Abstract
Bowel perforation can lead to significant bacterial spillage, which may then cause septic peritonitis, characterized by a systemic inflammatory response and organ dysfunction. There are several reports that have shown that the development of peritoneal adhesions is dependent on inflammatory cytokine levels and that these adhesions can reduce bacterial spread, possibly by sealing off the cecum in the cecal ligation and puncture (CLP) model of septic peritonitis. There have not, however, been any studies that have utilized a strategy to accelerate tissue repair in order to seal off the injured cecum and reduce bacterial spread as well as ameliorate systemic inflammation. In the present study, we demonstrate that the administration of anti-gamma interferon (IFN-γ) antibody (1.2 mg/kg of body weight, intravenously) accelerated tissue repair via increased fibrin deposition 12 and 24 h after CLP in rats. This increase in fibrin deposition was associated with peritoneal adhesion 24 h after CLP and a reduction in bacterial load compared to the bacterial load of rats given irrelevant antibody. Plasma fibrin levels, however, were not altered after IFN-γ antibody administration, suggesting that the inhibition of IFN-γ activity specifically increased fibrin deposition to the site of injury. Furthermore, plasma interleukin-6, used as a marker of systemic inflammatory response, was reduced in CLP rats given IFN-γ antibody compared to that found in those given irrelevant antibody. These results suggest that the early inhibition of IFN-γ activity in the CLP model is beneficial by accelerating fibrin deposition in cecal tissue to prevent bacterial spread and reduce the systemic inflammatory response. Importantly, increased fibrin deposition in the ceca was not associated with increased plasma fibrin whereas the latter may have detrimental effects associated with coagulation disorders.
Bacterial peritonitis can result from bowel perforation due to the leakage of bacteria into the peritoneal cavity. The local infection may lead to significant increases in the release of proinflammatory mediators such as cytokines, arachidonic acid metabolites, and free radicals. Prolonged increase in the systemic levels of these proinflammatory mediators is a hallmark of overt sepsis and may cause organ injury. The cecal ligation and puncture (CLP) model is an established model in which bacterial load within the peritoneal cavity has been well quantified (7, 16, 21). With respect to this, early formation of peritoneal adhesions has generally been shown to be protective by “walling off” the cecum and inhibiting bacterial spread (7, 23, 28). The peritoneal adhesions associated with CLP-induced injury appear to be tumor necrosis factor (TNF) dependent, as the use of anti-TNF antibodies reduces peritoneal adhesions and increases bacterial load (7). Additionally, use of interleukin-1 (IL-1β) antibody also appears to prevent adhesion formation in rats (11). Interestingly and conversely, work from our laboratory has shown that the inhibition of gamma interferon (IFN-γ) by using a monoclonal antibody increases peritoneal adhesions and decreases bacterial load (23). The mechanisms of this accelerated adhesion formation have not been clearly defined. These latter results suggest that the inhibition of IFN-γ has a beneficial effect opposite TNF-α (or IL-1β), which can augment bacterial containment after CLP.
Adhesion formation has been widely reported after abdominal surgery (10) and is defined as the pathological bonds between surfaces of the peritoneal cavity formed during the scarring of peritoneal injury. Adhesions can be caused by surgical lesions, infections, bowel perforations, and inflammatory pathologies. Cellularly, peritoneal adhesions are formed after an injury to the parietal or visceral peritoneum which leads to exposure of the basal layer of the mesothelium to surrounding tissues. This injury leads to the activation of a sequential cascade of events, characterized by the activation of fibrin from fibrinogen and deposition of the fibrin matrix with later collagen synthesis.
IFN-γ plays a critical role in host defense and is primarily produced by T-cell lymphocytes and natural killer (NK) cells (26, 31). As a major activator of macrophages, IFN-γ activates several important macrophage functions, including antimicrobial activity and antigen presentation to lymphocytes through the induction of major histocompatibility complex II (12, 20). There is evidence implicating IFN-γ in the lethality of acute endotoxemia, as the use of antibodies against IFN-γ ameliorates lethality in this model (5). In addition, IFN-γ-receptor-deficient mice are resistant to endotoxic shock (1). In the only two reports using a model of gram-negative bacteremia, antibodies to IFN-γ were also successful in decreasing lethality (13, 27) while the use of recombinant IFN-γ increased mortality in the CLP model of sepsis (17). On the other hand, the use of IFN-γ in immune-suppressed patients increased macrophage HLA-DR expression and lipopolysaccharide-stimulated TNF expression in vitro (4) while the use of IFN-γ gene knockout mice in a model of fecal peritonitis caused a much-elevated mortality rate compared to wild-type mice (32). Taken together, these results indicate that IFN-γ may be proinflammatory but may also be of critical importance in host defense after the onset of sepsis. It should also be emphasized that the role of IFN-γ in models of bacterial sepsis (such as CLP) is more complex than that of endotoxemic models because of its potentially pivotal role in bacterial clearance.
In addition to its immunoregulatory functions, IFN-γ has been reported to decrease the level of collagen synthesis after tissue injury (9, 18) and prolong wound repair. Thus, it is plausible that the inhibition of IFN-γ activity can accelerate tissue repair after injury. Furthermore, in the setting of CLP-induced peritonitis, it may also be plausible that enhanced tissue repair prevents excessive bacterial leakage, subsequent peritonitis, and sepsis. It may also be possible that, in a model of bacterial peritonitis such as the CLP model, which is dependent on the leakage of bacteria from the punctured cecum, the beneficial effects of accelerating tissue repair by inhibiting IFN-γ activity override the possibly deleterious immune-suppressive effects of this strategy.
We hypothesized that, in the CLP model of peritonitis, inhibition of IFN-γ will accelerate tissue repair in the CLP, walling off the cecum and reducing bacterial load as well as ameliorating the release of proinflammatory cytokines. We postulate that the mechanism by which the inhibition of IFN-γ enhances tissue repair is through increasing fibrin deposition in the peritoneal cavity and at the surface of the cecum. To test these hypotheses, we have compared fibrin deposition, adhesion formation, and peritoneal bacterial load in CLP rats with and without the administration of monoclonal antibody against rat IFN-γ (1.2 mg/kg of body weight, intravenously). Systemic levels of the proinflammatory cytokine IL-6 as well as of IFN-γ were also measured as markers of systemic inflammation.
MATERIALS AND METHODS
The experiments described here were performed in adherence to National Institutes of Health guidelines on the use of experimental animals. All experiments were performed in adherence to protocols approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey.
CLP.
CLP was performed on male Sprague-Dawley rats (250 to 275 g each) as previously described (2). Rats were anesthetized with ketamine-xylazine (60 mg of ketamine/kg, 10 mg of xylazine/kg). A 2-cm-long midline incision was made in the abdomen to expose the cecum. Fecal matter was massaged into the cecum. The distal two-thirds of the cecum was ligated with 4.0-thickness surgical silk. Using an 18-gauge needle, the cecum was punctured through and through, making two holes. Some fecal matter was massaged out of the cecum through these holes. The cecum was placed back into the abdomen, which was then closed. Saline (2 ml/100 g) was injected to replace any fluids lost during surgery. The rats were placed on a heating pad until recovery. In sham control rats, the cecum was exposed and massaged but not ligated or punctured. CLP rats were injected with either specific mouse anti-rat IFN-γ antibody or irrelevant antibody (mouse immunoglobulin G1 [MOPC-21; Sigma Chemical Co.]). The IFN-γ antibody is a neutralizing, purified mouse anti-rat monoclonal antibody (Biosource). The antibodies were injected intravenously immediately after surgery. Rats were sacrificed 12 or 24 h after surgery. No rats died within 12 h. At 24 h, 3 out of 20 CLP rats died while 1 out of 18 CLP plus IFN-γ antibody rats died. The peritoneal cavity was subjected to lavage with 8 ml of saline containing sodium citrate (0.38% final volume), and fluid was collected for the measurement of bacterial load and immunoreactive fibrin.
Scoring of adhesion formation.
The amount of adhesion formation was scored by using the following criteria: 0, no adhesions observed; 1, intestinal adhesions on one surface; 2, adhesions on two surfaces of the cecum; 3, cecum enclosed by adhesions. All observations were made by observers who were blind to the treatment groups.
Measurement of peritoneal bacterial load.
To determine bacterial load in the peritoneum, the peritoneal cavity was subjected to lavage with 8 ml of sterile saline. Serial dilutions (1:10) were made. Fifty microliters of each dilution was then plated on tryptic soy agar plates (Fisher Scientific), as previously published by our laboratory and other investigators (16). The plates were incubated overnight at 37°C, and CFU were counted by operators who were blind to the different treatment groups. Results are expressed as CFU/milliliter. It has been noted that there are significant numbers of anerobes in this model of peritonitis, as previously reported (16), but the primary purpose of this study was to examine if IFN-γ inhibition would accelerate tissue repair (by increasing fibrin deposition) and decrease bacterial load. Thus, we assumed that the leakage of bacteria into the peritoneal cavity was similar between aerobes and anerobes.
Histology.
The cecum samples were dehydrated with increasing concentrations of ethanol (30 to 100%). They were then placed in xylene for 3 h. Samples were embedded in paraffin wax overnight. The samples were then cut into 8-μm-thick sections and placed on slides. To deparaffinize the sections, the slides were placed in xylene and decreasing concentrations of ethanol (100 to 70%).
Slides were placed in phosphate-buffered saline (PBS) containing 2% milk and 0.05% Tween for 30 min. Slides were incubated with primary antibody, which cross-reacted with rat fibrinogen (American Diagnostica) for 1 h at 37°C. They were then washed in PBS and incubated for 30 min at 37°C with secondary antibody (universal biotinylated secondary antibody from Vector Laboratory). Slides were washed again in PBS. The streptavidin-peroxidase complex working solution was added for 5 min. After another washing, the peroxidase substrate solution DAB (3,3-diaminobenzadine) was added to the slides until the desired color intensity developed. The slides were then washed in tap water, and hematoxylin counterstain was added for 3 min. After this, slides were washed in tap water for 5 min and allowed to dry overnight in air.
Slides were scanned under a Nikon digital camera DXM1200 microscope, and images were obtained by using Nikon ACT-1 version 2.00 software interfaced with the microscope. Ten high-powered fields from five histological sections (from five different rat cecums) in each group of rats were scored to estimate the relative amounts of fibrin deposition. To do this, sections were scored according to the size and number of immuno-positive stained areas. A score from 1 to 5 was then given to each slide. Scoring was performed by an operator blind to the treatment groups.
Western blot analysis.
Plasma samples were diluted 40 times with saline. Peritoneal lavage samples were diluted 10 times with saline. All samples were well mixed with equal volumes of 2× Laemmli buffer (0.125 M Tris-Cl [pH 6.8]-4% sodium dodecyl sulfate-20% glycerol, without β-mercaptoethanol). Mixed samples were denatured at 95°C for 5 min. Samples, standard markers, and authentic fibrinogen were loaded into 4 to 15% Tris-HCl gradient gels (purchased from Bio-Rad) and electrophoresed at 100 V for 1.5 h. Gels were transferred to 0.45-μm-thick nitrocellulose membranes under electrical conditions of 60 V for 1.5 h. The membranes were blocked in Tris-buffered solution containing 0.1% Tween (T-TBS; pH 7.6, containing 5% nonfat milk powder) at 4°C overnight before incubation with polyclonal primary antibody (American Diagnostica) diluted 1,000 times. After washing in T-TBS, membranes were incubated in secondary antibody (horseradish peroxidase-linked donkey anti-rabbit immunoglobulin) diluted 1,000 times and washed thoroughly again in T-TBS. Membranes were blotted dry and then placed in enhanced chemiluminescence Western blotting detection reagent (Bio-Rad) for 1 min. To visualize the specific protein, membranes were exposed on Kodak X-OMAT film for 10 s to 10 min according to the density of fluorescence. The darkness of each band reflects the amount of fibrin in each corresponding sample. The density of the bands was quantified by using a computerized imaging system (Pharmacia).
Cytokine and plasminogen activator inhibitor 1 (PAI-1) measurements.
A total of 5 ml of blood was drawn from the inferior vena cava into syringes (containing sodium citrate at a final concentration of 0.38%) for the measurement of plasma IFN-γ and IL-6. After collection, blood was centrifuged at 350 × g to obtain plasma. The plasma was collected and stored at −80°C in 1-ml aliquots. Enzyme-linked immunosorbent assay kits for IFN-γ and IL-6 were purchased from Endogen. Assays were performed according to the manufacturer's instructions.
PAI-1 activity was measured by using a chromogenic assay kit (Biopool, Ventura, Calif.) according to the manufacturer's instructions.
Statistical analyses.
Summary statistics are expressed as the mean ± the standard error of the mean (SEM). The data were analyzed by one-way analysis of variance for comparisons between multiple groups. If there was a significant difference between groups, Dunnet's t test for pairwise comparisons was then used. A Mann-Whitney rank sum test was used to compare scatterplots for CFU. Groups were deemed to be significantly different from one another when P was <0.05. Values that were more than 3 standard deviations from the mean were omitted.
RESULTS
Peritoneal adhesions.
At 12 h postsurgery, there was no evidence of peritoneal adhesions in any of the groups of rats. On the other hand, 24 h postsurgery, there was a significant increase in the number of peritoneal adhesions in the CLP rats given IFN-γ antibody (2.35 ± 0.15, n = 17, P < 0.001) compared to that in rats given irrelevant antibody (MOPC-21; 1.36 ± 0.18, n = 18). In rats treated with IFN-γ antibody, 7 out of 17 rats had an adhesion score of 3, where the adhesion completely sealed the cecum. Compared to this, only 3 out of 18 CLP rats given irrelevant antibody had an adhesion score of 3. The peritoneal adhesions were confined to the area surrounding the cecum.
Bacterial load.
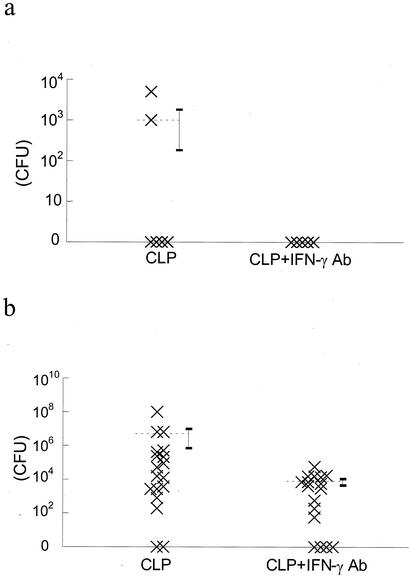
There was detectable growth of bacterial colonies from peritoneal lavage samples taken from only two out of six CLP rats given irrelevant antibody 12 h after CLP surgery (Fig. 1a). There was no detectable growth of bacteria from any samples taken from the CLP rats given anti-IFN-γ antibody (Fig. 1a). At 24 h after surgery, there was a substantial number of CFU from CLP rats given irrelevant antibody (Fig. 1b). Administration of IFN-γ antibody significantly decreased the number of CFU cultured from lavage samples (Fig. 1b) compared to that for CLP rats given irrelevant antibody.
FIG. 1.
(a) Scatterplot of bacterial load (CFU/ml) in peritoneal lavage fluid of CLP rats given irrelevant antibody (CLP) and CLP rats given IFN-γ antibody (CLP + IFN-γ Ab) 12 h after surgery. Two out of six CLP rats had detectable bacterial growth, while none of the CLP rats given IFN-γ antibody had detectable growth. (b) At 24 h after surgery, there was a substantial bacterial load in CLP rats. Bacterial load was significantly reduced in CLP rats given IFN-γ antibody (P < 0.05; Mann-Whitney rank sum test).
Western blot of fibrin deposition.
Western blots were performed to measure the presence of soluble fibrinogen products in peritoneal fluid as well as in plasma.
Peritoneal lavage fluid.
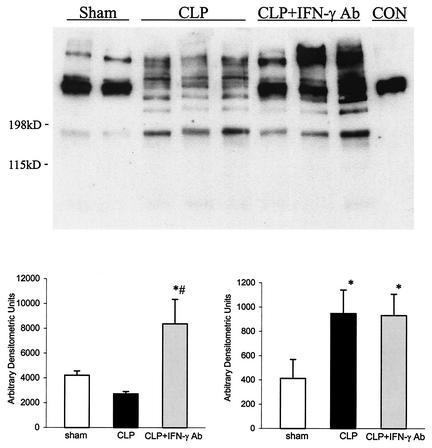
At 12 h after surgery, there was clear evidence of fibrinogen products in the peritoneal lavage fluid of sham control rats, as shown by the presence of immunoreactive fibrinogen obtained from Western blot analysis with specific antibody that cross-reacted with rat fibrinogen (Fig. 2). The immunoblot shows that, in sham controls, most if not all the fibrinogen products were greater than 200 kDa. Comparison with control fibrinogen showed that authentic fibrinogen was greater than 200 kDa, while breakdown products from fibrinolysis were between 198 and 115 kDa. Western blots of peritoneal fluid from positive control rats (CLP plus MOPC-21) revealed that there was little soluble fibrinogen product greater than 200 kDa, but there was a clear presence of fibrinogen products with molecular masses between 198 and 115 kDa, suggesting the presence of fibrinogen breakdown products. In contrast, peritoneal fluid from CLP rats given IFN-γ antibody had both a larger fibrinogen product (>200 kDa), which comigrated with authentic rat fibrinogen, as well as a smaller fibrinogen product. These results suggest that, in the peritoneal fluid from CLP rats given anti-IFN-γ antibody, there was fibrin deposition in the presence of ongoing fibrinolytic activity.
FIG. 2.
Representative Western blot of soluble fibrinogen products from sham controls, CLP plus irrelevant antibody (CLP), and CLP plus IFN-γ antibody (CLP + IFN-γ Ab) 12 h after surgery. Authentic fibrinogen (CON) was approximately 200 kDa in size. Densitometric analysis showed that CLP rats given IFN-γ antibody had more fibrinogen products larger than 200 kDa as well as products smaller than 200 kDa than those from CLP rats given irrelevant antibody. Values are means ± SEM. *, P < 0.05 compared to that for sham controls; #, P < 0.05 compared to that for CLP. For sham controls, n = 4; for CLP, n = 6; and for CLP + IFN-γ Ab, n = 6.
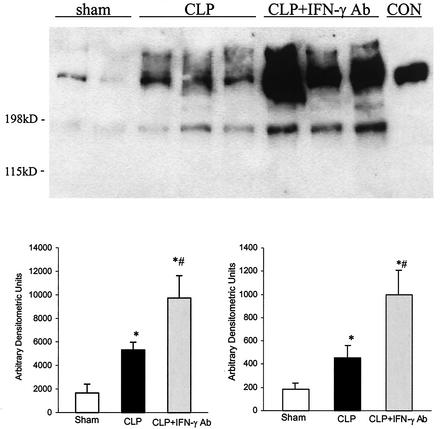
At 24 h after surgery, there were increased levels of both large and smaller soluble fibrinogen products in CLP plus MOPC-21 rats compared to those for sham controls (Fig. 3). In the group of CLP rats given the IFN-γ antibody, the amount of soluble fibrinogen products greater than 200 kDa was more than that in sham controls but was variable and not significantly different from that from those with the CLP plus irrelevant antibody. On the other hand, there was a greater amount of smaller fibrinogen products (<200 kDa) than in the other two groups.
FIG. 3.
Representative Western blot of soluble fibrinogen products from sham controls, CLP plus irrelevant antibody (CLP), and CLP plus IFN-γ antibody (CLP + IFN-γ Ab) 24 h after surgery. Authentic fibrinogen (CON) was approximately 200 kDa in size. Densitometric analysis showed that CLP rats given IFN-γ antibody had more fibrinogen products larger than 200 kDa as well as smaller than 200 kDa than those from CLP rats given irrelevant antibody. Values are means ± SEM for sham controls (n = 6), CLP (n = 9), and CLP + IFN-γ antibody (n = 9). *, P < 0.05 compared to that for sham controls; #, P < 0.05 compared to that for CLP.
Plasma.
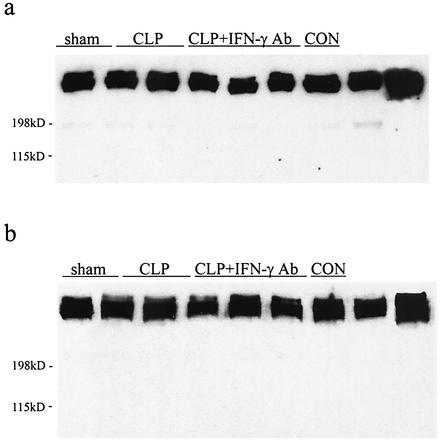
Immunoblots of rat plasma revealed that there were no detectable soluble fibrinogen products greater in size than authentic fibrinogen in the plasma of all groups both at 12 and 24 h after surgery (Fig. 4). Product that comigrated with authentic fibrinogen was similar in all groups, while smaller fibrinogen products were not consistently detectable.
FIG. 4.
(a) Representative Western blot of soluble fibrinogen (CON) in plasma of sham controls, CLP plus irrelevant antibody (CLP), and CLP plus IFN-γ antibody (CLP + IFN-γ Ab) 12 h after surgery. (b) Representative Western blot of soluble fibrinogen in plasma of sham controls, CLP, and CLP + IFN-γ Ab. The results suggest that there is no difference between groups in the amount of soluble fibrinogen in plasma.
Histology.
Measurements of soluble fibrinogen product in the peritoneal lavage fluid provide information about fibrin exudation and fibrin deposition. This, however, does not provide information on fibrin matrix formation at the specific site of injury. Thus, we performed a histological analysis of fibrin formation on sections of cecal tissue.
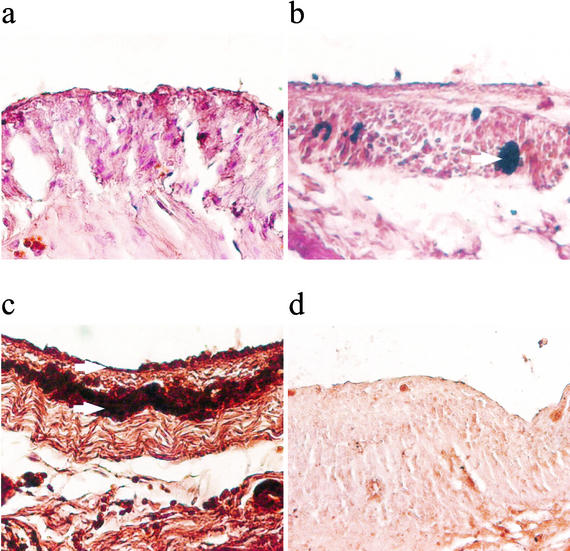
At 24 h postsurgery, there was no evidence of large quantities of fibrin deposition in the serosal surface of the ceca from sham controls (Fig. 5a). There was a significantly increased amount of immunoreactive fibrinogen staining in the cecal tissue of CLP rats given irrelevant antibody (CLP plus MOPC-21) (Fig. 5b). In the cecal tissue from CLP rats given the IFN-γ antibody, there were substantial amounts of immunoreactive fibrinogen (Fig. 5c). The areas of fibrin deposition were much larger in IFN-γ-treated rats than in other groups. To ensure that the changes we observed were not a result of nonspecific binding, we also stained slides by replacing the primary antibody with nonspecific antisera (Fig. 5d). There was no positive staining of fibrin in these slides, indicating that the positive staining for fibrin that we observed was specific. Quantitation of the fibrin immunostaining showed a significant increase in the positive immunostaining of IFN-γ-antibody-treated rats. The fibrinogen immunostaining scores ranged from 1 to 5, where 5 showed extensive immunoreactive staining and 1 showed the least immunoreactive staining of five sections (from five different rats). The immunostaining scores (means ± SEM) were as follows: for sham controls, 1.2 ± 0.2; for CLP plus MOPC-21, 1.8 ± 0.5; for CLP plus IFN-γ antibody, 3.3 ± 0.4 (P < 0.05 compared to that for sham controls and P < 0.05 compared to that for CLP).
FIG. 5.
Representative slides showing immunostaining of fibrinogen in cecal tissue 24 h after surgery of sham controls (a), CLP plus irrelevant antibody (b), and CLP plus IFN-γ antibody (c) and in the section from a CLP not incubated with primary antibody against fibrinogen but instead with irrelevant antibody (d). Arrows point to areas of dark brown staining showing fibrinogen staining. The photos show that there was increased fibrin deposition in cecal tissue 24 h after CLP compared to that in sham controls. IFN-γ antibody administration further increased fibrin deposition in cecal tissue. The staining was specific for fibrin, as there was virtually no positive staining in slides that were not incubated with primary antibody.
Plasma cytokine levels.
In order to examine the effects of antibody treatment on the systemic inflammatory response, we measured levels of IFN-γ and IL-6 in plasma (6, 24, 28). Both these cytokines and, in particular, IL-6 have been reported to be increased early in sepsis.
IFN-γ levels.
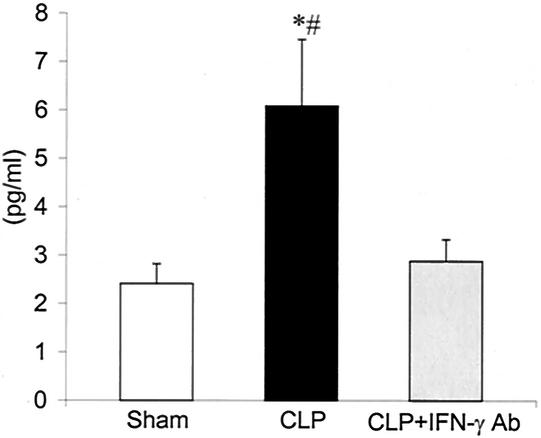
Plasma IFN-γ levels were increased in CLP plus MOPC-21 rats, compared to those in sham controls, 12 h after surgery (Fig. 6). Treatment with IFN-γ antibody significantly reduced IFN-γ levels. At 24 h after surgery, IFN-γ levels were not consistently detectable in the plasma from rats in all groups (data not shown). These results would suggest that, in our model, there is an increase in plasma IFN-γ levels 12 h after CLP but that levels drop by 24 h, consistent with the results reported by Steinhauser et al. (28).
FIG. 6.
Levels of IFN-γ in plasma from sham controls, CLP plus irrelevant antibody (CLP), and CLP plus IFN-γ antibody (CLP + IFN-γ Ab) 12 h after surgery. Values are means ± SEM for sham (n = 6), CLP (n = 9), and CLP + IFN-γ Ab (n = 9). *, P < 0.05 compared to that for sham controls; #, P < 0.05 compared to that for CLP + IFN-γ Ab.
IL-6 levels.
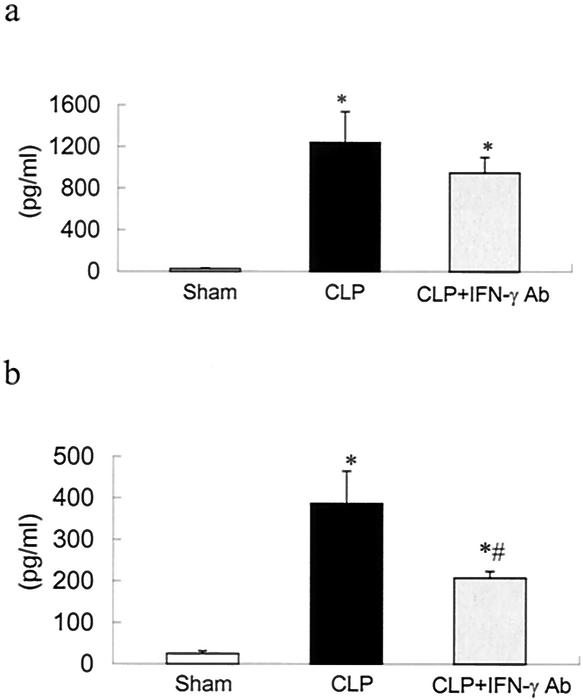
Plasma IL-6 levels were greatly increased in CLP plus MOPC-21 rats, compared to those in sham controls, 12 h after surgery (Fig. 7a). Plasma IL-6 levels were significantly reduced in animals given IFN-γ antibody compared to those in positive controls, and levels were not different from sham controls. At 24 h after surgery, the profile was similar in that levels in plasma in CLP rats were increased, compared to those in sham controls, and treatment with IFN-γ antibody significantly reduced levels (Fig. 7b).
FIG. 7.
Levels of IL-6 in plasma from sham controls, CLP plus irrelevant antibody (CLP), and CLP plus IFN-γ antibody (CLP + IFN-γ Ab) 12 h after surgery. Values are means ± SEM for sham (n = 6), CLP (n = 9), and CLP + IFN-γ Ab (n = 9). (b) Levels of IL-6 in plasma from sham controls, CLP, and CLP + IFN-γ Ab 24 h after surgery. Values are means ± SEM for sham (n = 14), CLP (n = 17), and CLP + IFN-γ Ab (n = 15). *, P < 0.05 compared to that for sham controls; #, P < 0.05 compared to that for CLP.
Peritoneal PAI-1 levels.
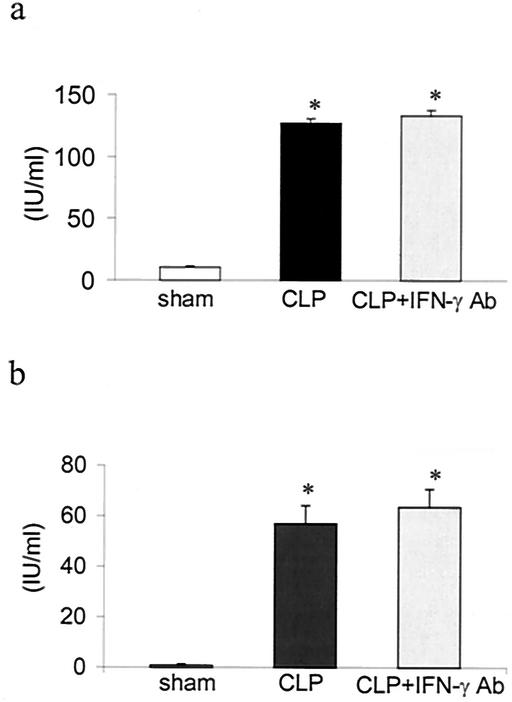
Plasma PAI-1 levels were significantly raised 12 and 24 h in CLP rats compared to those in sham controls. CLP rats given IFN-γ antibody had peritoneal PAI-1 levels that were significantly higher than those for sham controls but were similar to those for CLP rats given irrelevant antibody (Fig. 8).
FIG. 8.
Activity of PAI-1 in peritoneal lavage samples from sham controls, CLP plus irrelevant antibody (CLP), and CLP plus IFN-γ antibody (CLP + IFN-γ Ab) 12 h after surgery. Values are means ± SEM for sham (n = 6), CLP (n = 9), and CLP + IFN-γ Ab (n = 9). (b) Activity of PAI-1 in peritoneal lavage samples from sham controls, CLP, and CLP + IFN-γ Ab 24 h after surgery. Values are means ± SEM for sham (n = 9), CLP (n = 14), and CLP + IFN-γ Ab (n = 15). *, P < 0.05 compared to that for sham controls.
DISCUSSION
In the present study, we provided evidence demonstrating that the use of an antibody to IFN-γ accelerated fibrin deposition at the site of injury, which was associated with reduced bacterial leakage in a rat CLP model of bacterial peritonitis. Taken together, these results would strongly suggest that the increased fibrin deposition after IFN-γ inhibition reduces bacterial load by walling off the cecum. Concomitant to this, inhibition of IFN-γ activity also reduces the circulating levels of IL-6 and IFN-γ.
Work from our laboratory as well as other investigators has shown that the development of peritoneal adhesions as a result of tissue repair is beneficial to the host to prevent bacterial spread in septic peritonitis (7, 23). Indeed, Echtenacher and colleagues (7) demonstrated that the use of anticoagulants reduces peritoneal adhesions, increases bacterial load in the peritoneal cavity, and significantly increases mortality. Increases in proinflammatory mediators such as TNF-α and IL-1 have been implicated in the formation of peritoneal adhesions (11). On the other hand, the actions of TNF-α and IL-1 have been linked to organ dysfunction (14, 15). Thus, the potential therapeutic administration of such proinflammatory cytokines to accelerate tissue repair would be questionable. In contrast, a decrease in IFN-γ levels has been correlated with peritoneal adhesions in humans (3). Thus, it appears plausible that blocking IFN-γ activity will result in accelerated tissue repair. To the best of our knowledge, this is the first report where an anti-inflammatory procedure, i.e., antibody against IFN-γ, has been shown to increase fibrin deposition, accelerate tissue repair, and reduce bacterial load.
This tissue repair process is linked to the coagulation cascade, where the activation of tissue factor initiates the activation of proteolytic coagulation factors, culminating in the thrombin-dependent activation of fibrinogen to fibrin (29). The transient activated fibrinogen, termed fibrin monomer, is made up of three chains, namely, the α, β, and γ polypeptide chains. Fibrin monomer can undergo a process to form larger fibrin oligomers and/or be degraded to various breakdown products by fibrinolytic enzymes such as plasmin. At 12 and 24 h after surgery, our Western blot results clearly showed that, in the peritoneal lavage fluid of CLP rats given IFN-γ antibody, there was a significant increase in fibrinogen products with molecular masses greater than or equal to that of authentic fibrinogen, suggesting that there was an increased deposition of fibrin oligomers to the peritoneal cavity. Additionally, smaller fibrinogen products (smaller than authentic fibrinogen) were increased in all CLP rats compared to those for sham controls. These latter results indicate that there was ongoing fibrinolysis. At 12 h after surgery, IFN-γ-antibody-treated CLP rats had absolute levels of fibrinogen breakdown products similar to those for CLP rats given irrelevant antibody, suggesting that the fibrinolytic activity was similar in both groups of rats. Similarly, at 24 h after surgery, the relative amounts of soluble fibrinogen product (both >200 and <200 kDa) were greater in CLP rats given IFN-γ antibody than those for the CLP rats given irrelevant antibody. Histological analysis showed that, at 24 h after surgery, there was more fibrin matrix formed on the serosal surface of the cecal tissue of IFN-γ-antibody-treated rats than on that of CLP rats given irrelevant antibody, supporting the notion that there was fibrin deposition onto injured cecal tissue surfaces.
The continued deposition of fibrin to the site of injury is maintained by the persistent stimulation of tissue factor via proinflammatory mediators such as cytokines (TNF-α, IL-1β, and IL-6) and/or by antifibrinolytic agents such as PAI-1 (25, 29). Excessive formation of activated fibrin may lead to hypercoagulation and disseminated intravascular coagulation. Our data show that the early systemic inflammatory response was ameliorated after the administration of IFN-γ antibody, as evidenced by a decrease in the levels of IL-6 and IFN-γ in plasma 12 h after surgery. These results would suggest that the impetus to promote fibrin activation and deposition (i.e., increased production of proinflammatory cytokines) may be ameliorated. As the inhibition of IFN-γ also increased fibrin deposition, the results suggest that the mechanism by which the inhibition of IFN-γ increases fibrin deposition is not mediated by an increase in proinflammatory cytokines. Additionally, our results showed that soluble fibrin in blood is not significantly elevated after IFN-γ antibody administration compared to that for CLP rats not given specific antibody. These latter results provide further evidence that ongoing and excessive fibrin formation in the circulation did not occur with IFN-γ antibody administration. These data support the notion that the administration of IFN-γ antibody increases fibrin deposition at the site of injury without significantly affecting the levels of soluble fibrin in circulating blood. Our results, however, do not provide information as to the mechanism by which IFN-γ antibody increases fibrin deposition in the peritoneal cavity. The two possible mechanisms are increased coagulation with the efficient exudation of fibrin to the site of injury and/or decreased fibrinolytic activity. The former mechanism is possibly more likely, as the immunoblots of soluble fibrin in peritoneal fluid clearly showed the increased presence of fibrinogen breakdown products (Fig. 2 and 3). Furthermore, our data showing that there was no increase in PAI-1 levels in antibody-treated rats compared to that for rats given irrelevant antibody supports the postulate that the most likely mechanism for the increased fibrin deposition in IFN-γ-treated rats is due to increased fibrin formation. Ongoing studies with specific markers for the two processes are currently being conducted.
With increased fibrin deposition in the cecal tissue and in the peritoneal cavity, there may be an increased likelihood of abscess formation in the long-term, as increased fibrin deposition with ongoing infection and/or inflammation predisposes the host to this condition. Indeed, it has been reported that abdominal adhesions and abscesses occur extensively after 7 days in the CLP model (8). Our results, however, showed that, after IFN-γ antibody administration, both the systemic inflammatory response and the bacterial load were significantly reduced. This leads us to speculate that it may be possible that, with a reduced inflammatory response and decreased bacterial load, the likelihood of abscess formation may be reduced. It must, however, be recognized that fibrin deposition may be increased in organs remote from the site of injury such as the lung or liver. Fibrin deposition in such sites would be detrimental, and an investigation into this possibility is currently being conducted.
The production of IFN-γ by T lymphocytes and NK cells is stimulated by the cytokine IL-12 (30). Thus, our results showing that inhibition IFN-γ activity increases fibrin deposition and ameliorates bacterial load is in contrast to that of Steinhauser and colleagues (28), who reported that the administration of anti-IL-12 antibody increases bacterial load after CLP in mice. The authors also showed that the increase in peritoneal bacterial load appeared to be due to insufficient tissue repair of the cecal wall. This discrepancy may be due to differences in the fact that IL-12 may have actions that are independent of IFN-γ. Support for this notion stems from the report that IL-12 can restore normal resistance to bacterial challenge after burn injury better than IFN-γ (22). Additionally, IL-12 can suppress inducible nitric oxide synthase (iNOS) expression in a model of immune-complex-induced lung injury where iNOS expression is dependent on IFN-γ formation (19). Another possibility is the difference in the time of administration. In the above-mentioned study, anti-IL-12 antibody (28) was given 6 h before surgery was performed, while in our experiments, the anti-IFN-γ antibody was administered immediately after surgery. Despite the discrepancy in the results, this report fully supports the postulate that tissue repair is important in bacterial containment after CLP.
Recently, IL-6 has been shown to be a proinflammatory mediator that is highly positively correlated with an increased risk of mortality in the murine model of CLP (24). IL-6 is a pleiotropic cytokine that is essential for host defense but, at high levels, can initiate organ dysfunction (21). Our results showing that there was a rise in plasma IL-6 levels 12 and 24 h after CLP that could be ameliorated by IFN-γ antibody demonstrate that the production of IL-6 is modulated and/or regulated by IFN-γ. Here, it should be emphasized that our results only provide evidence that anti-IFN-γ antibody administration can reduce the levels of early systemic proinflammatory response markers, as evidenced by the reduction in circulating IFN-γ and IL-6 levels. These results should not be used to indicate a beneficial role for the specific blockade of IL-6.
In summary, our studies indicate a beneficial effect of early IFN-γ antibody administration after CLP where the peritoneal bacterial load as well as the systemic inflammatory response were significantly ameliorated. These effects were associated with increased fibrin deposition in the peritoneum, which plausibly would aid in sealing off the cecal wall to reduce bacterial spread. This action appeared to occur without an increase in plasma-soluble fibrin, which may have detrimental effects, predisposing the host to hypercoagulation-associated problems.
Acknowledgments
This work was supported by grants from NIH (HL 58172) and the American Heart Association-Heritage affiliate (0050890T) and an award from the Lindback Foundation (K.Y.).
We thank Robert Nagele for help in sectioning.
Editor: F. C. Fang
REFERENCES
- 1.Car, B. D., V. M. Eng, B. Schnyder, L. Ozmen, S. Huang, P. Gallay, D. Heumann, M. Aguet, and B. Ryffel. 1994. Interferon-gamma receptor deficient mice are resistant to endotoxic shock. J. Exp. Med. 179:1437-1444. [DOI] [PMC free article] [PubMed]
- 2.Chaudry, I. H., K. A. Wichterman, and A. E. Baue. 1979. Effect of sepsis on tissue adenine nucleotide levels. Surgery 85:205-211. [PubMed] [Google Scholar]
- 3.Chegini, N., H. Rong, B. Bennett, and K. I. Stone. 1999. Peritoneal fluid cytokine and eicosanoid levels and their relation to the incidence of peritoneal adhesion. J. Soc. Gynecol. Investig. 6:153-157. [DOI] [PubMed] [Google Scholar]
- 4.Docke, W. D., F. Randow, U. Syrbe, D. Krausch, K. Asadullah, P. Reinke, H. D. Volk, and W. Kox. 1997. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat. Med. 3:678-681. [DOI] [PubMed] [Google Scholar]
- 5.Doherty, G. M., J. R. Lange, H. N. Langstein, H. R. Alexander, C. M. Buresh, and J. A. Norton. 1992. Evidence for IFN-γ as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J. Immunol. 149:1666-1670. [PubMed] [Google Scholar]
- 6.Ebong, S. J., D. R. Call, G. Bolgos, D. E. Newcomb, J. I. Granger, M. O'Reilly, and D. G. Remick. 1999. Immunopathologic responses to non-lethal sepsis. Shock 12:118-126. [DOI] [PubMed] [Google Scholar]
- 7.Echtenacher, B., K. Weigl, N. Lehn, and D. N. Mannel. 2001. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect. Immun. 69:3550-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghellai, A. M., A. F. Stucchi, D. J. Lynch, K. C. Skinner, M. J. Colt, and J. M. Becker. 2000. Role of hyaluronate-based membrane in the prevention of peritonitis-induced adhesions. J. Gastrointest. Surg. 4:310-315. [DOI] [PubMed] [Google Scholar]
- 9.Gurujeyalakshmi, G., and S. N. Giri. 1995. Molecular mechanisms of antifibrotic effect of interferon-γ in bleomycin-mouse model of lung fibrosis: downregulation of TGF-β and procollagen I and III gene expression. Exp. Lung Res. 21:791-794. [DOI] [PubMed] [Google Scholar]
- 10.Hellebrekers, B. W. J., T. C. M. Trimbos-Kemper, J. B. M. Z. Trimbos, J. J. Emeis, and T. Kooistra. 2000. Use of fibrinolytic agents in the prevention of post-operative adhesion formation. Fertil. Steril. 74(2):203-212. [DOI] [PubMed] [Google Scholar]
- 11.Kaidi, A. A., M. Nazzal, T. Gurchumelidze, M. A. Ali, E. J. Dawe, and Y. J. Silva. 1995. Preoperative administration of antibodies against tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) and their impact on peritoneal adhesion formation. Am. Surg. 61:569-572. [PubMed] [Google Scholar]
- 12.Keller, R., P. Joller, R. Keist, H. Binz, and P. H. van der Meide. 1988. Modulation of major histocompatibilty complex (MHC) expression by interferons and microbial agents. Scand. J. Immunol. 28:113-121. [DOI] [PubMed] [Google Scholar]
- 13.Kohler, J., D. Heumann, G. Garotta, D. LeRoy, S. Bailat, C. Barras, J.-D. Baumgartner, and M. P. Glauser. 1993. IFN-γ involvement in the severity of gram-negative infections in mice. J. Immunol. 151:916-921. [PubMed] [Google Scholar]
- 14.Kumar, A., V. Thota, L. Dee, J. Olson, E. Uretz, and J. E. Parillo. 1996. Tumor necrosis factor-α and IL-1β are responsible for the in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 183:949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meldrum, D. R. 1998. Tumor necrosis factor in the heart. Am. J. Physiol. 274:R577-R595. [DOI] [PubMed] [Google Scholar]
- 16.Mercer-Jones, M. A., M. Heinzelmann, J. C. Peyton, D. J. Wickel, M. Cook, and W. G. Cheadle. 1997. The pulmonary inflammatory response to experimental fecal peritonitis: relative roles of tumor necrosis factor-α and endotoxin. Inflammation 21:401-417. [DOI] [PubMed] [Google Scholar]
- 17.Miles, R. H., T. P. Paxton, D. J. Dries, and R. L. Gamelli. 1994. Interferon-gamma increases mortality following cecal ligation and puncture. J. Trauma 36:607-611. [DOI] [PubMed] [Google Scholar]
- 18.Miles, R. H., T. P. Paxton, D. Zacheis, D. J. Dries, and R. L. Gamelli. 1994. Systemic administration of interferon-γ impairs wound healing. J. Surg. Res. 56:288-294. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan, M. S., R. L. Warner, J. L. Foreback, T. P. Shanley, and P. A. Ward. 1997. Protective effects of IL-4, IL-10, IL-12 and IL-13 in IgG immune complex-induced lung injury: role of endogenous IL-12. J. Immunol. 159:3483-3489. [PubMed] [Google Scholar]
- 20.Nathan, C. F., H. W. Murray, M. E. Wiebe, and B. Y. Rubin. 1983. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and anti-microbial activity. J. Exp. Med. 158:670-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberholzer, A., C. Oberholzer, and L. L. Moldawer. 2001. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16:83-96. [DOI] [PubMed] [Google Scholar]
- 22.O'Suilleabhain, C., S. T. O'Sullivan, J. L. Kelly, J. Lederer, J. A. Mannick, and M. L. Rodrick. 1996. Interleukin-12 treatment restores normal resistance to bacterial challenge after burn injury. Surgery 120:290-292. [DOI] [PubMed] [Google Scholar]
- 23.Qiu, G., C. Wang, R. Smith, K. Harrison, and K. Yin. 2001. Role of IFN-γ in bacterial containment in a model of intra-abdominal sepsis. Shock 16:425-429. [DOI] [PubMed] [Google Scholar]
- 24.Remick, D. G., G. R. Bolgos, J. Siddiqui, J. Shin, and J. A. Nemzek. 2002. Six at six: interleukin-6 measured 6h after the initiation of sepsis predicts mortality over 3 days. Shock 17:463-467. [DOI] [PubMed] [Google Scholar]
- 25.Salgado, A., J. L. Boveda, J. Monasterio, R. M. Segura, M. Mourelle, J. Gomez-Jimenez, and R. Peracaula. 1994. Inflammatory mediators and their influence on hemostasis. Haemostasis 24(2):132-138. [DOI] [PubMed] [Google Scholar]
- 26.Seki, S., S.-I. Osada, S. Ono, S. Aosasa, Y. Habu, T. Nishikage, H. Mochizuki, and H. Hiraide. 1998. Role of liver NK cells and peritoneal macrophages in gamma interferon and interleukin-10 production in experimental bacterial peritonitis in mice. Infect. Immun. 66:5286-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva, A. T., and J. Cohen. 1992. Role of interferon-γ in experimental gram-negative sepsis. J. Infect. Dis. 166:331-335. [DOI] [PubMed] [Google Scholar]
- 28.Steinhauser, M. L., C. M. Hogaboam, N. W. Lukacs, R. M. Strieter, and S. L. Kunkel. 1999. Multiple roles for IL-12 in a model of acute septic peritonitis. J. Immunol. 162:5437-5443. [PubMed] [Google Scholar]
- 29.Tapper, H., and H. Herwald. 2000. Modulation of hemostatic mechanisms in bacterial infectious diseases. Blood 96:2329-2337. [PubMed] [Google Scholar]
- 30.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 31.Young, H. A., and K. J. Hardy. 1990. Interferon-γ: producer cells, activation stimuli, and molecular genetic regulation. Pharmacol. Ther. 45:137-151. [DOI] [PubMed] [Google Scholar]
- 32.Zantl, N., A. Uebe, B. Neumann, H. Wagner, J. R. Siewert, B. Holzmann, C. D. Heidecke, and K. Pfeffer. 1998. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect. Immun. 66:2300-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]