer between strain 1 and strain 2, an rdxA mutation arose independently in both strains.
Region around rdxA.
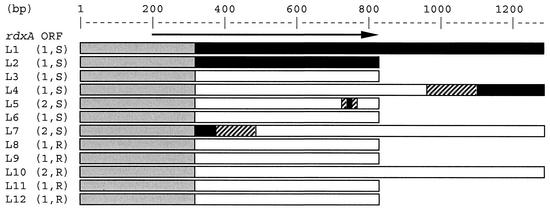
In order to determine the length of the recombinations between strain 1 and strain 2, the DNA sequence on both sides of the rdxA ORF was determined for two isolates from strain 1 (L1 with allele B and L4 with allele A), as well as two isolates from strain 2 (L7, which contains the 5′ end of allele B and the complementing part of allele A, and isolate L10, which contains allele A). The results are shown in Fig. 2. Downstream, another crossover between the two alleles was identified: after a perfect homology of 145 bp between the four sequences, the sequences of the strain 1 isolates L4 and L1 were identical over a length of at least 205 bp, with eight mismatches between strain 1 (L1 and L4) and strain 2 (L7 and L10). This is concordant with ancestral combinations of strain 1 with allele B and strain 2 with allele A. Upstream of the rdxA ORF, however, the sequences of L1, L4, L7, and L10 were identical over a length of >1.7 kbp (results not shown). It is unlikely that the original sequences of strain 1 and 2 were already identical before their cohabitation, because in this region there is only 94% conservation between these strains and the published genome sequences (2, 21). Apparently, a previous, major recombination event had replaced the original sequence in one of the strains, which results in an identical sequence in all four isolates. In an attempt to find a copy of the other original sequence, from the remaining eight isolates a fragment of 800 bp upstream of rdxA was sequenced. Their sequences were identical to the one in L1, L4, L7, and L10. Thus, the second ancestral sequence is not present in our set of 12 isolates.
FIG. 2.
Distribution of alleles A and B in isolates L1 to L12. Numbers in parentheses indicate the genotype (1 or 2). S and R, metronidazole susceptibility and resistance, respectively. The arrow shows the rdxA ORF. Open bars, allele A; solid bars, allele B; hatched bars, crossover sequence; gray bars, identical sequence. Strain L4 and strain L7 contain recombined sequences. The point mutations that cause resistance in the resistant isolates L8 to L12 are not shown.
Most observations on natural horizontal gene transfers concern transfer of genetic elements that are mobile by nature, such as plasmids or transposons. Horizontal gene transfer of nonmobile elements is only rarely observed outside experimental settings (16). It is usually inferred from sequence analysis of unrelated isolates of mucosal pathogens that are competent for DNA exchange by natural transformation: Streptococcus pneumoniae, Neisseria spp., Haemophilus influenzae, and H. pylori. In some cases, the recombining sequences must have been over 1 kb long (5, 6). For H. pylori, its isolated habitat in the gastric mucosa with prolonged contact between different strains provides the unique opportunity to isolate recombining strains together. Kersulyte et al. identified recombination events between two H. pylori strains from the same patient that involve up to 400 bp (13). Falush et al. investigated genetic relationships of sequential isolates of H. pylori from a single patient and estimated the mean size of the recombination fragments to be 417 bp (7).
Here, we demonstrate that multiple homologous recombination events occur in a single locus during cohabitation of two strains and that these events result in a molecular patchwork between two ancestral sequences. Since the duration of the contact is not known, we can make no estimate of the frequency of recombination. The sequences upstream of the rdxA ORF are identical for at least 1.7 kb, which suggests that a large fragment was transferred and integrated in the ancestors of all isolates and that H. pylori can exchange DNA fragments long enough to contain one or more complete genes. The lack of polymorphic sites for over 1.7 kb in this area obscures the second recombination site for two of the isolates, L4 and L7 (Fig. 2). Two crossovers had taken place, at 145 and 77 bases of perfect homology. This is in accordance with experimental data which indicate that recombination efficiency is highly dependent on the presence of a perfect homology of sufficient length (18). The molecular patchwork that is described here illustrates the frequent recombination during cocolonization of H. pylori strains that leads to the panmyctic population structure of this species.
Acknowledgments
We thank Raymond Pot (Department of Gastroenterology and Hepatology, Erasmus University Medical Center, Rotterdam, The Netherlands) for his assistance with DNA sequencing.
Editor: J. T. Barbieri
REFERENCES
- 1.Achtman, M., T. Azuma, D. E. Berg, Y. Ito, G. Morelli, Z. J. Pan, S. Suerbaum, S. A. Thompson, A. van der Ende, and L. J. van Doorn. 1999. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 32:459-470. [DOI] [PubMed] [Google Scholar]
- 2.Alm, R. A., L.-S. L. Ling, D. T. Moir, and B. L. King. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 3.Arents, N. L., L. C. Smeets, A. A. van Zwet, J. C. Thijs, E. J. van der Wouden, A. de Jong, J. E. Degener, and J. G. Kusters. 2001. Implications of the simultaneous presence of metronidazole-susceptible and -resistant Helicobacter pylori colonies within a single biopsy specimen. Eur. J. Clin. Microbiol. Infect. Dis. 20:418-420. [DOI] [PubMed] [Google Scholar]
- 4.Atherton, J. C., R. M. J. Peek, K. T. Tham, T. L. Cover, and M. J. Blaser. 1997. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112:92-99. [DOI] [PubMed] [Google Scholar]
- 5.Bowler, L. D., Q. Y. Zhang, J. Y. Riou, and B. G. Spratt. 1994. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: natural events and laboratory simulation. J. Bacteriol. 176:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowson, C. G., T. J. Coffey, C. Kell, and R. A. Whiley. 1993. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol. Microbiol. 9:635-643. [DOI] [PubMed] [Google Scholar]
- 7.Falush, D., C. Kraft, N. S. Taylor, P. Correa, J. G. Fox, M. Achtman, and S. Suerbaum. 2001. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. USA 98:15056-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go, M. F., V. Kapur, D. Y. Graham, and J. M. Musser. 1996. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J. Bacteriol. 178:3934-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin, A., D. Kersulyte, G. Sisson, S. V. Vanzanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 10.Israel, D. A., N. Salama, U. Krishna, U. M. Rieger, J. C. Atherton, S. Falkow, and R. M. Peek, Jr. 2001. Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc. Natl. Acad. Sci. USA 98:14625-14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong, J. Y., A. K. Mukhopadhyay, J. K. Akada, D. Dailidiene, P. S. Hoffman, and D. E. Berg. 2001. Roles of FrxA and RdxA nitroreductases of Helicobacter pylori in susceptibility and resistance to metronidazole. J. Bacteriol. 183:5155-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong, J. Y., A. K. Mukhopadhyay, D. Dailidiene, Y. Wang, B. Velapatino, R. H. Gilman, A. J. Parkinson, G. B. Nair, B. C. Wong, S. K. Lam, R. Mistry, I. Segal, Y. Yuan, H. Gao, T. Alarcon, M. L. Brea, Y. Ito, D. Kersulyte, H. K. Lee, Y. Gong, A. Goodwin, P. S. Hoffman, and D. E. Berg. 2000. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high-level metronidazole resistance in Helicobacter pylori. J. Bacteriol. 182:5082-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kersulyte, D., H. Chalkauskas, and D. E. Berg. 1999. Emergence of recombinant strains of Helicobacter pylori during human infection. Mol. Microbiol. 31:31-43. [DOI] [PubMed] [Google Scholar]
- 14.Kuipers, E. J., D. A. Israel, J. G. Kusters, M. M. Gerrits, J. Weel, A. van der Ende, R. W. van der Hulst, H. P. Wirth, J. Hook-Nikanne, S. A. Thompson, and M. J. Blaser. 2000. Quasispecies development of Helicobacter pylori observed in paired isolates obtained years apart from the same host. J. Infect. Dis. 181:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon, D. H., J. A. Pena, M. S. Osato, J. G. Fox, D. Y. Graham, and J. Versalovic. 2000. Frameshift mutations in rdxA and metronidazole resistance in North American Helicobacter pylori isolates. J. Antimicrob. Chemother. 46:793-796. [DOI] [PubMed] [Google Scholar]
- 16.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 17.Salaun, L., C. Audibert, G. Le Lay, C. Burucoa, J. L. Fauchere, and B. Picard. 1998. Panmictic structure of Helicobacter pylori demonstrated by the comparative study of six genetic markers. FEMS Microbiol. Lett. 161:231-239. [DOI] [PubMed] [Google Scholar]
- 18.Shen, P., and H. V. Huang. 1986. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics 112:441-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suerbaum, S., J. M. Smith, K. Bapumia, G. Morelli, N. H. Smith, E. Kunstmann, I. Dyrek, and M. Achtman. 1998. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:12619-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tankovic, J., D. Lamarque, J. C. Delchier, C. J. Soussy, A. Labigne, and P. J. Jenks. 2000. Frequent association between alteration of the rdxA gene and metronidazole resistance in French and North African isolates of Helicobacter pylori. Antimicrob. Agents Chemother. 44:608-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 22.van der Ende, A., E. A. Rauws, M. Feller, C. J. Mulder, G. N. Tytgat, and J. Dankert. 1996. Heterogeneous Helicobacter pylori isolates from members of a family with a history of peptic ulcer disease. Gastroenterology 111:638-647. [DOI] [PubMed] [Google Scholar]
- 23.van Doorn, L. J., C. Figueiredo, R. Rossau, G. Jannes, M. van Asbroek, J. C. Sousa, F. Carneiro, and W. G. Quint. 1998. Typing of Helicobacter pylori vacA gene and detection of cagA gene by PCR and reverse hybridization. J. Clin. Microbiol. 36:1271-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Doorn, L. J., C. Figueiredo, R. Sanna, A. Plaisier, P. Schneeberger, W. de Boer, and W. Quint. 1998. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology 115:58-66. [DOI] [PubMed] [Google Scholar]
- 25.Wang, Y., K. P. Roos, and D. E. Taylor. 1993. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J. Gen. Microbiol. 139:2485-2493. [DOI] [PubMed] [Google Scholar]