Abstract
Cells of the innate immune system and their mediators were studied at the single-cell level in the rectums of pediatric and adult patients with Shigella infection to better understand why children are at higher risk for severe infection. Adult patients had increased infiltration of mucosal mast cells (MMC) at the acute stage (3 to 5 days after the onset of diarrhea) and eosinophils in early convalescence (14 to 16 days after onset). Increased expression of stem cell factor and prostaglandin H synthase-1 (PGHS-1) was associated with increased tryptase-Ki67-double-positive MMC in the acute stage and increased apoptosis of MMC, which led to a rapid decline in early convalescence. The eosinophils demonstrated increased expression of major basic protein (MBP), eotaxin, and CCR3, as well as increased necrotic death. The neutrophils showed enhanced α-defensin and lactoferrin expression in the acute phase. In contrast to adults, the pediatric patients demonstrated delayed accumulation of mast cells and eosinophils, while α-defensin expression persisted during convalescence. In contrast, neutrophil counts and lactoferrin expression were reduced in children compared to adults. The results suggest that children with shigellosis have a persistent activation of the innate immune response in the convalescent phase, indicating delayed elimination of Shigella antigens compared to adults.
Shigellosis, an infectious enteritis, is caused by one of the four species of Shigella: Shigella dysenteriae, Shigella flexneri, Shigella sonnei, or Shigella boydii. In adults, the illness is generally self-limiting, while in young children it can lead to complications and even death, especially as a sequel to chronic illness or malnutrition. It is the most common cause of dysentery and a leading cause of death in children <5 years old in underdeveloped countries and is therefore a major public health concern.
The hallmark of Shigella infection is epithelial cell invasion through induction of phagocytosis, rapid intra- and intercellular growth, and eventual death of the host cells (35). As a consequence, an extensive inflammatory response occurs, leading to trafficking of neutrophils, macrophages, lymphocytes, natural killer cells, and other inflammatory cells and accumulation of proinflammatory molecules in the local milieu (3, 20, 26, 29, 35). However, few data are available on the role of the innate cells, such as mast cells and eosinophils, in shigellosis (20, 24). Most early cells are known to be neutrophils; however, mast cells can also provide an early defense against an invading pathogen, being strategically located at the host-environment interface. Mast cells have been shown to play a role in innate immunity in defined animal models of bacterial infection (1, 13, 14) and may also have a role in specific immune responses (18). It remains to be determined whether mast cells participate in innate immune responses in vivo in the protection of the human host against bacteria. Both mast cells and eosinophils are resident in the gastrointestinal mucosa. They are usually considered to be involved in chronic inflammatory processes, such as inflammatory bowel disease and gastrointestinal allergy (39), since these cells may be recruited from circulation to the inflammatory site, where they may modulate both the innate and antigen-specific immune responses (33).
Increased production of mediators of the innate immune system, including lactoferrin, myeloperoxidase, superoxide dismutase, nitric oxide, and eicosanoids (prostaglandin E2, 8-iso-prostaglandin F2α, and leukotriene B4) in patients with acute shigellosis has been reported (27). However, the role of innate cells that secrete these mediators has not been studied at the local site in clinical shigellosis. Since the onset of adaptive immune responses is delayed and reduced in magnitude in pediatric patients with shigellosis compared to that in adult patients (28), we hypothesized that innate immunity necessary for priming adaptive responses may also be delayed or reduced in children with shigellosis.
MATERIALS AND METHODS
Patients.
A preliminary selection of all presumptive cases of Shigella infection with occult blood and mucus in the stool and with a history of 0 to 4 days of diarrhea was done at the outpatient clinic of the Clinical Research Service Center of the International Centre for Diarrhoeal Diseases Research, Bangladesh (ICDDRB), Centre for Health and Population Research in Dhaka, Bangladesh. Stool samples were examined by direct microscopy for the presence of cyst and vegetative forms of intestinal parasites and ova of helminths and cultured for Salmonella, Shigella, and Aeromonas species, Vibrio cholerae O1 and O139, and Campylobactor jejuni. Patients with stool culture-confirmed shigellosis were included in the study. Signed informed consent was obtained from each adult patient (n = 20; age range, 18 to 45 years) or the guardian of each child (n = 20; age range, 3 to 8 years) according to the guidelines of the ethical review committee at ICDDRB. All patients received pivmecillinam immediately after admission as empirical therapy, were released from the hospital when diarrhea subsided (usually 3 to 4 days), and were requested to return for follow-up visits. The patients underwent physical examination as well as routine clinical investigation. Healthy adults (n = 15 males; age range, 18 to 45 years) living in urban slums where diarrheal diseases are endemic and of socioeconomic and nutritional status similar to those of the patients were recruited as healthy controls. Signed informed consent was obtained from each participant. Individuals with a history of infection and fever within the previous 3 months were excluded. Physical and clinical investigations were carried out as for the patients.
Sample collection.
Rectal biopsy samples, taken 10 to 12 cm from the anus, were obtained upon sigmoidoscopy (Olympus, Tokyo, Japan) from adult patients on the day of admission (3 to 5 days after the onset of diarrhea), 11 days later (14 to 16 days after onset), and 30 days later (33 to 35 days after onset). For pediatric patients, biopsy specimens were obtained on the day of admission, 30 days later, and 60 days later (63 to 65 days after onset). Healthy rectal tissues obtained from pediatric patients after 60 days from the time of onset of diarrhea served as healthy control tissues. At each time point, a total of six pieces from rectal pinch biopsies were obtained. Two pieces were fixed in buffered formalin and embedded in paraffin, and sectioning was done in a microtome (RM 2055; Leica, Heidelberg, Germany) at 3-μm thickness; the sections were mounted on glass slides (Superfrost*/plus; Menzel-Glaser, Germany), dried overnight at 37°C, and kept at room temperature for use. Two pieces were collected in Histocon (Histolab, Goteberg, Sweden), snap frozen in liquid nitrogen, and kept at −70°C until they were used for cryostat sectioning (Leica CM 3000). Two pieces of tissue were fixed in 2.5% gluteraldehyde, postfixed in osmium tetroxide, and embedded in araldite. Ultrathin sections were cut on an LKB UM4 ultramicrotome with a diamond knife (Diatome, Switzerland), stained with uranyl acetate and lead citrate, and examined with a Philips (Eindhoven, The Netherlands) EM201C electron microscope. Blood samples were also obtained from the patients 3 to 5, 8 to 10, 14 to 16, and 33 to 35 days after the onset of diarrhea and were used for another study. Data for an eosinophil count in blood, however, was used in the present study (Excell 22 hematology analyzer; DANAM, Dallas, Tex.).
Histology.
The paraffin sections were deparaffinized and stained with hematoxylin and eosin, and coded sections were read by the pathologist (M.M.) using criteria described earlier (3, 20, 29). For counting of eosinophils, hematoxylin- and eosin-stained sections were used; the counting was done manually using an ocular micrometer (Olympus) fitted into the eyepiece of the microscope (Olympus system microscope model BH-2), and data were calculated as the number of cells per 100-μm2 field area. For the enumeration of mast cells and neutrophils, an enzyme-histochemical technique (naphthol-3-hydroxy-2-naphthoic acid-o-toluidine-d-chloroacetate as the substrate and a pararosaniline dye) was used to detect chloroacetate esterase activity (9). Mast cells and neutrophils were counted in the same way as eosinophils. For staining, computerized image analysis techniques could not be applied, as the colors of the granules in neutrophils, mast cells, and eosinophils were very similar and only the morphologies were different.
Antibodies.
The following antibodies were used for immunohistochemical staining: anti-human tryptase (Dako A/S, Glostrup, Denmark), anti-human chymase (NeoMarkers; Lab Vision Corp., Fremont, Calif.), rabbit polyclonal anti-major basic protein (MBP) (Pharmingen International, San Diego, Calif.), rabbit polyclonal anti-human eotaxin (Serotec Ltd., Oxford, England), anti-human CCR3 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), goat polyclonal anti-human stem cell factor (SCF) (R&D Systems Europe, Ltd., Abingdon, United Kingdom), anti-human interleukin 3 (IL-3) (Santa Cruz Biotechnology), anti-human IL-5 (Pharmingen), anti-human α-defensins (Serotec), anti-prostaglandin H-synthase 1 (PGHS-1) (Oxford Medical Research, Oxford, Mich.), rabbit polyclonal anti-lactoferrin (Ltf) (Dako), and anti-Ki67 (Dako).
Immunohistochemistry.
Frozen rectal tissues were sectioned at 6-μm thickness and fixed with 2% formaldehyde (pH 7.4; Sigma Chemicals, St. Louis, Mo.), followed by a few washes with Earl's balanced salt solution (Gibco Ltd., Paisley, Scotland). The slides were air dried and kept at −20°C until they were used. Immunohistochemical staining for cytokines and protein markers was performed by the saponin procedure as previously described for cytokines (26, 27). Briefly, after the endogenous peroxidase activity was quenched, the sections were incubated overnight with specific antibodies (for IL-3, IL-5, α-defensin, eotaxin, CCR3, SCF, PGHS-1, and MBP; 2 to 4 μg/ml). After the sections were washed, biotinylated goat anti-mouse immunoglobulin G1 (IgG1) or IgG2b (1:300; Caltag Laboratories, South San Francisco, Calif.) or anti-rabbit Ig (1:600; Vector Laboratories, Burlingame, Calif.) was added, and the sections were incubated for 30 min. This was followed by incubation with avidin-biotin-horseradish peroxidase complex (Dako), and later, the color reaction was developed using diaminobenzidine (brown). As a control, specific antibodies were replaced by irrelevant isotype-matched antibodies.
The paraffin sections were deparaffinized and hydrated and were given microwave treatment in citrate buffer (pH 6.0) for 15 min followed by washing in phosphate buffer (pH 7.2) (for Ltf staining) or were incubated with 0.1% trypsin in Tris-buffered saline (pH 7.6) (Sigma) for 15 min at 37°C and washed with Tris-buffered saline (for chymase and tryptase staining). Primary antibodies were added at optimum dilutions for 2 (anti-tryptase) or 18 (anti-chymase and -Ltf) h, followed by sequential incubation in biotinylated goat anti-rabbit (Dako)- anti-mouse (Caltag) and avidin-biotin complex (Dako), respectively. The color reaction was developed using diaminobenzidine. After being counterstained in hematoxylin and eosin, the slides were mounted in paramount (BDH Chemicals, Poole, Dorset, England).
The procedure described above was repeated for double staining using specific antibodies of different isotypes and IgG subclasses and biotinylated Ig conjugates. The step of blocking endogenous peroxidase activity was omitted. The slides were incubated with extravidin-alkaline phosphatase conjugate (1:800; Sigma) and were subsequently developed by incubating them in the Fast Red substrate system (Dako) for 20 to 30 min, counterstained with hematoxylin for 3 to 10 s, and mounted. Two-color staining was performed for simultaneous detection of (i) chymase and tryptase, (ii) MBP and eotaxin, (iii) SCF and chymase, (iv) Ki67 and tryptase, (v) Ki67 and MBP, (vi) apoptotic cells (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick labeling [TUNEL]) and tryptase, and (vii) apoptotic cells and MBP. Apoptotic cells were visualized by the TUNEL method using the ApoTACS in situ diaminobenzidine apoptosis detection kit (R&D Systems, Minneapolis, Minn.) (25). For double staining, TUNEL was performed first, followed by staining with anti-tryptase or anti-MBP antibodies.
Computerized image analysis for detection of immunostaining.
Immunohistochemical staining of specific enzymes in rectal tissues was examined with a DMLB microscope (Leica Wetzlar GmbH, Bensheim, Germany) equipped with a three-chip charged couple device color camera (Sony Corp., Tokyo, Japan). Each image was examined in a Leica Qwin image analysis system (Leica Cambridge Ltd., Cambridge, United Kingdom) that was directed by a personal computer system. The standards were set for positive as well as negative cells. The positive staining of enzymes in rectal-tissue sections were defined by computer-assisted analysis of video microscopic images as described earlier (27). Each image was analyzed using a program routine (Tissue Includer) especially designed for these experiments. The acquired image was divided into 512 by 512 pixels, and each pixel was expressed in square micrometers (area) after calibration with the current magnification. The data acquired were imported into Microsoft (Redmond, Wash.) Excel and Sigma Stat (Jandal Scientific, San Rafael, Calif.). The positive immunostaining as assessed by computer-assisted analysis of video microscopic images was determined for the studied proteins and enzymes in patients and in healthy controls. For each tissue section, at least 20 0.4 × 105-μm2 fields were investigated at ×40 magnification, and the average was used for each protein or enzyme staining in each tissue section. The automated video microscopic analysis allowed the quantification of positive immunoreactivity relative to the total cell area of the tissue section, and the results were expressed as a percentage for the ratio of positive pixels to total pixels.
Statistical analyses.
The data were processed using Microsoft Excel 2000. Statistical analyses were performed by the Wilcoxon- Kruskal-Wallis test, the Mann-Whitney U test, and chi-square or Fischer's exact test where appropriate using Sigma Stat for Windows version 2.03. The data were expressed as medians with 25th and 75th percentiles. Probabilities were regarded as significant if they reached a 95% level of confidence (P < 0.05).
RESULTS
Stool culture and microscopy.
Stool culture revealed that 16 adult patients had infections due to S. flexneri and 4 had S. dysenteriae type 1 infections. Among pediatric patients, 13 were infected with S. flexneri and 7 were infected with S. dysenteriae type 1. The majority of S. flexneri serotypes were 2b, followed by 2a, 1b, 6, and Y. Special emphasis was given to stool microscopy for detection of intestinal parasites or helminths in patients as well as healthy controls. Examination of stools showed that 4 of 20 adult and 5 of 20 pediatric patients had Trichurius trichiura infections, 3 of 20 adults and 4 of 20 children had Ascaris lumbricoides infections, 5 of 20 adults and 3 of 20 children had Giardia lamblia infections, and 2 of 20 adults had Blastocystosis hominis infections in the acute stage. Stool microscopy in the convalescent stage showed similar frequencies of intestinal parasites or helminths in the adult and pediatric patients (Table 1). Among adult healthy controls, 4 of 15 had A. lumbricoides infections, 3 of 15 had G. lamblia infections, and 2 of 15 had T. trichiura infections. The enteric parasite carrier rates in healthy controls and patients were not different. The patients and the healthy controls were not treated for the parasitic infections, since they appeared to be chronically infected and were asymptomatic. The median duration of symptoms among adult patients was 5 days (range, 4 to 6 days; 25th and 75th percentiles), and among pediatric patients it was 6 days with (range, 5 to 7 days).
TABLE 1.
Numbers of Shigella-infected patients and healthy controls with intestinal parasites as copathogens at different intervals after onset of diarrhea
| Subjects | No. of subjects with parasitesa
|
|||
|---|---|---|---|---|
| 3-5 days | 14-16 days | 30-35 days | Healthy controls | |
| Children | 5/20 (25) | 7/20 (35) | 4/20 (20) | 5/20 (25) |
| Adults | 5/20 (25) | 7/20 (35) | 5/20 (25) | 4/15 (26.6) |
Ratio of number of subjects with intestinal parasites and total subjects. Percentages are given in parentheses.
Different accumulations of mast cells and eosinophils in the rectum in adult and pediatric patients.
Adult patients with shigellosis had increased infiltration of the rectal mucosa with mast cells (chloroacetate esterase positive) in the acute stage (3 to 5 days after the onset of diarrhea) that declined rapidly during convalescence in <30 days to healthy control levels (P < 0.03) (Table 2). In contrast, the number of mucosal mast cells in pediatric patients was high in the acute stage and persisted to the convalescent stage (33 to 35 days after onset). The mucosal mast cell count in the convalescent stage was significantly higher than in healthy controls (a biopsy specimen obtained 63 to 65 days after onset was considered a healthy control).
TABLE 2.
Comparative analysis of number of cells in rectal tissues of patients at different time intervals after onset of diarrhea and age-matched healthy controlsa
| Subjects | Days after onset | No. of mast cells | No. of Eosinophils | No. of Neutrophils |
|---|---|---|---|---|
| Adults | 3-5 | 17.8 (8.6-26)b,c | 33.9 (27.7-56.7)d | 9.2 (1-23)b,c,d |
| 14-16 | 7 (5-14.5) | 46.9 (31.5-58.9)d | 0 (0-0.9) | |
| 30-35 | 9 (6.4-14.7) | 39.7 (31-53.4)5 | 0.7 (0.2-2) | |
| Healthy controls | 12.3 (4.6-17.5) | 19 (14.8-28.8) | 0.4 (0-1.1) | |
| Children | 3-5 | 19.4 (13.8-26) | 33.4 (15.7-47)c | 2.6 (1.6-8.4)d |
| 30-35 | 21 (14-28)d | 75.7 (44-106.5)d | 0.9 (0.3-2.8) | |
| Healthy controls | 11.5 (9-18) | 23 (7.6-42) | 0.3 (0-1.06) |
Mast cells and neutrophils stained by the chloroacetate method and eosinophils stained by the hematoxylin-and-eosin method were counted manually using an ocular micrometer (Olympus) fitted into the eyepiece, and data were calculated as the number of cells per 100-μm2 field area. The data are given as median frequencies of stained cells per square millimeter with 25th and 75th percentiles in parentheses. Probability values were determined by the Wilcoxon signed-rank test comparing differences in the cell numbers at different time within the same group of patients and by the Mann-Whitney U test comparing patients to healthy controls.
Significant difference versus 14 to 16 days.
Significant difference versus 30 to 35 days.
Significant difference versus controls.
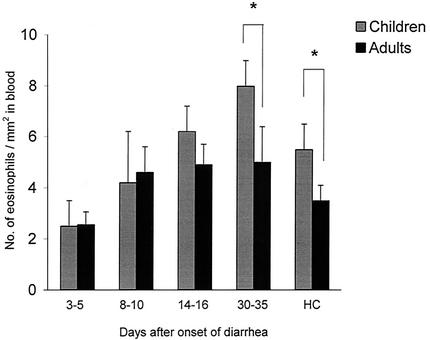
In adult patients, the frequency of eosinophils in the rectum was significantly higher throughout the study period than in healthy controls (P < 0.005), and peak numbers were seen 14 to 16 days after the onset of diarrhea (Table 2). In contrast, this peak was significantly delayed and highest in children 33 to 35 days after onset. The eosinophil count in blood was also highest in the convalescent stage in both adult and pediatric patients and was significantly higher than in the respective acute stage (Fig. 1). Pediatric patients had significantly higher eosinophil counts in blood in the convalescent stage than adult patients (P < 0.006). Healthy children also had higher eosinophil counts in blood than healthy adults.
FIG. 1.
Comparison of eosinophil counts in blood in pediatric and adult patients with shigellosis at various times after onset of diarrhea. The eosinophil counts in children (shaded bar) and in adults (solid bar) are given as geometric means with standard errors. Blood samples were collected 3 to 5, 8 to 10, 14 to 16, and 33 to 35 days after the onset of diarrhea. HC, healthy controls. The asterisks indicate a significant difference between adult and pediatric patients (P < 0.05).
Analyses by stratification of patients for presence and absence of intestinal parasites did not reveal any significant difference in the numbers of mast cells and eosinophils and expression of MBP in the rectum and eosinophils in blood (data not shown).
The number of neutrophils was increased only in the acute stage in both adult and pediatric patients, although it was much lower in the children (Table 2).
Increased expression of innate mediators in the acute stage.
A granular pattern of chymase and tryptase was evident in mast cells in the rectal tissue. Tissue expression of tryptase and chymase was high 3 to 5 days after onset in adult patients and returned to normal levels by 14 to 16 days after onset (Table 3). Double staining of mast cells for the tryptase and chymase enzymes revealed that the numbers of double-positive cells gradually increased from the acute stage to the convalescent stage (data not given). Expression of SCF, a mast cell growth factor and a mediator of mast cell chemotaxis, was localized mostly to the mast cells or macrophages in the lamina propria and was occasionally detected on the apical surfaces of the epithelial cells. SCF expression was increased in the acute stage and returned to control levels during convalescence. A similar picture was also seen in pediatric patients for chymase, tryptase, and SCF.
TABLE 3.
Quantitative comparison of various mediators expressed by cells of the innate immune system in the rectal mucosa from adult patients and in healthy age-matched controls at different time intervals after the onset of diarrheaa
| Mediators and receptors | % Positive area
|
|||
|---|---|---|---|---|
| Adults
|
Healthy controls | |||
| 3-5 days | 14-16 days | 30-35 days | ||
| Chymase | 1.4 (1-1.6)b,d | 0.8 (0.55-0.9) | 0.9 (0.4-1.4) | 0.65 (0.37-0.7) |
| Tryptase | 1.6 (1.5-2.5)b,d | 0.73 (0.4-1) | 1 (0.7-2.3) | 0.6 (0.4-1) |
| SCF | 3 (1.9-5)d | 2 (1-4.5) | 2 (0.9-3.4) | 1.6 (1.3-1.9) |
| MBP | 1 (0.8-2) | 1.2 (0.9-1.5) | 2.1 (1.7-5)d | 0.3 (0.3-1.2) |
| Eotaxin | 0.6 (0.2-1.4)d | 0.2 (0.1-0.4) | 0.12 (0.04-1.2)d | 0.01 (0.02-0.08) |
| CCR3 | 0.9 (0.6-1.5)b,d | 0.4 (0.2-0.5) | 0.2 (0.05-0.9) | 0.15 (0.1-0.3) |
| PGHS-1 | 0.5 (0.3-0.7)b,a,d | 0.05 (0.02-0.17) | 0.2 (0.1-0.2) | 0.05 (0.03-0.1) |
| Defensins | 7.7 (4.9-10.2)b,c,d | 2.7 (1.4-3.3) | 0.9 (0.7-1.5) | 0.2 (0-0.2) |
| Ltf | 1.03 (0.74-1.6)b,c,d | 0.2 (0.18-0.37) | 0.1 (0.1-0.2) | 0.19 (0.12-0.2) |
The average area studied for each section was 12.6 × 105 μm (±10%). Quantification of the immunoreaction-positive area relative to the total tissue section was done by a computerized image-analyzing technique, and the results are expressed as a percentage for the ratio of the positive area to the total area. Data are given as medians, with 25th and 75th percentiles in parentheses. Probability values were determined by the Wilcoxon signed-rank test comparing data at different times within the same group of patients and by the Mann-Whitney U test comparing patients to healthy controls.
Significant difference versus 14 to 16 days.
Significant difference versus 30 to 35 days.
Significant difference versus controls.
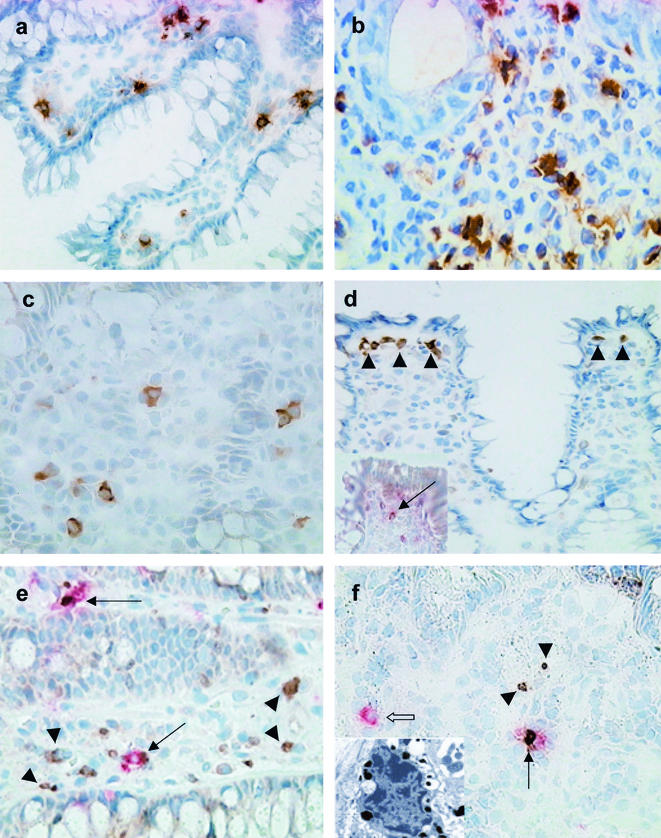
MBP expression by eosinophils, together with the eosinophil count, was significantly increased in the rectum 33 to 35 days after the onset of diarrhea in both adult and pediatric patients (Tables 3 and 4 and Fig. 2a). Expression of eotaxin (Fig. 2b) persisted up to the convalescent stage, while that of CCR3 (Fig. 2c), the receptor for eotaxin, was elevated only in the acute stage compared to controls (Tables 3 and 4). However, tissue expression of IL-3 and IL-5 did not increase in either group of patients in comparison to healthy controls (data not shown). PGHS-1 is constitutively expressed by the cells in the gut and is a key and rate-limiting enzyme in the arachidonic acid pathway for the synthesis of eicosanoids (19). The PGHS-1-positive cells were located predominantly just beneath the surface epithelium in the lamina propria (Fig. 2d). During acute shigellosis, expression of PGHS-1 was markedly increased in both children and adults compared to the convalescent stage, as well as healthy controls. Double staining revealed some chymase-positive cells expressing PGHS-1 close to the epithelial layer (Fig. 2d, inset).
TABLE 4.
Quantitative comparison of various mediators expressed by cells of the innate immune system in rectal mucosa from pediatric patients at acute and convalescent stages of disease and in healthy controlsa
| Mediators and receptors | % Positive area
|
||
|---|---|---|---|
| Children
|
Healthy controls | ||
| 3-5 days | 30-35 days | ||
| Chymase | 0.8 (0.56-1.2)c | 0.7 (0.4-0.86) | 0.3 (0.2-0.4) |
| Tryptase | 1.76 (0.6-2.6)c | 1.1 (0.6-1.6) | 0.7 (0.5-0.8) |
| SCF | 3 (1.6-3.7)b | 1.2 (0.8-1.4) | 1 (0.9-1.5) |
| MBP | 1 (0.6-2.6) | 2.8 (2.2-4.6)c | 0.4 (0.25-0.6) |
| Eotaxin | 0.5 (0.15-0.6)c | 0.22 (0.13-0.8) | 0.01 (0.01-0.1) |
| CCR3 | 1.3 (0.95-1.5)c | 0.8 (0.6-1) | 0.6 (0.3-0.7) |
| PGHS-1 | 0.3 (0.3-0.9)c | 0.17 (0.08-0.6) | 0.14 (0.05-0.2) |
| Defensins | 8.4 (6.3-12)b,c | 6 (3.9-6.8)c | 0.2 (0.2-1) |
| Ltf | 0.56 (0.43-1.44)b,c | 0.13 (0.1-0.31) | 0.07 (0.02-0.13) |
The average area studied for each section was 12.5 × 105 μm2 (±10%). Quantification of immunoreaction-positive area relative to the total tissue section was done by a computerized image-analyzing technique, and the results are expressed as a percentage for the ratio of the positive area to the total area. The data are given as medians, with 25th and 75th percentiles in parentheses. Probability values were determined by the Wilcoxon signed-rank test comparing data at different times within the same group of patients and by the Mann-Whitney U test comparing patients to healthy controls.
Significant difference versus 30 to 35 days.
Significant difference versus controls.
FIG.2.
Rectal biopsy sections from adult and pediatric patients showing localization of eosinophils, mast cells, and various mediators, as well as proliferating and apoptotic mast cells, during the course of Shigella infection. (a) Eosinophils expressing MBP were significantly increased in the rectum of a Shigella-infected pediatric patient during late convalescence (30 to 35 days after the onset of disease). Magnification, ×380. (b) Eotaxin expression was markedly upregulated during acute Shigella infection (3 to 5 days after the onset of diarrhea) in a pediatric patient. Eotaxin expression was localized to the eosinophils, as well as neutrophils, as is evident from the morphological appearance. Magnification, ×855. (c) Immunohistochemical staining revealed increased expression of CCR3 in rectal tissue during acute Shigella infection (3 to 5 days after the onset of disease) in an adult. The staining was localized primarily to the cytoplasm of the cells. Magnification, ×570. (d) Immunohistochemical staining delineates cells underlying the surface epithelium expressing PGHS-1 during acute dysentery (3 to 5 days after the onset of disease) in an adult patient. The positive cells were in close proximity to each other (arrowheads). The inset shows a chymase-positive mast cell (deep pink) expressing PGHS-1 (brown) just below the surface epithelium, as shown by double staining (arrow). Magnification, ×380. (e) During the early stage of acute Shigella infection (3 to 5 days after the onset of diarrhea), increased numbers of Ki67-positive cells (brown; arrowheads) were present in the lamina propria and in the crypt epithelial lining in a pediatric patient. Chymase-positive mast cells (deep pink) also stained for anti-Ki67 antibody (brown), indicating active proliferation of mast cells (arrows). Magnification, ×570. (f) During acute shigellosis (3 to 5 days after the onset of diarrhea), apoptotic (TUNEL-positive [brown; arrowheads]) cells were seen in the lamina propria in an adult patient. Double immunohistochemical staining showed an apoptotic (brown) chymase-positive mast cell (deep pink, thin arrow) in the proximity of an unaffected mast cell (deep pink; open arrow) in the lamina propria. Magnification, ×380. In the inset, ultrastructural analysis reveals an apoptotic mast cell. The mast cell shows nuclear pyknosis, without cytoplasmic-organelle swelling, suggestive of early apoptosis. Magnification, ×7,980.
Expression of the microbicidal factors Ltf and α-defensin, released primarily by neutrophils, was considerably increased concurrent with increased neutrophil accumulation during the acute stage. The tissue expression of both proteins decreased in adults during convalescence, while in pediatric patients, α-defensin expression remained elevated up to 1 month after the onset of diarrhea (Table 4).
Proliferation and death of mast cells and eosinophils.
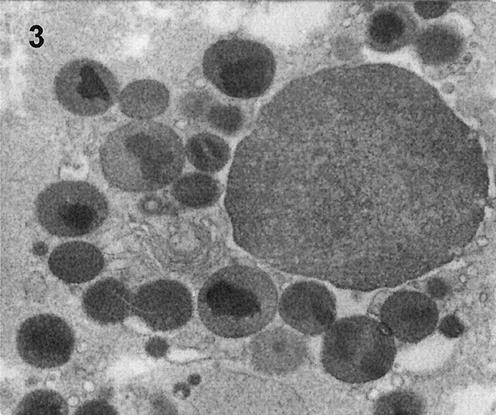
A vast majority of tryptase-positive mast cells stained for Ki67 in adults, with fewer such cells in pediatric patients in the acute stage (Fig. 2e). This indicated active proliferation of mast cells in the acute stage, especially in adults. Intriguingly, in the acute stage, increased TUNEL-tryptase-double positive mast cells (Fig. 2f and inset of electron micrograph) were also seen mostly in adults and to a lesser extent in children. Thus, increased proliferation and increased apoptotic death of mast cells occurred simultaneously in the acute stage and were more prominent in adults. On the other hand, we could not detect Ki67-MBP double-positive cells in the convalescent stage, though the number of MBP-positive eosinophils had increased. We were also unable to detect apoptotic eosinophils by immunostaining; however, electron microscopic examination showed extensive necrosis of eosinophils (Fig. 3) in the acute stage that decreased during convalescence, accompanied by an increase in the eosinophil cell count during convalescence (Table 5).
FIG. 3.
Electron micrograph of a necrotic eosinophil in the lamina propria in the rectal mucosa during acute Shigella infection (3 to 5 days after the onset of diarrhea) in a pediatric patient. Magnification, ×19,000.
TABLE 5.
Mast cells showing ultrastructural evidence of degranulation and activation in rectal biopsies of adults and children with shigellosis
| Features of mast cellsa | % of cells with featureb
|
||||||
|---|---|---|---|---|---|---|---|
| Adults
|
Children
|
||||||
| 3-5 (n = 30)c | 14-16 (n = 31) | 30-35 (n = 24) | Healthy controls (n = 20) | 3-5 (n = 26) | 30-35 (n = 33) | Healthy controls (n = 38) | |
| GT-particle | 23 | 16 | 25 | 5 | 12 | 36 | 21 |
| GT-scroll | 50 | 68e | 29d | 70 | 62d | 30e | 16 |
| GT-mixed | 27 | 16e | 46 | 25 | 27d | 33d | 63 |
| Normal | 23d | 26d | 8d | 65 | 4d | 15d | 53 |
| PMD | 20 | 34 | 54d,e | 15 | 46d | 52d | 8 |
| AND | 47d | 35 | 25 | 15 | 42 | 30 | 34 |
| Lipid bodies | 43d | 45d | 21 | 10 | 62d | 27e | 26 |
GT, granule type.
Chi-square test or Fischer's exact test was used in comparing the differences between patients on different days and healthy controls.
Number of days after onset of diarrhea. n, total number of must cells studied.
Significant difference versus controls.
Significant difference versus days 3 to 5.
Ultrastructaral analyses of mast cells in the rectum.
Ultrastructural studies were performed to study the degranulation and activation patterns of mast cells to better understand their mechanism of action during acute shigellosis. In adult patients, there was a gradual decline in the percentage of normal inactive mast cells from the acute to the convalescent stages. The percentage of normal mast cells was significantly higher in the acute stage than in the convalescent stage (Table 5). The granules in mast cells were mostly scroll type, with a small percentage of mixed type. The piecemeal type of degranulation (PMD) from the membrane-bound granules was observed, with partially empty to completely empty granule chambers in the mast cells. PMD was markedly higher in mast cells 14 to 16 days after the onset of diarrhea compared to that in the acute stage or in the healthy controls and persisted even 30 to 35 days after onset (Table 5). On the other hand, the anaphylactic type of degranulation (AND), though evident throughout the study period, was markedly elevated in the acute stage compared to that in the healthy controls. The prevalence of lipid bodies was strikingly higher in mast cells in the acute and early convalescent stages compared to that in the healthy controls (P < 0.03) (Table 5). Interestingly, apoptotic mast cells were also detected by electron microscopic analysis in the rectum during acute shigellosis (Fig. 2f, inset).
Unlike adults, pediatric patients had significantly lower numbers of normal inactive mast cells throughout the study period (3 to 5 and 30 to 35 days after onset) than healthy controls. Scroll-type granules were significantly more numerous in pediatric patients than in controls. As seen in adult patients, PMD was remarkably elevated in the acute and convalescent stages in pediatric patients compared to healthy controls (P < 0.003) (Table 5). Similar to adults, AND in mast cells was slightly higher in the acute stage but was not significantly different from that in the controls. The prevalence of lipid bodies in mast cells was noticeably increased in the acute stage compared to controls (P < 0.01), with a rapid decline during convalescence.
Ultrastructural analyses of eosinophils in shigellosis.
In adult patients with shigellosis, surprisingly, inactive normal eosinophils were very few throughout the study period (Table 6), while in healthy adults, 75% of the total eosinophils were found in a resting state. MBP is the major constituent of the cores, while the matrix contains many eosinophil proteins and cytokines. Combined release of the contents of both the matrix and core in a PMD pattern was significantly increased in the late convalescent stage (30 to 35 days after onset) compared to controls (Table 6). This indicated the release of MBP, as well as other proteins and cytokines, in the local milieu, although the release of matrix proteins alone was also evident in some adult patients. AND by eosinophils was almost absent in shigellosis. Lipid bodies were significantly upregulated throughout the study period in adults compared to the healthy controls. Extensive necrotic death of eosinophils was noted within 3 to 5 days after the onset of diarrhea, and it decreased during late convalescence (P < 0.003) (Fig. 3).
TABLE 6.
Eosinophils showing ultrastructural evidence of degranulation, activation, and death in rectal biopsies of adults and children with shigellosis
| Features of eosinophils | % of cells with featurea
|
||||||
|---|---|---|---|---|---|---|---|
| Adults
|
Children
|
||||||
| 3-5 (n = 24)b | 14-16 (n = 44) | 30-35 (n = 21) | Healthy controls (n = 20) | 3-5 (n = 20) | 30-35 (n = 30) | Healthy controls (n = 25) | |
| PMD matrix only | 50 | 47.7 | 52.4 | 20 | 40 | 43.3 | 28 |
| PMD core and matrix | 4 | 16 | 28.6c,d | 0 | 10 | 13.3 | 0 |
| Necrosis | 33.3c | 20.5c | 5d | 0 | 30 | 30 | 8 |
| Lipid bodies | 66.6a | 9d | 24d | 10 | 25 | 26.6 | 40 |
| Normal | 12.5c | 9c | 14c | 75 | 15c | 3c | 64 |
Chi-square test or Fischer's exact test was used in comparing the differences between patients on different days and healthy controls.
Number of days after onset of disease. n, number of eosinophils studied in each group.
Significant difference versus controls.
Significant difference versus days 3 to 5.
In pediatric patients with acute shigellosis, 15% of the total eosinophils were inactive normal eosinophils that were replaced by activated cells during convalescence. The release of core and matrix contents was also evident in children, though not significantly different from that in healthy controls (Table 6). The most striking feature in children was the high frequency of eosinophils with necrotic injury in the acute stage that persisted even in the convalescent stage. However, the trend in PMD, lipid bodies, and necrosis, though similar to that in adults, was not significantly different from that in healthy controls. In fact, lipid bodies were more prominent in the controls than in the patients. This could be due to the fact that the healthy tissue samples were obtained from each patient 2 months after clinical recovery, accentuating the possibility of a lingering effect.
DISCUSSION
The study indicates that mast cells and eosinophils were upregulated in acute shigellosis, though the accumulation of neutrophils, mast cells, and eosinophils and the release of mediators were somewhat different in adult and pediatric patients.
There was an early enrichment of mast cells in the site of infection in the rectal mucosa in both adult and pediatric patients, as was evident from naphthol chloroacetate staining and mast cell enzyme expression. Double staining showed accumulation of Ki67-positive mast cells in the rectum, suggesting active proliferation of these cells. Early infiltration suggests that mast cells may directly encounter the bacteria (15, 17), activate endothelial cells (10), and recruit leukocytes, as well as antigen-processing and -presenting cells (18). Tumor necrosis factor alpha (TNF-α) and chymase released by activated mast cells have been shown to be critical for neutrophil chemotaxis, in addition to potentiating neutrophil bactericidal properties (5, 14, 42). SCF, a mast cell growth factor and a mediator of mast cell chemotaxis, was increased in acute shigellosis, which may have promoted the growth, proliferation, and survival of mast cells (21). However, despite this finding, we observed increased apoptotis in mast cells in the acute stage. Reduced Bcl-2 expression during acute shigellosis may promote mast cell apoptosis (25). The sharp decline in tissue expression of SCF in the convalescent stage was in accordance with the reduction in numbers of mast cells in adults during that time. Since Ki67-positive mast cells were not as prominent in children as in adults, it may be that the persistence of mast cells in children was not due to proliferation of local mast cells alone but also to local recruitment from other body sites (37), as seen in vitro (12). It may also be that the released chymase noticed in mast cells participates in local mast cell accumulation in a positive-feedback fashion via the ability of chymase to process cell membrane-bound SCF (36). Mast cell-derived chymase and tryptase stimulate epithelial cell proliferation and may be important in the healing process during convalescence in shigellosis (2).
Lipid bodies in the cytoplasm of human mast cells contain PGHS and are reservoirs of arachidonyl phospholipid (40). The increased prevalence of lipid bodies in mast cells and the enhanced tissue expression of PGHS-1 was suggestive of increased activity of the arachidonic acid pathway (6, 24). This was further strengthened by an earlier report of increased release of the eicosanoids prostaglandin E2 and leukotriene B4 in stool in acute shigellosis (27). In the present study, we could locate a few chymase-positive mast cells expressing PGHS-1 in the rectum by double staining. SCF has been shown to induce PGHS-I mRNA and protein in bone marrow-derived mast cells, leading to increased eicosanoid formation (34), which was also found in the present study. Increased PMD in early convalescence suggests a slow and regulated release of mediators from the secondary granules in mast cells that may occur as a result of specific antigen-induced continuous activation of mast cells in the microenvironment for a prolonged period (16).
We observed that the response of eosinophils to Shigella infection was prolonged and persistent, especially in children, both in the peripheral blood and in the rectal tissue (Table 1 and Fig. 1). Eosinophil localization to the lamina propria is critically regulated by eotaxin and IL-5 (32). An initial upregulation of eotaxin, the primary eosinophil chemottractant, and its receptor, CCR3, during acute shigellosis suggested increased recruitment of eosinophils and mast cells to the site of infection, since both cell types express CCR3 (31). However, eosinophils persisted for a long time even after downregulation of eotaxin and CCR3 in pediatric patients during convalescence. Eotaxin is also a potent activator of respiratory burst in eosinophils (7). Eosinophil homing to the mucosal site may occur by eotaxin-dependent, as well as eotaxin-independent, pathways (22, 32), such as α4β7 integrin, which is expressed by gastrointestinal eosinophils and is partly responsible for eosinophil homing (4). IL-5 is important for eosinophil growth and differentiation, and a combined effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-5 was seen in regulating eosinophil levels in the gastrointestinal tract (22, 32). Nevertheless, in the present study there was a baseline level of tissue expression of IL-5 that remained unchanged throughout the study period. This suggests the involvement of other chemokines, such as GM-CSF, in the regulation of eosinophil trafficking to and accumulation in the gastrointestinal tract during recovery from shigellosis (25). The slow protracted release of granule contents by eosinophils and their persistence in the rectum for a prolonged period in children may have a critical role in bactericidal activity (23), induction of antigen-specific T-cell responses (8, 33), and wound repair and tissue remodeling by synthesizing transforming growth factor β1-3. Mast cells are known to enhance eosinophil survival by releasing TNF-α and GM-CSF (11). However, extensive necrotic injury and death of eosinophils, which decreased but was still evident in children 1 month after onset, were noted early in the disease process even with an excess of TNF-α and GM-CSF (26, 30). The presence of parasites as copathogens during the course of shigellosis was apparently not responsible for the accumulation of eosinophils and mast cells in the rectum in convalescence.
Infiltration of neutrophils into the rectal mucosa was much higher in adults and resolved quickly during convalescence, while in pediatric patients it persisted for a month. Synchronously, expression of α-defensin, released primarily by neutrophils, remained upregulated in the convalescent stage in pediatric patients. This could be due to the persistence of mast cells, which in turn may help to maintain a sustained pool of neutrophils at the local site for various functions. α-Defensins have been shown to be chemotactic for mononuclear cells and neutrophils; they can induce CD45RA+ and CD8 T-lymphocyte migration for direct bactericidal activity (5), are known to initiate adaptive immune responses by recruiting immature dendritic cells to sites of infection (41), and may be involved in wound repair (38).
Increased numbers of eosinophils and mast cells have been implicated in inflammatory and allergic diseases and in parasitic infections. However, the present study demonstrated upregulation of activated degranulating mast cells and eosinophils in shigellosis, an infectious colitis. The accumulation of mast cells in the rectum was short-lived in adults, while in pediatric patients they persisted for a prolonged period. In contrast to adults, neutrophils were significantly less pronounced in pediatric patients. Perhaps due to the delayed and reduced onset of specific immune responses in children with Shigella infection (28), the innate cells play a critical role in immune surveillance for a prolonged period, thereby participating in the development of adaptive immune responses and tissue remodeling, as well as the persistence of chronic pathology at the mucosal site (27).
Acknowledgments
We gratefully acknowledge the participation of the healthy controls and patients in this study.
This study was conducted at the ICDDRB, Center for Health and Population Research, with the support of grants from the Swedish Agency for Research Cooperation with developing countries (Sida/SAREC; grant no. 98-05440).
Editor: F. C. Fang
REFERENCES
- 1.Abraham, S. N., and R. Malaviya. 2000. Mast cell modulation of the innate immune response to enterobacterial infection. Adv. Exp. Med. Biol. 479:91-105. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, S. N., and R. Malaviya. 1997. Mast cells in infection and immunity. Infect. Immun. 65:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand, B. S., V. Malhotra, S. K. Bhattacharya, P. Datta, D. Datta, D. Sen, M. K. Bhattacharya, P. P. Mukherjee, and S. C. Pal. 1986. Rectal histology in acute bacillary dysentery. Gastroenterology 90:654-660. [DOI] [PubMed] [Google Scholar]
- 4.Artis, D., N. E. Humphreys, C. S. Potten, N. Wagner, W. Muller, J. R. McDermott, R. K. Grencis, and K. J. Else. 2000. Beta7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur. J. Immunol. 30:1656-1664. [DOI] [PubMed] [Google Scholar]
- 5.Chertov, O., D. Yang, O. M. Howard, and J. J. Oppenheim. 2000. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol. Rev. 177:68-78. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak, A. M. 1997. New aspects of mast cell biology. Int. Arch. Allergy Immunol. 114:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Elsner, J., R. Hochstetter, D. Kimmig, and A. Kapp. 1996. Human eotaxin represents a potent activator of the respiratory burst of human eosinophils. Eur. J. Immunol. 26:1919-1925. [DOI] [PubMed] [Google Scholar]
- 8.Islam, D., B. Wretlind, A. Lindberg, and B. Christensson. 1996. Changes in the peripheral blood T-cell receptor V beta repertoire in vivo and in vitro during shigellosis. Infect. Immun. 64:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jolly, S., J. Detilleux, F. Coignoul, and D. Desmecht. 2000. Enzyme-histochemical detection of a chymase-like proteinase within bovine mucosal and connective tissue mast cells. J. Comp. Pathol. 122:155-162. [DOI] [PubMed] [Google Scholar]
- 10.Krishnaswamy, G., J. Kelley, D. Johnson, G. Youngberg, W. Stone, S. K. Huang, J. Bieber, and D. S. Chi. 2001. The human mast cell: functions in physiology and disease. Front. Biosci. 6:D1109-D1127. [DOI] [PubMed]
- 11.Levi-Schaffer, F., V. Temkin, V. Malamud, S. Feld, and Y. Zilberman. 1998. Mast cells enhance eosinophil survival in vitro: role of TNF-alpha and granulocyte-macrophage colony-stimulating factor. J. Immunol. 160:5554-5562. [PubMed] [Google Scholar]
- 12.Lin, T. J., T. B. Issekutz, and J. S. Marshall. 2000. Human mast cells transmigrate through human umbilical vein endothelial monolayers and selectively produce IL-8 in response to stromal cell-derived factor-1 alpha. J. Immunol. 165:211-220. [DOI] [PubMed] [Google Scholar]
- 13.Malaviya, R., and S. N. Abraham. 2000. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. J. Leukoc. Biol. 67:841-846. [DOI] [PubMed] [Google Scholar]
- 14.Malaviya, R., T. Ikeda, E. Ross, and S. N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381:77-80. [DOI] [PubMed] [Google Scholar]
- 15.Malaviya, R., T. Ikeda, E. A. Ross, B. A. Jakschik, and S. N. Abraham. 1995. Bacteria-mast cell interactions in inflammatory disease. Am. J. Ther. 2:787-792. [DOI] [PubMed] [Google Scholar]
- 16.Malaviya, R., E. Ross, B. A. Jakschik, and S. N. Abraham. 1994. Mast cell degranulation induced by type 1 fimbriated Escherichia coli in mice. J. Clin. Investig. 93:1645-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malaviya, R., E. A. Ross, J. I. MacGregor, T. Ikeda, J. R. Little, B. A. Jakschik, and S. N. Abraham. 1994. Mast cell phagocytosis of FimH-expressing enterobacteria. J. Immunol. 152:1907-1914. [PubMed] [Google Scholar]
- 18.Malaviya, R., N. J. Twesten, E. A. Ross, S. N. Abraham, and J. D. Pfeifer. 1996. Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells. J. Immunol. 156:1490-1496. [PubMed] [Google Scholar]
- 19.Masferrer, J. L., B. S. Zweifel, K. Seibert, and P. Needleman. 1990. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J. Clin. Investig. 86:1375-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathan, M. M., and V. I. Mathan. 1986. Ultrastructural pathology of the rectal mucosa in Shigella dysentery. Am. J. Pathol. 123:25-38. [PMC free article] [PubMed] [Google Scholar]
- 21.Mekori, Y. A., C. K. Oh, and D. D. Metcalfe. 1995. The role of c-Kit and its ligand, stem cell factor, in mast cell apoptosis. Int. Arch. Allergy Immunol. 107:136-138. [DOI] [PubMed] [Google Scholar]
- 22.Mishra, A., S. P. Hogan, J. J. Lee, P. S. Foster, and M. E. Rothenberg. 1999. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Investig. 103:1719-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson, T., P. Andersson, M. Bodelsson, M. Laurell, J. Malm, and A. Egesten. 2001. Bactericidal activity of human eosinophilic granulocytes against Escherichia coli. Infect. Immun. 69:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulimood, A. B., M. M. Mathan, and V. I. Mathan. 1998. Quantitative and ultrastructural analysis of rectal mucosal mast cells in acute infectious diarrhea. Dig. Dis. Sci. 43:2111-2116. [DOI] [PubMed] [Google Scholar]
- 25.Raqib, R., C. Ekberg, P. Sharkar, P. K. Bardhan, A. Zychlinsky, P. J. Sansonetti, and J. Andersson. 2002. Apoptosis in acute shigellosis is associated with increased production of Fas/Fas ligand, perforin, caspase-1, and caspase-3 but reduced production of Bcl-2 and interleukin-2. Infect. Immun. 70:3199-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raqib, R., A. A. Lindberg, B. Wretlind, P. K. Bardhan, U. Andersson, and J. Andersson. 1995. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect. Immun. 63:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raqib, R., S. M. Mia, F. Qadri, T. I. Alam, N. H. Alam, A. K. Chowdhury, M. M. Mathan, and J. Andersson. 2000. Innate immune responses in children and adults with shigellosis. Infect. Immun. 68:3620-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raqib, R., F. Qadri, P. Sarker, S. M. Mia, P. J. Sansonnetti, M. J. Albert, and J. Andersson. 2002. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand. J. Immunol. 55:414-423. [DOI] [PubMed] [Google Scholar]
- 29.Raqib, R., F. P. Reinholt, P. K. Bardhan, A. Karnell, and A. A. Lindberg. 1994. Immunopathological patterns in the rectal mucosa of patients with shigellosis: expression of HLA-DR antigens and T-lymphocyte subsets. APMIS 102:371-380. [DOI] [PubMed] [Google Scholar]
- 30.Raqib, R., B. Wretlind, J. Andersson, and A. A. Lindberg. 1995. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stool than to plasma. J. Infect. Dis. 171:376-384. [DOI] [PubMed] [Google Scholar]
- 31.Romagnani, P., A. De Paulis, C. Beltrame, F. Annunziato, V. Dente, E. Maggi, S. Romagnani, and G. Marone. 1999. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am. J. Pathol. 155:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothenberg, M. E. 2001. Gastrointestinal eosinophils. Allergy 56(Suppl. 67):21-22. [DOI] [PubMed] [Google Scholar]
- 33.Rothenberg, M. E., A. Mishra, E. B. Brandt, and S. P. Hogan. 2001. Gastrointestinal eosinophils in health and disease. Adv. Immunol. 78:291-328. [DOI] [PubMed] [Google Scholar]
- 34.Samet, J. M., M. B. Fasano, A. N. Fonteh, and F. H. Chilton. 1995. Selective induction of prostaglandin G/H synthase I by stem cell factor and dexamethasone in mast cells. J. Biol. Chem. 270:8044-8049. [DOI] [PubMed] [Google Scholar]
- 35.Sansonetti, P. J. 1998. Pathogenesis of shigellosis: from molecular and cellular biology of epithelial cell invasion to tissue inflammation and vaccine development. Jpn. J. Med. Sci. Biol. 51(Suppl.):S69-S80. [DOI] [PubMed]
- 36.Tomimori, Y., T. Muto, H. Fukami, K. Saito, C. Horikawa, N. Tsuruoka, K. Yamashiro, M. Saito, N. Sugiura, M. Sumida, S. Kakutani, and Y. Fukuda. 2002. Mast cell chymase regulates dermal mast cell number in mice. Biochem. Biophys. Res. Commun. 290:1478-1482. [DOI] [PubMed] [Google Scholar]
- 37.Trautmann, A., A. Toksoy, E. Engelhardt, E. B. Brocker, and R. Gillitzer. 2000. Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J. Pathol. 190:100-106. [DOI] [PubMed] [Google Scholar]
- 38.van Wetering, S., P. J. Sterk, K. F. Rabe, and P. S. Hiemstra. 1999. Defensins: key players or bystanders in infection, injury, and repair in the lung? J. Allergy Clin. Immunol. 104:1131-1138. [DOI] [PubMed] [Google Scholar]
- 39.Weller, P. F. 1991. The immunobiology of eosinophils. N. Engl. J. Med. 324:1110-1118. [DOI] [PubMed] [Google Scholar]
- 40.Weller, P. F., and A. M. Dvorak. 1994. Lipid bodies: intracellular sites for eicosanoid formation. J. Allergy Clin. Immunol. 94:1151-1156. [DOI] [PubMed] [Google Scholar]
- 41.Yang, D., Q. Chen, O. Chertov, and J. J. Oppenheim. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68:9-14. [PubMed] [Google Scholar]
- 42.Zhang, Y., B. F. Ramos, and B. A. Jakschik. 1992. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science 258:1957-1959. [DOI] [PubMed] [Google Scholar]