Abstract
Colonization of the cardiovascular endothelium by viridans group streptococci can result in infective endocarditis and possibly atherosclerosis; however, the mechanisms of pathogenesis are poorly understood. We investigated the ability of selected oral streptococci to infect monolayers of human umbilical vein endothelial cells (HUVEC) in 50% human plasma and to produce cytotoxicity. Planktonic Streptococcus gordonii CH1 killed HUVEC over a 5-h period by peroxidogenesis (alpha-hemolysin) and by acidogenesis but not by production of protein exotoxins. HUVEC were protected fully by addition of supplemental buffers and bovine liver catalase to the culture medium. Streptococci were also found to invade HUVEC by an endocytic mechanism that was dependent on polymerization of actin microfilaments and on a functional cytoskeleton, as indicated by inhibition with cytochalasin D and nocodazole. Electron microscopy revealed streptococci attached to HUVEC surfaces via numerous fibrillar structures and bacteria in membrane-encased cytoplasmic vacuoles. Following invasion by S. gordonii CH1, HUVEC monolayers showed 63% cell lysis over 4 h, releasing 64% of the total intracellular bacteria into the culture medium; however, the bacteria did not multiply during this time. The ability to invade HUVEC was exhibited by selected strains of S. gordonii, S. sanguis, S. mutans, S. mitis, and S. oralis but only weakly by S. salivarius. Comparison of isogenic pairs of S. gordonii revealed a requirement for several surface proteins for maximum host cell invasion: glucosyltransferase, the sialic acid-binding protein Hsa, and the hydrophobicity/coaggregation proteins CshA and CshB. Deletion of genes for the antigen I/II adhesins, SspA and SspB, did not affect invasion. We hypothesize that peroxidogenesis and invasion of the cardiovascular endothelium by viridans group streptococci are integral events in the pathogenesis of infective endocarditis and atherosclerosis.
Viridans group streptococci comprise a large proportion of the commensal bacteria that colonize oral surfaces (20, 24, 25). These bacteria frequently enter the bloodstream following trauma to oral tissues (12, 17, 41, 58) and can then adhere to surfaces of abnormal or previously damaged heart valves (15, 21, 29, 47) or become implanted in arterial atherosclerotic plaques (11). Streptococci growing on heart valve surfaces (causing infective endocarditis) become encased in a matrix of fibrin and platelets, which form macroscopic verrucous lesions and can lead to valve perforation, abnormalities in cardiac conduction, valve ring abscesses, pericarditis, aneurysm of the sinus of Valsalva, and release of peripheral emboli (21, 56). Viridans group streptococci are the most common cause of native valve endocarditis in humans, accounting for 45 to 80% of cases (5, 55). A variety of virulence factors have been implicated in the initial colonization of bacteria to cardiac valve surfaces (1, 32, 49, 50, 54), but those responsible for the ultimate destruction of underlying tissues are not well understood (1, 2, 28, 35, 48).
Experimental infective endocarditis in laboratory animals usually involves placement of an indwelling intracardiac catheter to cause endocardial damage that permits subsequent streptococcal colonization and infection (8, 19, 27, 43, 47); intravenous injection of bacteria in noncatheterized animals seldom results in cardiac infection. The requirement for the presence of a foreign body and the relatively large bacterial inoculum required for infection have raised concern about the relevance of these animal models to the natural disease in humans (2, 30, 51). In addition, animal models do not facilitate studies of the action of putative virulence factors during the early events in infection. Therefore, the present study was undertaken to define a simple, in vitro infection model using human endothelial cells and human plasma. In addition to the species relevance, the human model described here offers a much broader range of experimental conditions than do laboratory animals and allows for optimum quantification of experimental data during bacterial colonization, host cell invasion, and subsequent cell killing.
MATERIALS AND METHODS
Bacteria.
Bacteria (Table 1), were grown at 37°C in TSBY medium, composed of tryptic soy broth (Becton Dickinson and Co., Cockeysville, Md.) supplemented with 0.5% yeast extract (Difco Laboratories, Detroit, Mich.). Prior to their use in the infection assay, streptococci were passaged in 50/50 medium (pH 7.4), which was composed of equal volumes of CS-C basal medium (serum free and growth factor free) (Cell Systems, Kirkland, Wash.) and a pool of fresh-frozen human plasma. The 50/50 medium also contained the standard CPD anticoagulant (American Red Cross, Buffalo, N.Y.) and containing 85 mM HEPES buffer, 44 mM NaHCO3, and 100 U of heparin (Sigma Aldrich) per ml, adjusted to pH 7.4. The addition of heparin to the medium was necessary to prevent plasma clotting caused by the metabolism of the citric acid anticoagulant by some of the streptococci. Growth media were also supplemented, when necessary, with 5 μg of erythromycin per ml or 5 μg of chloramphenicol per ml (Sigma-Aldrich). Streptococcus gordonii strain CH1 (Challis) was chosen as the paradigm for most of the experiments reported here because it is virulent in the rat model of infection (59), several adhesion-deficient mutants were available for comparison (14, 39, 40), and it produces large amounts of the alpha-hemolysin (hydrogen peroxide) (3, 4).
TABLE 1.
Bacterial species and strains used in this study and their invasion of HUVEC monolayers
| Bacterium | Description | Source or reference | HUVEC invasiona |
|---|---|---|---|
| S. gordonii | |||
| DL1 (Challis) | Wild-type reference strain | 52 | 17 ± 5 |
| EM230 | DL1 hsa::ermAM | 52 | 4 ± 2 |
| OB219 | DL1 sspA′ sspB′::ermAM | 14 | 20 ± 4 |
| OB220 | DL1 sspA::ermAM | 14 | 19 ± 6 |
| OB235 | DL1 cshA3::ermAM | 40 | 2 ± 0.4 |
| OB271 | DL1 cshB2::ermAM | 40 | 2 ± 1 |
| OB277 | DL1 cshA31::cat cshB2::ermAM | 40 | 2 ± 0.6 |
| CH1 (Challis) | Wild-type reference strain | 59 | 82 ± 15 |
| AMS12 | CH1 with 1.7-kb Hind fragment of gtfG replaced with lacZ/Ermr | 59 | 14 ± 0.5 |
| M5 | Wild type from human dental plaque | 28 | 5 ± 2.5 |
| S. sanguis | |||
| L22 | Wild type from human dental plaque | 28 | 76 ± 11 |
| L52 | Wild type from human dental plaque | 28 | 9 ± 2 |
| L74 | Wild type from human dental plaque | 28 | 52 ± 8 |
| L79 | Wild type from human dental plaque | 28 | 14 ± 5 |
| 133-79 | Wild type from human endocarditis | 28 | 21 ± 5 |
| 2017-78 | Wild type from human endocarditis | 28 | 25 ± 3 |
| 10556 | Wild type from human endocarditis | ATCC | 98 ± 18 |
| S. oralis | |||
| KS32AR | Wild type from dental plaque | 8 | 9 ± 8 |
| 16532AR | Wild type from dental plaque | 8 | 3 ± 2 |
| S. mitis | |||
| NCTC 10712 | Wild type from dental plaque | 8 | 23 ± 6 |
| OP51 | Wild type from dental plaque | 8 | 35 ± 9 |
| S. mutans | |||
| UA159 | Wild type from dental plaque | 60 | 26 ± 7 |
| S. salivarius | |||
| 13419 | Wild type from human saliva | ATCC | 2 ± 1 |
| E. coli | |||
| HB101 | Hybrid of strains K-12 and B, transformable strain | 7 | <0.01 |
Average number of intracellular bacteria (× 104) per HUVEC monolayer ± standard deviation of the mean (n = 6).
ATCC, American Type Culture Collection.
Cell culture.
Human umbilical vein endothelial cells (HUVEC) were purchased from Cell Systems and grown routinely in CS-C medium containing fetal calf serum and growth factor. For the infection assay, 5 × 104 HUVEC in 0.2 ml of CS-C medium without growth factor (Cell Systems) were seeded into each well of a gelatin-coated 48-well plate and incubated for 20 h at 37°C in 5% CO2 to establish a confluent monolayer. Fifty percent human plasma (50/50 medium) was chosen for the subsequent coculture experiments with streptococci because it was the highest concentration of plasma that supported maximum viability of HUVEC monolayers over 48 h.
Infection of HUVEC monolayers.
Streptococci were grown overnight in 50/50 medium without exogenous catalase and were harvested during mid-logarithmic growth phase by centrifugation; the various streptococcal strains had generation times between 150 and 195 min under these conditions. The bacterial pellets were suspended to the indicated cell densities in fresh 50/50 medium, readjusted to pH 7.4, and added to HUVEC monolayers in 48-well plates. In one series of experiments, the HUVEC-streptococcus cocultures were incubated for up to 5 h at 37°C in 5% CO2. The culture medium and planktonic bacteria were removed, and the HUVEC monolayers were washed once with CS-C basal medium. HUVEC were detached from the plastic surface using 0.1 ml of trypsin-EDTA solution (PRG-2 solution; Cell Systems) for 3 to 5 min; then 0.1 ml of 50/50 medium was added. The suspended HUVEC were counted microscopically in a hemacytometer in the presence of trypan blue dye to determine percent viability and total cell numbers. In some experiments, the 50/50 medium was supplemented with 10,000 U of bovine liver catalase (Sigma Aldrich) per ml to inactivate the alpha-hemolysin of viridans group streptococci.
In a second series of experiments, the ability of viridans streptococci to enter HUVEC was determined using a modification of the antibiotic protection assay of Isberg and Falkow (31). The HUVEC-streptococcus cocultures, in 48-well plates, were immediately centrifuged at 800 × g for 10 min and then incubated for the indicated time intervals. After the medium and planktonic bacteria were removed and discarded, 0.4 ml of 50/50 medium, containing 200 μg of gentamicin, 20 μg of penicillin G, and 10,000 U of catalase per ml (46), was added to each well, and the plates were incubated for 1 h at 37°C in 5% CO2 to kill residual extracellular bacteria. Preliminary experiments showed that these conditions were sufficient to kill 2 × 108 streptococci per ml in 1 h. The resulting monolayers were treated in the following sequence: one 30-s wash was performed with 0.4 ml of EDTA solution (PRG-1; Cell Systems); 0.1 ml of trypsin-EDTA solution (PRG-2; Cell Systems) was added to each well, and the plates were incubated for 10 min at 37°C; 0.4 ml of 0.1% Tween 20 was added, and the mixture was incubated at 37°C for 20 min; and finally the well contents (0.5 ml) were pipetted vigorously several times to complete the lysis of HUVEC and to liberate intracellular streptococci. A 100-μl volume was removed from each well, serially diluted in ice-cold TSBY, and spread onto TSBY agar plates. The CFU of invasive bacteria were enumerated after the plates had been incubated for 48 h at 37°C in 5% CO2. Invasion is expressed as total CFU per monolayer (5 × 104 HUVEC) or as a percentage of the initial bacterial inoculum. All assays were performed in triplicate and repeated at least twice.
To determine the ability of S. gordonii CH1 to multiply after penetrating HUVEC, the invasion assay was modified as follows. After centrifugation and removal of planktonic bacteria from the cocultures, the monolayers were incubated at 37°C in 50/50 medium containing 40 μg of gentamicin per ml and 4 μg of penicillin per ml, the minimum lethal concentration for 2 × 107 streptococci per ml. After 1 h, the culture medium was removed and replaced with fresh 50/50 medium devoid of antibiotics. At the indicated times, the culture medium was collected and centrifuged at 225 × g for 5 min to separate intact HUVEC (pellet) from planktonic bacteria (supernatant fluid). The monolayer and detached HUVEC were lysed and analyzed for intracellular streptococci using the standard assay. Planktonic bacteria were enumerated by CFU on agar plate medium.
Invasion inhibitors.
HUVEC monolayers were incubated for 30 min at 37°C in the presence of selected chemical inhibitors prior to coculture with streptococci. One exception was for nocodazole, which was preincubated with HUVEC on ice for 1 h to disrupt microtubules (45) followed by 30 min at 37°C prior to the assay. Each inhibitor was also present during the 2-h coculture period. Protease inhibitor cocktail (Sigma Aldrich), containing 104 mM AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride], 0.08 mM aprotinin, 2.1 mM leupeptin, 3.6 mM bestatin, 1.5 mM pepstatin A, and 1.4 mM E-64, was added to the 50/50 culture medium at a final dilution of 1:1,000. In each case, control HUVEC monolayers were treated with the inhibitors in the absence of streptococci and tested for cell viability using the trypan blue exclusion assay. The concentration reported for each inhibitor in this study was the maximum tolerated by uninfected HUVEC monolayers without loss of viability.
Electron microscopy.
S. gordonii CH1 was added to a HUVEC monolayer growing on a glass coverslip, at a 2,000:1 ratio of bacteria to host cells. The coculture was centrifuged at 800 × g for 10 min and then incubated for 1 h at 37°C in 5% CO2. The supernatant fluid was removed, and the monolayer was washed once with CS-C basal medium to remove planktonic streptococci. The monolayers were fixed with 2% glutaraldehyde in 0.05 M sodium cocodylate buffer (pH 7.4) and then postfixed in 0.5% osmium tetroxide-0.8% potassium ferricyanide-0.15% tannic acid-2% uranyl acetate. The samples were dehydrated in a graded acetone series and embedded in Embed-Araldite. Sections were cut with a diamond knife on a Reichert Ultracut E ultramicrotome, stained with saturated aqueous uranyl acetate and Reynold's lead citrate, and examined with a JEOL 100CX-II electron microscope at magnifications of ×4,000, ×8,000, and ×27,000.
Statistical analysis.
Student's t test with Welch correction (GraphPad/InStat, San Diego, Calif.) was used to compare differences in HUVEC invasion by selected isogenic streptococcal strains, and statistical significance was achieved at P < 0.05.
RESULTS
Killing of HUVEC during coculture.
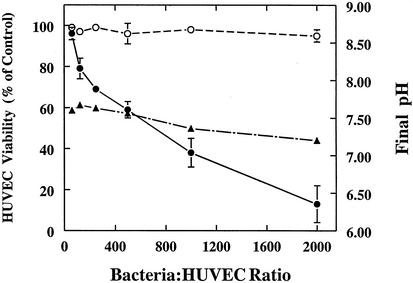
S. gordonii CH1 was examined initially for its ability to produce, in 50% human plasma, extracellular products that are detrimental to cultured HUVEC. This pathogenic mechanism was considered likely to occur in endocarditis since large numbers of viridans streptococci can accumulate in valvular lesions. Streptococci growing in human plasma were coincubated for 5 h with HUVEC monolayers at multiplicities of infection (MOI) of 50 to 2,000 (Fig. 1). In the absence of exogenous catalase, HUVEC numbers in the monolayers decreased in a dose-dependent manner, reaching 87% loss at the highest MOI. The percent viability of HUVEC making up the residual monolayer in each coculture was 70 to 89%, whereas the noninfected control cultures were 92 to 96% viable. Similar rates of HUVEC loss were observed during coculture with S. gordonii DH1, S. sanguis 10556 and S. mitis OP51 (data not shown). In an independent series of cocultures with S. gordonii CH1, the HUVEC monolayers were totally protected at all MOI by the addition of 10,000 U of bovine liver catalase, indicating that the cytotoxic effect seen in the first set of cocultures was due to the production of H2O2, which is the alpha-hemolysin of viridans group streptococci (3). The endogenous catalase in the fresh-frozen human plasma was unavoidably inactivated by freeze-thawing. Identical cytotoxic effects were induced in HUVEC monolayers by the addition of pure H2O2; 50% destruction of the monolayers was achieved in 5 h with 18 nmol of H2O2 per well. Acidogenesis did not contribute to cell death in Fig. 1; the pH decreased slowly from 7.45 to 7.21 during the 5-h assay, a pH range that supported maximum viability of HUVEC monolayers in control experiments. Separate experiments, however, revealed that failure to fortify the buffering system of the 50/50 culture medium resulted in rapid acidogenesis (final pH, 5.9 to 6.5) due to lactic acid production by the streptococci, which caused complete loss of the monolayers. When HUVEC were cultured in the absence of streptococci for 5 h in 50/50 medium at different pHs, 50% of the monolayer was lost at pH 6.8. Other experiments showed that bacterial viability was necessary for maximum effect; cocultures conducted in 50/50 medium containing 50 μg of penicillin G per ml or 50 μg of erythromycin per ml resulted in 65 and 74% reduction in streptococcus-induced damage of HUVEC monolayers, respectively, and coculture with heat-killed bacteria (70°C for 30 min) produced no HUVEC damage. Addition of filter-sterilized, spent 50/50 medium obtained from an aerated 5-h S. gordonii culture resulted in 54% destruction of HUVEC monolayers (data not shown), and addition of bovine liver catalase to this spent medium completely eliminated the cytotoxic effects, indicating that only H2O2 was responsible. Spent medium from an anaerobically grown culture, which does not support peroxidogenesis by viridans streptococci, caused a loss of only 5 to 8% of the monolayer at pH 7.4; therefore, no other extracellular, cytotoxic products were detected in the plasma medium after growth of S. gordonii.
FIG. 1.
Killing of HUVEC by planktonic bacteria. S. gordonii CH1 was cocultured with HUVEC monolayers, at the indicated cell ratios, in 50/50 medium with (○) and without (•) bovine liver catalase. The pH of the culture medium (▴) was determined when the monolayers were harvested after 5 h at 37°C in 5% CO2. Values represent means and standard deviations (n = 3).
Electron microscopy.
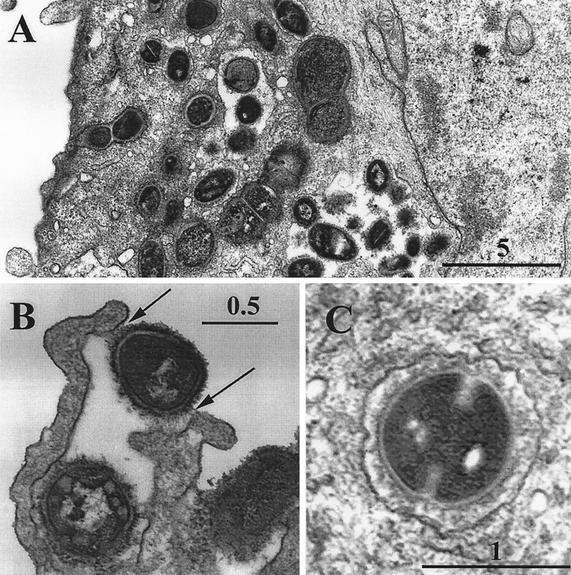
To determine the nature of the bacterium-host cell interactions during coculture, HUVEC monolayers were obtained after incubation with S. gordonii CH1 (at an MOI of 2,000) and examined by electron microscopy (Fig. 2). Streptococci were seen in intimate contact with HUVEC surfaces, adhering by a fibrillar network that appeared to elicit the formation of endocytic processes (Fig 2B, arrows). Each HUVEC was seen to have numerous cytoplasmic vacuoles containing bacteria (Fig. 2A), some of which appeared to be actively dividing (Fig. 2C).
FIG. 2.
Transmission electron micrographs of HUVEC after coculture with S. gordonii CH1. The micrographs show numerous intracellular bacteria (A) within membrane-bound vacuoles (C) and (B) streptococci attached to the HUVEC surface through tufts of cell wall fibrillae (arrows), which appear to result in endocytic activity by the HUVEC.
Bacterial invasion of HUVEC.
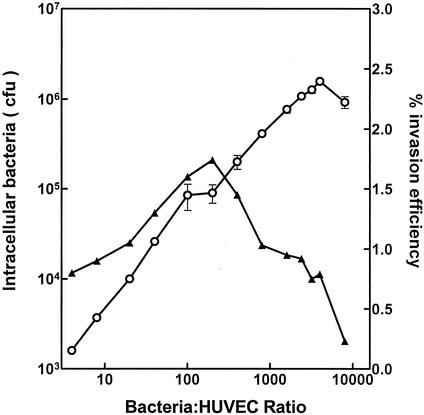
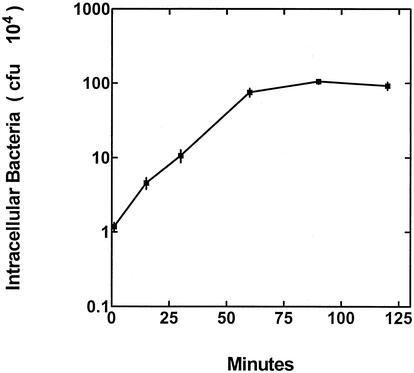
The ability of S. gordonii CH1 to enter cultured HUVEC in 50/50 medium was determined using an antibiotic protection assay. The increase in the numbers of intracellular streptococci, attained over 2 h, was found to be nearly linear within the MOI range of 4 to 6,000 (Fig. 3); however, the most efficient MOI was 200, as indicated by the highest percentage (1.7%) of the initial streptococcal inoculum that entered the HUVEC. A subsequent experiment, using a MOI of 2,000, showed that the rate of streptococcal invasion of HUVEC was linear during the first 60 min after the centrifugation step and that an extension of the incubation time did not result in larger numbers of intracellular bacteria (Fig. 4).
FIG. 3.
HUVEC invasion by S. gordonii CH1 in cocultures at different cell ratios (○). The percentage of the initial streptococcus population that invaded the HUVEC (▴) was calculated as an indicator of infection efficiency in each coculture. Values represent the means and standard deviations (n = 3).
FIG. 4.
S. gordonii CH1 invasion of HUVEC at different time points during coculture in which the bacterium/HUVEC ratio was 2,000:1. Values represent the means and standard deviation (n = 3).
Growth of intracellular bacteria.
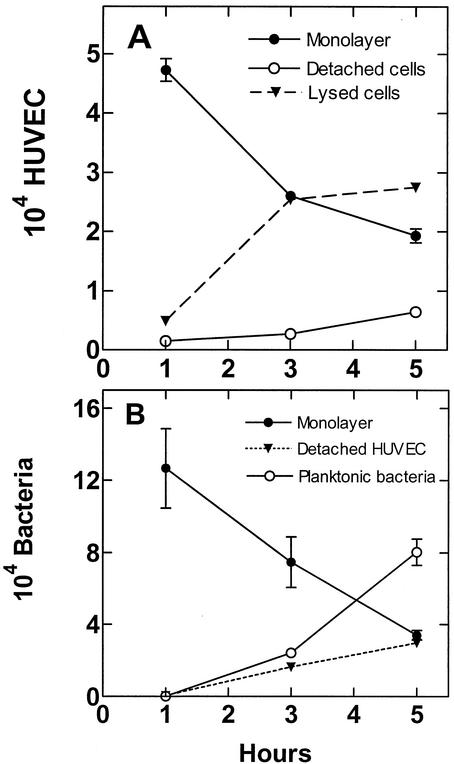
The fates of infected HUVEC monolayers and intracellular S. gordonii were determined in cocultures in which the penicillin-gentamicin mixture (MLC) was removed after 1 h and replaced with 50/50 medium devoid of antibiotics. Previous experiments had shown that this antibiotic treatment was sufficient to kill 100% of planktonic bacteria within the first 1 h. Over the ensuing 4-h incubation, the number of HUVEC decreased 63% through detachment and lysis (Fig. 5A), resulting in a 64% reduction in the number of total intracellular streptococci (Fig. 5B). The presence of extraneous catalase in the culture medium did not protect the HUVEC monolayer from destruction by intracellular streptococci. Analyses of the culture medium revealed increasing numbers of planktonic bacteria and detached HUVEC containing intracellular streptococci. The sum of intracellular and planktonic bacteria at the 5-h point was 114% of the intracellular bacteria detected in the monolayer at the outset (1 h), indicating that the streptococci did not grow much in the HUVEC cytoplasm or culture medium. The increase in the number of planktonic bacteria (Fig. 5B) between 1 and 5 h was 518-fold, which would represent more than nine generations if they were derived from the initial 1-h planktonic population. Since S. gordonii CH1 has a generation time of 2.5 h in the 50/50 medium, this magnitude of increase can be explained only by release of bacteria from dying HUVEC and from limited bacterial cell division.
FIG. 5.
Fate of HUVEC following invasion by S. gordonii CH1. Monolayers were infected with streptococci at a cell ratio of 2,000:1, treated with a gentaminin-penicillin mixture for 1 h to kill extracellular bacteria, and incubated in antibiotic-free 50/50 medium for an additional 4 h. (A) Numbers of HUVEC in the monolayer, floating in the culture medium, and lost to lysis. (B) Numbers of streptococci present in the HUVEC monolayer, in the detached HUVEC, and free in the culture medium. Values represent the means and standard deviations (n = 3).
Effect of chemical inhibitors.
The effects of selected inhibitors of eukaryotic cell function were tested to ascertain the abilities of these inhibitors to inhibit the invasion of HUVEC monolayers by S. gordonii CH1 (Table 2). The results obtained with cytochalasin D and nocodazole indicate that fully functional cytoskeletal elements, both microfilaments and microtubules, are required in HUVEC for efficient uptake of S. gordonii in endocytic vacuoles. Staurosporine, at concentrations known to inhibit both protein kinase C and myosin light-chain kinase and to result in a loss of myosin II from cytoskeletal structures (33), did not diminish streptococcal invasion. Interruption of ATP synthesis with sodium azide or inhibition of protease activity also reduced the amount of bacterial invasion. It should be emphasized that the maximum concentrations of inhibitors used in this experiment were limited by the ability of HUVEC monolayers to tolerate them in the absence of streptococci; at least 90% of the monolayers remained viable in these control monolayers. None of these inhibitors, at the indicated concentrations, adversely affected the viability or growth of streptococci in independent tests.
TABLE 2.
Effects of selected inhibitors of HUVEC function on invasion by S. gordinii CH1
| Inhibitor | Amt | HUVEC target | % Inhibitiona |
|---|---|---|---|
| Cytochalasin D | 0.25 μg/ml | Actin microfilament and myosin II | 99 ± 0.1 |
| Nocodazole | 10 μg/ml | Microtubule polymerization | 51 ± 17 |
| Staurosporine | 0.2 μg/ml | Protein kinase C and myosin light-chain kinase | 0 |
| Cycloheximide | 0.2 μg/ml | Protein synthesis | 0 |
| Sodium azide | 325 μg/ml | Oxidative phosphorylation | 31 ± 4 |
| Protease inhibitorsb | 1:1,000 | Cellular proteases | 52 ± 4 |
(Number of internalized streptococci observed in the presence of the indicated inhibitor divided by the number of internalized bacteria observed in the absence of inhibitor) × 100. Values represent the means ± standard deviation.
Protease inhibitor cocktail (Sigma Aldrich), containing AEBSF, aprotinin, leupeptin, bestatin, pepstatin A and E-64 (see Materials and Methods), used at a final dilution of 1:1,000.
HUVEC invasion by other oral streptococci and the role of surface proteins.
The relative abilities of selected species and strains of oral streptococci to invade cultured HUVEC were determined in 2-h cocultures (Table 1). Although all of the streptococci invaded HUVEC, there were considerable differences in the numbers of intracellular bacteria among the species and strains; the most successful were S. gordonii CH1, S. mitis OP51, and S. sanguis strains 10556, L22, and L74.
Comparison of isogenic pairs of S. gordonii CH1 indicated that deletion of any one of several surface localized proteins resulted in a significant reduction in the ability of the mutant bacteria to invade HUVEC; these proteins include glucosyltransferase (strain CH1 versus strain AMS12), P < 0.0005; sialic acid-binding protein Hsa (strain DL1 versus strain EM230), P < 0.005; and the hydrophobicity/coaggregation proteins CshA and CshB (strain DL1 versus strains OB235, OB271, and OB277), P < 0.05; but not the antigen I/II adhesins SspA and SspB (strain DL1 versus strains OB219 and OB220), P > 0.5.
DISCUSSION
This study shows that several species of oral viridans group streptococci can infect and kill cultured HUVEC by multiple virulence mechanisms. Planktonic bacteria, growing in 50% human plasma-18 mM glucose, quickly killed HUVEC by alpha-hemolysin (hydrogen peroxide) production and by lowering the pH by lactic acid production. S. gordonii (109 cells/ml) can produce 15 nmol of hydrogen peroxide and 18 nmol of lactic acid per min when the culture medium is fully oxygenated (4). We think that these experimental conditions are consistent with those of streptococci entrapped in the platelet-fibrin matrix of valvular vegetations in infective endocarditis, where bacteria can grow to 109 to 1010 cells per g of vegetation (29, 48). The density of the fibrin-platelet-streptococcal verruca in vivo appears to hinder the diffusion of therapeutic antibiotics into the lesion (18, 48) and, similarly, is expected to diminish the diffusion of toxic streptococcal products away from the endocardium, thus enhancing pathogenesis. Also, blood catalase is unlikely to reach bacterial H2O2 because of this permeability barrier. Streptococci most often colonize the mitral and aortic valves on the left side of the heart (5, 21, 51), where they are bathed in highly oxygenated blood that might support maximum production of hydrogen peroxide. It is tempting to speculate that the cytotoxic effects of streptococcal hydrogen peroxide might also inhibit host defense mechanisms on the left side of the heart, particularly neutrophil clearance of bacteria (21, 37, 51), whereas bacteria that colonize sites on the right side of the heart (22), where oxygen is limited, would be less peroxidogenic and thus more susceptible to the action of granulocytes.
Several species and strains of oral streptococci were found to invade cultured HUVEC to various extents. S. gordonii CH1 was among the most invasive strains and was found to adhere to host cells via fibrillar structures that resemble those first described by Beachey and Ofek (6) for S. pyogenes adhesion to human epithelial cells and are consistent with the participation of surface-localized protein adhesins in the present study. The intimate cell contact was sufficient to induce HUVEC to activate an endocytic mechanism to engulf the streptococci. This event depended on polymerization of actin filaments and fully functional microtubule structures, as indicated by the sensitivity of streptococcal invasion to inhibition by cytochalasin D and nocodazole, similar to the mechanism for S. agalactiae invasion (42, 46). The effects of cycloheximide on streptococcal invasion were less obvious because most concentrations induced morphological changes in HUVEC and caused their detachment from the monolayer during the invasion assay. The inhibitory effects of sodium azide and protease inhibitors confirmed the need for ATP and HUVEC proteases in the invasion process. Concentrations of the inhibitors that were tolerated by the HUVEC did not adversely affect bacterial viability.
Intracellular S. gordonii CH1 did not grow much (14%) during the 4-h period following streptococcal invasion of HUVEC monolayers, although the bacteria were in the logarithmic phase of growth (generation time, 2.5 h) in the 50/50 medium at the time of coculture. This somewhat unexpected observation might be explained by a possible failure of the HUVEC to fully protect the intracellular streptococci from inhibitory effects of gentamicin and penicillin during the initial 1-h exposure, although the intracellular bacteria grew well on the agar plate medium after HUVEC lysis. A second possible explanation is that the intracellular environment is nutritionally unfavorable for growth, causing the bacteria to undergo a stringent response. Alternatively, and perhaps most likely, multiplication of streptococci within cell chains might not be detected in the CFU assay, which cannot discriminate between single cells, cell chains, and bacteria adherent to host cell fragments. Similar observations of poor streptococcal growth in host cells were reported by Rubens and coworkers (42, 46), who found that the group B streptococcus S. agalactiae did not grow in respiratory epithelial cells or in brain microvascular endothelial cells following invasion. The most interesting and important findings of the present study are the abilities of viridans group streptococci to invade endothelial cells while suspended in human plasma, the high levels of streptococcal toxicity for the host cells, and the implication of these activities for cardiovascular disease in humans.
Comparison of isogenic pairs of S. gordonii indicated that several cell wall proteins were necessary for maximum bacterial invasion of HUVEC, presumably by mediating bacterial adhesion to host cells, the first step in pathogenesis. Streptococci in which particular genes encoding adhesin proteins had been inactivated did not completely lose their ability to invade HUVEC, indicating that other adhesion mechanisms can still mediate attachment, albeit at a diminished level. It is generally accepted that adhesion of streptococci to host surfaces involves the concerted action of multiple adhesins of different types and binding specificities (26). One protein detected in the present study is glucosyltransferase (GTF), a 153-kDa cell wall protein, which is responsible for biosynthesis of the glucan capsule (23, 27) and for the adhesion of streptococci to cultured HUVEC (54). Surface glucans have been implicated for many years in S. sanguis colonization of heart valves in experimental endocarditis (44, 49), and electron microscopy has confirmed the production of exopolysaccharides by streptococci in valvular vegetations in infected rabbits (38). Studies of viridans group streptococci, isolated from the blood of patients, have shown that polysaccharide production correlates closely with the pathogenicity of the bacteria in infective endocarditis (13). The ability of GTF to form heterologous complexes with extracellular polysaccharides (57) would enable it to assume a prominent superficial cellular location, where it could readily make contact with receptors on host cell surfaces. Our observations concerning the possible role of GTF in endothelial cell adhesion, invasion, and endocarditis, however, are inconsistent with the findings of Wells et al. (59), who reported that the GTF-deficient S. gordonii strain AMS12 colonized heart valves in a rat infection model as efficiently as did the isogenic wild-type S. gordonii CH1. This apparent discrepancy may be a consequence of the alternative adhesin molecules on streptococci, the high infective dosages of streptococci used, and the valvular damage caused by the intracardiac catheter used in the rat infection model.
The second surface protein of S. gordonii that appeared necessary for maximum invasion of HUVEC was the sialic acid-binding adhesin Hsa (203 kDa), which can also facilitate bacterium-mediated hemagglutination and bacterial adhesion to immobilized salivary sialoglycoconjugates in vitro (52). The presence of terminal sialic acid residues on glycoproteins and glycolipids of human cell surfaces predicts that this bacterial adhesin might be an important virulence factor in the pathogenesis of infective endocarditis. The relatively large size of the Hsa protein and the possibility that it may assume an α-helical conformation on the bacterial surface would enable it to extend into the aqueous environment as a prominent component of the fuzzy coat (Fig. 2B) that is characteristic of oral streptococci (52) and to interact freely with host cell surface receptors.
The cell surface hydrophobicity proteins CshA and CshB (259 and 245 kDa, respectively) of S. gordonii also appeared to facilitate bacterial invasion of HUVEC in this study. These antigenically related proteins have been reported to be responsible for cell wall hydrophobicity of S. gordonii, to mediate streptococcal coaggregation with Actinomyces naeslundii in the oral environment (40), and to mediate streptococcal adhesion to immobilized fibronectin. Our findings on the role of these proteins in host cell invasion are consistent with those of Lowrance et al. (34), who determined that S. sanguis adhesion to tissue-localized fibronectin was an important event in streptococcal colonization of heart valves and possibly the pathogenesis of infective endocarditis.
The human infection model, composed of HUVEC monolayers and human plasma as described in this communication, provides a convenient system to evaluate early events in host- bacterium interactions that are relevant to both infective endocarditis and atherosclerosis. Growth of bacteria in human plasma prior to their coincubation with host cells ensures that virulence genes are fully induced in this environment at the time of coculture. Future analyses of endothelial cell cultures, stimulated by coculture with streptococci, may reveal host cell responses that contribute to inflammation and blood coagulation at sites of infection. Vascular endothelial cells are known to produce tissue factor (thromboplastin) in response to a variety of stimuli, including interleukin-1, tumor necrosis factor alpha, interferon, various mitogens, bacterial and virus infection, and platelet binding (reviewed in reference 10). Tissue factor is the primary initiator of the extrinsic pathway of blood coagulation and is considered to be a major stimulus for fibrin deposition in heart valve vegetations (16) and atherosclerotic plaque (10, 61). Endothelial cells also produce platelet-activating factor in response to bacterial stimuli (36), including H2O2. Platelet-activating factor affects the adhesion and transmigration of leukocytes across vessel walls and can activate the endothelium itself by interacting with specific receptors, causing changes in cell shape by rearrangement of the cytoskeleton (9). Future work will focus on the ability of viridans group streptococci to stimulate vascular endothelial cells to produce proinflammatory cytokines and to induce intravascular coagulation.
Acknowledgments
This work was supported by Public Health Service grant R01-DE05696 from the National Institute of Dental and Craniofacial Research.
We thank Thaddeus M. Szczesny for performing the electron miscroscopy and Robert A. Burne, Donald B. Clewell, Howard F. Jenkinson, Frank A. Scannapieco, and Yukihiro Takahashi for providing various Streptococcus strains and species.
Editor: A. D. O'Brien
REFERENCES.
- 1.Baddour, L. M. 1994. Virulence factors among gram-positive bacteria in experimental endocarditis. Infect. Immun. 62:2143-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour, L. M., G. D. Christensen, J. H. Lowrance, and W. A. Simpson. 1989. Pathogenesis of Experimental endocarditis. Rev. Infect. Dis. 11:452-463. [DOI] [PubMed] [Google Scholar]
- 3.Barnard, J. P., and M. W. Stinson. 1996. The alpha hemolysin of Streptococcus gordonii is hydrogen peroxide. Infect. Immun. 64:3853-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard, J. P., and M. W. Stinson. 1999. Influence of environmental conditions on hydrogen peroxide formation by Streptococcus gordonii. Infect. Immun. 67:6558-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayless, R., C. Clark, C. M. Oakley, W. Sommerville, A. G. W. Whitfield, and S. E. J. Young. 1987. The microbiology and pathogenesis of infective endocarditis. Br. Heart J. 50:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beachey, E. H., and I. Ofek. 1976. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J. Exp. Med. 143:759-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-464. [DOI] [PubMed] [Google Scholar]
- 8.Brown, A. E., J. D. Rogers, E. M. Haase, P. M. Zelasko, and F. A. Scannapieco. 1999. Prevalence of the amylase-binding protein A gene (abpA) in oral streptococci. J. Clin. Microbiol. 37:4081-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussolino, F., and G. Camussi. 1995. Platelet-activating factor produced by endothelial cells, a molecule with autocrine and paracrine properties. Eur. J. Biochem. 229:327-337. [PubMed] [Google Scholar]
- 10.Camerer, E., A.-B. Kolstø, and H. Prydz. 1996. Cell biology of tissue factor, the principal initiator of blood coagulation. Thrombosis Res. 8:1-41. [DOI] [PubMed] [Google Scholar]
- 11.Chiu, B. 1999. Multiple infections in carotid atherosclerotic plaques. Amer. Heart J. 138:S534-S536. [DOI] [PubMed] [Google Scholar]
- 12.Coulter, W. A., A. Coffy, I. D. F. Saunders, and A. M. Emmerson. 1990. Bacteremia in children following dental extraction. J. Dent. Res. 69:1691-1695. [DOI] [PubMed] [Google Scholar]
- 13.Dall, L., and B. L. Herndon. 1990. Association of cell-adherent glycocalyx and endocarditis production by viridans group streptococci. J. Clin. Microbiol. 28:1698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuth, D. R., Y. Duan, W. Brooks, A. R. Holmes, R. McNab, and H. F. Jenkinson. 1996. Tandum genes encode cell surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol. Microbiol. 20:403-413. [DOI] [PubMed] [Google Scholar]
- 15.Douglass, C. W. I., J. Heath, K. K. Hampton, and F. E. Preston. 1993. Identity of viridans streptococci isolated from cases of infective endocarditis. J. Med. Microbiol. 39:179-182. [DOI] [PubMed] [Google Scholar]
- 16.Drake, T. A., G. M. Rodgers, and M. A. Sande. 1984. Tissue factor is a major stimulus for vegetation formation in enterococcal endocarditis in rabbits. J. Clin. Investig. 73:1750-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durack, D. T. 1975. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J. Pathol. 115:81-89. [DOI] [PubMed] [Google Scholar]
- 18.Durack, D. T. 1995. Prevention of infective endocarditis. N. Engl. J. Med. 332:38-44. [DOI] [PubMed] [Google Scholar]
- 19.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53:44-49. [PMC free article] [PubMed] [Google Scholar]
- 20.Fransen, E. V. G., V. Pedrazzoli, and M. Kilian. 1991. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol. Immunol. 6:129-133. [DOI] [PubMed] [Google Scholar]
- 21.Freedman, L. R. 1987. The pathogenesis of infective endocarditis. J. Antimicrob. Chemother. 20:1-6. [DOI] [PubMed] [Google Scholar]
- 22.Garrison, P. K., and L. R. Freedman. 1970. Experimental endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J. Biol. Med. 42:394-410. [PMC free article] [PubMed] [Google Scholar]
- 23.Grahame, D. A., and R. M. Mayer. 1985. Purification and comparison of two forms of dextran sucrase from Streptococcus sanguis. Carbohydr. Res. 142:285-298. [DOI] [PubMed] [Google Scholar]
- 24.Hardy, J. M., and G. H. Bowen. 1974. The normal microbial flora of the mouth. Soc. Appl. Bacteriol. Symp. Ser. 3:47-83. [PubMed] [Google Scholar]
- 25.Hardy, J. M., and P. D. Marsh. 1978. Streptococci and the human oral flora. p. 157-206. In F. A. Skinner and L. B. Quesnel (ed.), Streptococci. Academic Press, Inc., New York, N.Y.
- 26.Hasty, D. L., I. Ofek, H. S. Courtney, and R. J. Doyle. 1992. Multiple adhesins of streptococci. Infect. Immun. 60:2147-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heraief, E., M. P. Glauser, and L. R. Freedman. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzberg, M. C., K. Gong, G. D. MacFarlane, P. R. Erickson, A. H. Soberay, P. H. Krebach, G. Manjula, K. Schilling, and W. H. Bowen. 1990. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect. Immun. 58:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hook, E. W., III, and M. A. Sande. 1974. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect. Immun. 10:1433-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imataka, K., Y. Kitahara, S. Naito, and J. Fujii. 1993. A new model for infective endocarditis of the mitral valve in rabbits. Am. Heart J. 125:1353-1357. [DOI] [PubMed] [Google Scholar]
- 31.Isberg, R. R., and S. Falkow. 1985. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature (London) 317:262-264. [DOI] [PubMed] [Google Scholar]
- 32.Juarez, Z. E., and M. W. Stinson. 1999. An extracellular protease of Streptococcus gordonii hydrolyzes type IV collagen and collagen analogues. Infect. Immun. 67:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolega, J., and S. Kumar. 1999. Regulatory light chain phosphorylation and the assembly of myosin II into the cytoskeleton of microcapillary endothelial cells. Cell Motil. Cytoskeleton 43:255-268. [DOI] [PubMed] [Google Scholar]
- 34.Lowrance, J. H., L. M. Baddour, and W. A. Simpson. 1990. The role of fibronectin binding in the rat model of experimental endocarditis caused by Streptococcus sanguis. J. Clin. Investig. 86:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning, J. E., E. B. H. Hume, N. Hunter, and K. W. Knox. 1994. An appraisal of the virulence factors associated with streptococcal endocarditis. J. Med. Microbiol. 40:110-114. [DOI] [PubMed] [Google Scholar]
- 36.Mathiak, G., D. Szewczyk, F. Abdullah, P. Ovadia, and R. Rabinovici. 1997. Platelet-activating factor (PAF) in experimental and clinical sepsis. Shock 7:391-404. [DOI] [PubMed] [Google Scholar]
- 37.Meddens, M. J. M., J. Thompson, H. Mattie, and R. van Furth. 1984. Role of granulocytes in the prevention and therapy of experimental Streptococcus sanguis endocarditis in rabbits. Antimicrob. Agents Chemother. 25:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills, J., L. Pullium, L. Dall, J. Marzouk, W. Wilson, and J. M. Costerton. 1984. Exopolysaccharide production by viridans streptococci in experimental endocarditis. Infect. Immun. 43:358-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNab, R., A. R. Holmes, J. M. Clark, G. W. Tannock, and H. F. Jenkinson. 1996. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect. Immun. 64:4204-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNab, R., H. F. Jenkinson, D. M. Loach, and G. W. Tannock. 1994, Cell-surface-associated polypeptides CshA and CshB of high molecular mass are colonization determinants in the oral bacterium Streptococcus gordonii. Mol. Microbiol. 14:743-754. [DOI] [PubMed] [Google Scholar]
- 41.Ness, P. M., and H. A. Perkins. 1980. Transient bacteremia after dental procedures and other minor manipulations. Transfusion 20:82-85. [DOI] [PubMed] [Google Scholar]
- 42.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perlman, B. B., and L. R. Freedman. 1971. Experimental endocarditis. III. Natural history of catheter induced staphylococcus endocarditis following catheter removal. Yale J. Biol. Med. 44:214-224. [PMC free article] [PubMed] [Google Scholar]
- 44.Ramirez-Ronda, C. H. 1978. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J. Clin. Investig. 62:805-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenshine, I., S. Ruschkowski, and B. B. Finlay. 1994. Inhibitors of cytoskeleton function and signal transduction to study bacterial invasion. Methods Enzymol. 236:467-476. [DOI] [PubMed] [Google Scholar]
- 46.Rubens, C. E., S. Smith, M. Hulse, E. Y. Chi, and G. van Belle. 1992. Respiratory epithelial cell invasion by group B streptococci. Infect. Immun. 60:5157-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santoro, J., and M. E. Levison. 1978. Rat model of experimental endocarditis. Infect. Immun. 19:915-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheld, W. M., and M. A. Sande. 1995. Endocarditis and intravascular infections, p. 740-783. In G. L. Mandell, J. E. Bennett, and R. Dolan (ed.), Principles and practice of infectious diseases. Churchill Livingstone, Inc., New York, N.Y.
- 49.Scheld, W. M., J. A. Valone, and M. A. Sande. 1978. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets and fibrin. J. Clin. Investig. 61:1394-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sommer, P., C. Gleyzal, S. Guerret, J. Etienne, and J.-A. Grimaud. 1992. Induction of a putative laminin-binding protein of Streptococcus gordonii in human infective endocarditis. Infect. Immun. 60:360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullum, P. M., T. A. Drake, and M. A. Sande. 1985. Pathogenesis of endocarditis. Am. J. Med. 78:110-115. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi, Y., K. Konishi, J. Cisar, and M. Yoshikawa. 2002. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect. Immun. 70:1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tardif, G., M. C. Sulavik, G. W. Jones, and D. B. Clewell. 1989. Spontaneous switching of the sucrose-promoted colony phenotype in Streptococcus sanguis. Infect. Immun. 57:3945-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vacca-Smith, A. M., C. A. Jones, M. J. Levine, and M. W. Stinson. 1994. Glucosyltransferase mediates adhesion of Streptococcus gordonii to human endothelial cells in vitro. Infect. Immun. 62:2187-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Meer, J. T. M., W. van Vianen, E. Hu, W. B. van Leeuwen, H. A. Valkenburg, J. Thompson, and M. F. Michel. 1991. Distribution, antibiotic susceptibility and tolerance of bacterial isolates in culture-positive cases of endocarditis in the Netherlands. Eur. J. Clin. Microbiol. Infect. Dis. 10:728-734. [DOI] [PubMed] [Google Scholar]
- 56.Varma, M. P. S., D. R. McCluskey, M. M. Khan, J. Cleland, H. O. O'Kane, and A. A. J. Adgey. 1986. Heart failure associated with infective endocarditis. A review of 40 cases. Br. Heart J. 55:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vickerman, M. N., D. B. Clewell, and G. W. Jones. 1991. Sucrose-promoted accumulation of growing glucosyltransferase varients of Streptococcus gordonii on hydroxyapatite surfaces. Infect. Immun. 59:3523-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanakunakorn, C., and J. Pantelakis. 1993. Alpha-hemolytic streptococcal bacteremia: A review of 203 episodes during 1980-1991. Scand. J. Infect. Dis. 25:403-408. [DOI] [PubMed] [Google Scholar]
- 59.Wells, V. G., C. L. Monro, M. C. Sulavik, D. B. Clewell, and F. L. Macrina. 1993. Infectivity of a glucan synthesis-defective mutant of Streptococcus gordonii (Challis) in a rat endocarditis model. FEMS Microbiol. Lett. 112:301-306. [DOI] [PubMed] [Google Scholar]
- 60.Wexler D. L., J. E. C. Penders, W. H. Bowen, and R. A. Burne. 1992. Characteristics and cariogenicity of a fructanase-defective Streptococcus mutans strain. Infect. Immun. 60:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcox, J. N., K. M. Smith, S. M. Schwartz, and D. Gordon. 1989. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. USA 86:2839-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]