Abstract
The prevalence of allergic diseases such as asthma has increased markedly over the past few decades. To evaluate the possible mutual influence of helminth infection and allergy, the combined effects of experimental allergic airway inflammation and infection with Strongyloides venezuelensis on various parasitological and inflammatory indices were evaluated in the rat. A challenge of immunized rats with aerosolized ovalbumin (OVA) resulted in eosinophilic inflammation that peaked 48 h after the challenge and was accompanied by airway hyperresponsiveness (AHR) to an intravenous acetylcholine challenge. S. venezuelensis infection concomitant with an OVA challenge of immunized rats resulted in prolonged pulmonary inflammation with increased eosinophil infiltration in bronchoalveolar lavage fluid but not in the lung tissue. These rats also showed a significant parasite burden reduction, especially during parasite migration through the lungs. However, the fecundity rates of worms that reached the intestine were similar in allergic and nonallergic animals. Despite airway inflammation, the increased responsiveness of the airways in the experimental asthma model was suppressed during parasite migration through the lungs (2 days). In contrast, parasite-induced AHR was unchanged 5 days after infection in immunized and challenged rats. In conclusion, infection with S. venezuelensis interfered with the onset of AHR following an antigen challenge of immunized rats. The ability of parasites to switch off functional airway responses is therapeutically relevant because we may learn from parasites how to modulate lung function and, hence, the AHR characteristic of asthmatic patients.
The global prevalence of allergic diseases such as asthma, the best-documented kind of allergic disease, has increased markedly over the past few decades (4, 15, 29). The overall increased frequency of asthma has been followed by an alarming increase in fatal and severe cases of the disease, especially in children (3). As a consequence, estimates in the United States have indicated billions of dollars lost in health care costs and reduced overall productivity due to asthma (32).
Although asthma has a genetic predisposition component (34), the fast increase in the incidence of asthma indicates that environmental factors are responsible for the epidemic behavior of the disease. Low educational and social levels, changes in certain types of air pollution (particulate, for example) and indoor exposure to allergens have been associated with asthma development (10, 33). Interestingly, the prevalence of asthma has risen especially in the developed parts of the world, where the overall air quality and socioeconomic level of the population have improved in recent years (4, 16). Therefore, the hygiene hypothesis initially proposed by Strachan (28) has attracted the most attention. The hygiene hypothesis argues that the diminished incidence of childhood infection observed in industrialized countries over the past century impairs the development of Th1 responses, thus increasing the tendency to allergic diseases (8). Experimental support for this hypothesis came from epidemiological studies showing an inverse correlation between levels of antibodies to orofecal microbes, such as hepatitis A virus and Toxoplasma gondii, and atopy (20). A similar correlation was also found between the rise in the incidence of allergies and the decline in cellular reactivity to mycobacteria in Japanese children (25). However, it was not all of the Th1-inducing stimuli that had an the immunomodulatory effect on atopy (27). Moreover, an increasing number of studies have shown that human populations with a high prevalence of helminth infection, the most potent natural Th2 response stimulant, generally have a low prevalence of atopy and allergic diseases (2, 17). Again, the inverse association between atopy and helminth infection was not always found (18, 19), suggesting that immunoregulation of allergies by parasite infection is more complex than just a matter of Th1-Th2 balance and is influenced by many factors, including the intensity and continuity of the infection and the infection site (36, 38).
The development of experimental models with which to understand the mechanism by which helminth infections modulate the development of allergic responses might result in new strategies by which to control allergic diseases. At the same time, this knowledge may also help in the evaluation of the relevance of allergic inflammation to worm elimination. We have recently described lung alterations induced by the nematode Strongyloides venezuelensis in the rat, its natural host. The obligatory migration of the parasite larvae through the lung induced eosinophilic inflammation, mucus production, increased local concentrations of total and specific immunoglobulin E (IgE), and airway hyperresponsiveness (AHR) (26). To evaluate the possible mutual influence of infection and allergy in our model, we have evaluated the combined effects of experimental allergic airway inflammation and infection with S. venezuelensis on various parasitological and inflammatory indices in the rat.
MATERIALS AND METHODS
Rats and parasite.
Male Wistar rats weighing 180 to 200 g and bred at the bioscience unit at Instituto Gonçalo Moniz (Fundação Oswaldo Cruz, Salvador, Brazil) were used in the experiments. During the investigation, rats were maintained at the animal facilities of the Federal University of Minas Gerais, fed with laboratory chow (Nuvilab; Colombo, Paraná, Brazil), and given tap water to drink ad libitum. The experimental procedures used received prior approval from the local animal ethics committee.
S. venezuelensis, a nematode parasite that obligatorily migrates through the host lungs before establishment in the duodenal mucosa, was used in the experiments. The nematode was isolated from Rattus norvegicus (5) and has been maintained in the Department of Parasitology, Federal University of Minas Gerais, by serial passage in Wistar rats.
Parasite infection model.
For the experiments described herein, S. venezuelensis infective filiform (L3) larvae were obtained from a charcoal culture of infected-rat feces and the infective larvae collected were counted and adjusted to 5,000 L3 larvae/ml of phosphate-buffered saline (PBS [NaCl at 136 mM, KCl at 2 mM, Na2HPO4 at 8 mM, KH2PO4 at 10 mM, pH 7.4]) for infection as previously described by Silveira et al. (26). For each infection, rats were inoculated subcutaneously with 1,500 infective larvae in 300 μl of PBS in the abdominal region and infectivity parameters were evaluated at 2, 5, 7, and 12 days after inoculation.
Experimental asthma model—immunization and challenge with OVA.
A protocol of immunization with ovalbumin (OVA) was developed to induce lung eosinophil inflammation and AHR in rats. The protocol, modified from that of Pereira de Siqueira et al. (21), consisted of immunization with a solidified egg white implant and a challenge with aerosolized OVA. Briefly, pasteurized and lyophilized hen's egg white was prepared at 10% in PBS and 45-μl samples were placed into 96-well plates and microwaved for 90 s. The solidified egg white was dehydrated in ethanol for 48 h, kept at 4°C, and hydrated in PBS for 2 h before implantation. Heat-coagulated hen's egg white was implanted into the subcutaneous tissue of the dorsal region of anesthetized Wistar rats (one 45-μl solidified sample/rat). A nasal antigen challenge was administrated once (14 days after OVA implantation) or twice (14 and 21 days after implantation) by exposure of rats to 2% aerosolized OVA for 30 min (OVA/OVA group). The aerosol was delivered into an adapted chamber by an ultrasonic nebulizer (Harvard Apparatus, Holliston, Mass.). Surgically treated, nonsensitized, PBS-aerosolized rats (PBS/PBS group); surgically treated, nonsensitized, and OVA-challenged rats (PBS/OVA group); and surgically implanted, OVA-sensitized, and PBS challenged rats (OVA/PBS) were used as controls. The animals were evaluated at 24, 48, and 72 h after the challenge.
Experimental asthma combined with parasite infection.
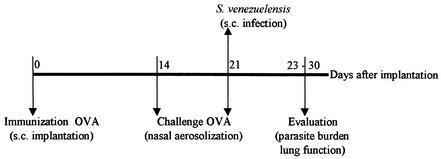
To verify the effect of natural nematode infections on the OVA-immunized lung, rats were subcutaneously infected with 1,500 S. venezuelensis larvae at the time of the second nasal OVA challenge (OVA/OVA infected group). The lung function of these rats was estimated at 2 and 5 days after parasite infection, time points of maximal allergen- and parasite-induced AHR, respectively (26). Lung cellular infiltration was analyzed at 2, 7, and 12 days after parasite infection, while the parasite burdens in the lung and small intestine were quantified at 2 and 7 days after parasite infection, respectively (Fig. 1). Surgically treated, nonsensitized, PBS-aerosolized, and parasite-infected rats (PBS/PBS infected group); surgically treated, nonsensitized, OVA-challenged, and parasite-infected rats (PBS/OVA infected group); and OVA-implanted and challenged but not infected rats (OVA/OVA not infected group) were also evaluated.
FIG. 1.
Scheme of parasite infection combined with OVA immunization and challenge.
Parasite burden.
Infectivity rates were determined by assessing fecal egg counts, numbers of larvae recovered from the lung, and numbers of worms recovered from the small intestine at 2 and 7 days after parasite infection in PBS/PBS infected and OVA/OVA infected mice. For worm recovery from the small intestine, the upper half of the small intestine was removed after sacrifice, washed, cut open longitudinally, and incubated in PBS at 37°C for 4 h. For recovery of larvae from the lung, the organ was removed, fragmented in PBS, and incubated for 2 h at 37°C. Worms that emerged from each organ were quantified by stereomicroscopy. Eggs eliminated with the host feces were diluted in a sugar-saturated solution (Sheater solution) and quantified in McMaster chambers (26). S. venezuelensis-infected hosts have only female worms in the small intestine; therefore, the number of eggs eliminated divided by the number of worms recovered from the intestine was used to calculate parasite fecundity at that time.
Lung function evaluation.
Changes in lung function were estimated by assessment of the responsiveness of the airways to increasing doses of intravenously injected acetylcholine. At defined time points after OVA immunization, parasite infection, or the combined protocol, rats—five rats from each experimental group—were anesthetized with thiopental sodium (40 mg/kg; Abbott Laboratories, São Paulo, Brazil) and the femoral artery, femoral vein, and trachea were cannulated. A three-way connector was attached to the tracheotomy tube; two ports were connected to the ventilator (with 10 ml of air/kg of animal at a rate of 90 breaths/min; Harvard Apparatus), and one was attached to a pressure transducer (Physiological Pressure Transducer; Ohmeda). Acetylcholine (10 to 300 μg/kg in 100 μl of sterile PBS, pH 7.4; Sigma Chemical Co., Poole, Dorset, United Kingdom) was injected through a cannula inserted into the femoral vein, and the variation in intratracheal pressure was used as an indirect measurement of lung resistance, as detailed by Silveira et al. (26). Data are expressed as percent increases in intratracheal pressure in comparison with the baseline. There were no differences in baseline pressure among any of the groups analyzed (data not shown).
Collection of blood, BAL fluid, and bone marrow.
After measurement of lung function parameters, anesthetized rats were bled via the abdominal aorta and blood samples were used to estimate the circulating-cell composition. Bronchoalveolar lavage (BAL) was performed by intratracheal instillation of 5 ml of PBS containing 0.3% bovine serum albumin (PBS-BSA; Sigma) and a protease inhibitor cocktail (one tablet in 50 ml; Boehringer Mannheim, Mannheim, Germany). The BAL fluid was centrifuged (200 × g for 7 min), and aliquots of the supernatant were kept at −70°C until further analysis. The cell pellet from the BAL fluid was resuspended in 1 ml of PBS-BSA.
Bone marrow cells were flushed from the right femur of each rat by injecting 5 ml of PBS containing heparin (50 IU/ml). The recovered solution was vortexed gently and centrifuged at 200 × g for 7 min. The cell pellet was resuspended in 1 ml of PBS-BSA, and the total numbers of leukocytes and mature eosinophils were determined.
Total numbers of leukocytes in blood, BAL fluid, and bone marrow lavage fluid were estimated in a Neubauer chamber. Cytospin slides prepared from BAL fluid and bone marrow samples and blood smears were stained with May-Grünwald-Giemsa stain. Standard morphological criteria were used to differentiate at least 200 cells/slide by light microscopy.
EPO assay.
The eosinophil peroxidase (EPO) assay was used to estimate the eosinophil numbers in lung tissue and BAL fluid (7). After flushing of the pulmonary artery with 20 ml of PBS, the left lung was weighed, chopped, and homogenized in PBS (5% [wt/vol]) with a tissue homogenizer (Power Gen 125; Fisher Scientific, Pittsburgh, Pa.). The homogenate was centrifuged (3,000 × g for 10 min), the red blood cells in the pellet were lysed, and cells were resuspended in PBS (pH 7.4) containing 0.5% hexadecyltrimethylammonium bromide (Sigma). The cell solution was homogenized again, and the homogenates were then freeze-thawed three times in liquid nitrogen and stored at −20°C until they were assayed. For the assay, samples of BAL fluid and lung tissue were spun and the supernatant was diluted 1:3 in PBS-hexadecyltrimethylammonium bromide. The assay was carried out with 96-well plates (Nalge Nunc International Co., Naperville, Ill.). Each sample was tested in triplicate by adding 75 μl of the sample/well and 75 μl of OPD substrate (1.5 mM o-phenylenediamine [Sigma] and 6.6 mM hydrogen peroxide in 75 mM Tris-HCl, pH 8.0)/well. The reaction was carried out at 20°C for 30 min and stopped with a 4 M sulfuric acid solution. Plates were read at 492 nm on a microplate reader (Emax; Molecular Devices), and results are shown as absorbance units.
Statistical analysis.
Data are reported as the mean ± the standard error of the mean (SEM) and were analyzed by one-way analysis of variance. In the latter analysis, P values were assigned by using the Student-Neumann-Keuls test. Differences with P values of <0.05 were considered significant.
RESULTS
Eosinophilic airway inflammation and AHR in a rat model of allergic asthma.
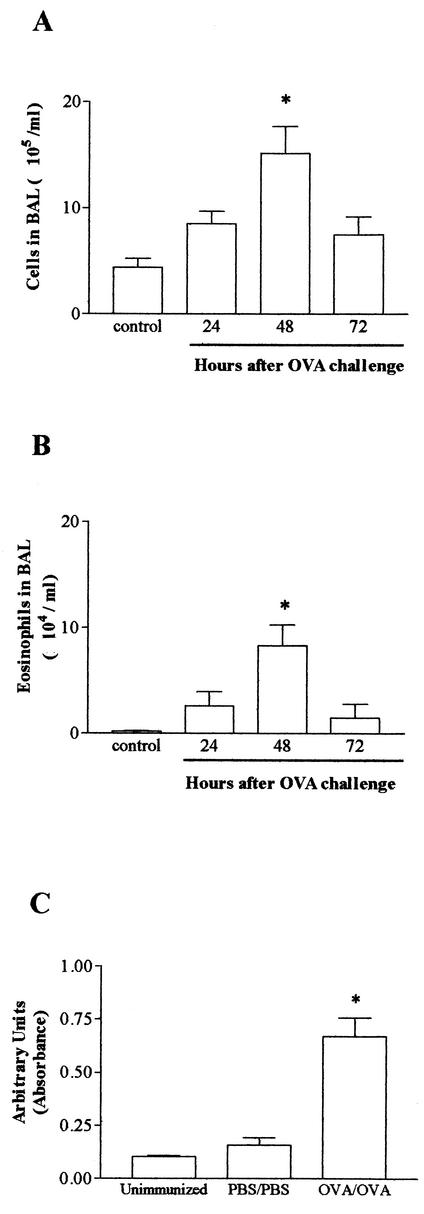
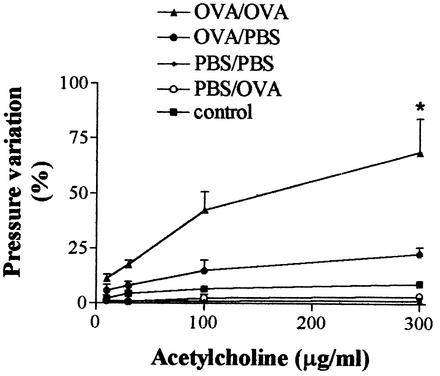
Immunization with a solidified egg white implant, followed by a single challenge with aerosolized OVA, induced total leukocyte and eosinophil increases in BAL fluid that peaked 48 h after the challenge (data not shown). However, pulmonary eosinophilia, as assessed by EPO levels in the tissue homogenate and visualized by histopathologic analysis, was discrete and there were only small variations in intratracheal pressure after injection of acetylcholine (data not shown). The inflammatory and functional responses were more intense when immunized rats were challenged twice with aerosolized OVA (at 14 and 21 days after the egg white implant). Therefore, this protocol was used throughout the experiments described below. Forty-eight hours after the second OVA aerosolization, there was a significant increase in the total numbers of leukocytes and eosinophils in blood and an increase in the number of eosinophils, but not in the number of total leukocytes, recovered from the bone marrow of OVA/OVA immunized rats (Table 1). In the lungs, a significant increase in the total numbers of leukocytes (Fig. 2A) and eosinophils (Fig. 2B) in the BAL fluid was also observed in OVA/OVA immunized animals. The presence of eosinophils in the lungs of OVA/OVA immunized rats was mirrored by a significant increase in the levels of EPO in tissue homogenate (Fig. 2C). AHR accompanied the eosinophilic airway inflammation observed in OVA/OVA immunized rats. The latter group of animals showed a significant leftward shift of the acetylcholine dose-response curve at 48 h after the last OVA challenge (Fig. 3). In PBS/OVA and OVA/PBS control animals, the variation in intratracheal pressure after acetylcholine was similar to that observed in nonimmunized rats (Fig. 3).
TABLE 1.
Numbers of total leukocytes and eosinophils recovered from bone marrow and blood of control (PBS/PBS) and allergic (OVA/OVA) rats at 48 h after the last DNA challenge
| Cell source and type (no.) | Mean no. of cells ± SEMa
|
|
|---|---|---|
| PBS/PBS | OVA/OVA | |
| Bone marrow | ||
| Leukocytes (106) | 31.4 ± 4.9 | 26.9 ± 3.3 |
| Eosinophils (105) | 3.1 ± 1.4 | 11.1 ± 3.5 |
| Blood | ||
| Leukocytes (106 ml) | 4.3 ± 0.2 | 9.1 ± 1.0 |
| Eosinophils (105 ml) | 0.1 ± 0.01 | 3.0 ± 0.9 |
Results are for five animals in each group. Values in bold are significantly different (P < 0.05) from those of control (PBS/PBS) rats.
FIG. 2.
Kinetics of leukocyte infiltration in the lungs of rats in an experimental model of allergic asthma. Animals were immunized with a solidified egg white implant and challenged 14 and 21 days later with aerosolized OVA. The numbers of total infiltrating leukocytes (A) and eosinophils (B) in BAL fluid were evaluated at 24, 48, and 72 h after the challenge. An EPO assay (C) was used to estimate the number of eosinophils in lung tissue. Each value represents the mean ± the SEM of six rats in each group. *, P < 0.05 compared to control (PBS-immunized and PBS-challenged) rats.
FIG. 3.
Changes in airway responsiveness to acetylcholine in an experimental rat model of allergic asthma. Animals were immunized with a solidified egg white implant (OVA) or underwent a sham operation (PBS). Fourteen and 21 days later, the animals received an aerosol challenge of OVA or PBS. A group of animals (control) was neither immunized nor challenged. Forty-eight hours after the last antigen challenge, changes in lung function were estimated by assessment of the responsiveness of the airways to increasing doses of intravenously injected acetylcholine. Each value represents the mean ± the SEM of six rats in each group. *, P < 0.05 compared to all other groups of animals.
Lung inflammation after parasite infection combined with experimental asthma.
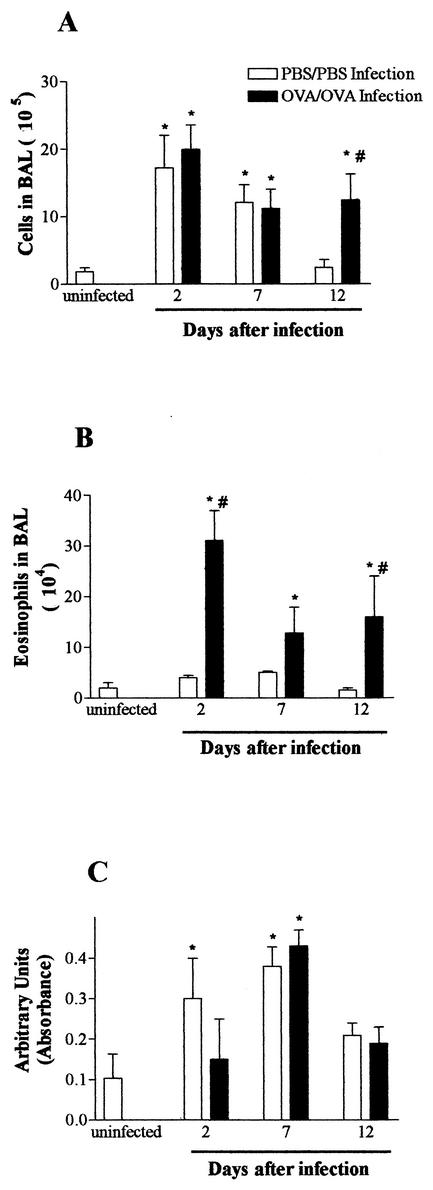
A transient eosinophilic airway inflammation accompanied by AHR has already been described after S. venezuelensis infection of rats (26). To study the relationship between allergic airway inflammation and the immune responses to nematode infection, experimental asthma immunization was combined with S. venezuelensis infection in such a way that parasite larvae were migrating through the lung tissue at the same time that the maximum response to OVA was measured (48 h after the second challenge; Fig. 1). Rats that received an OVA immunization and a challenge combined with a nematode infection (OVA/OVA infection) showed numbers of leukocytes in BAL fluid collected at 2 and 7 days postinfection similar to those of infected controls (PBS/PBS infection) (Fig. 4A). However, a significantly higher number of leukocytes was observed in BAL fluid collected from OVA/OVA infected rats 12 days postinfection than in that from PBS/PBS infected rats (Fig. 4A). OVA immunization and a challenge resulted in a significantly higher number of eosinophils in the BAL fluid of infected rats, especially at 2 days postinfection (Fig. 4B). Nevertheless, EPO levels in lung tissue were not different in OVA/OVA and PBS/PBS infected rats (Fig. 4C). There was also an increase in the number of eosinophils in the blood and bone marrow of OVA/OVA infected rats from days 2 to 12 after infection (data not shown). These increases were similar to that seen in the blood and bone marrow of uninfected, OVA-immunized, and challenged rats (Table 1).
FIG.4.
Kinetics of leukocyte infiltration in the lungs of rats infected with S. venezuelensis concomitant with induction of allergic airway inflammation. BAL fluid (A and B) and lung tissue (C) were obtained before (uninfected) and at 2, 7, and 12 days after a single S. venezuelensis infection (one subcutaneous inoculation of 1,500 L3 larvae). Animals were immunized with a solidified egg white implant and challenged 14 and 21 days later with aerosolized OVA (OVA/OVA) or subjected to a sham operation and challenged with saline (PBS/PBS). The last aerosol challenge coincided with S. venezuelensis infection. The total numbers of infiltrating leukocytes (A) and eosinophils (B) were evaluated in BAL fluid, and an EPO assay (C) was used to estimate the number of eosinophils in lung tissue. Each value represents the mean ± the SEM of 8 to 10 rats in each group. *, P < 0.05 compared to uninfected rats; #, P < 0.05 for PBS/PBS versus OVA/OVA rats.
Parasite infection combined with experimental asthma immunization leads to a diminished parasite burden.
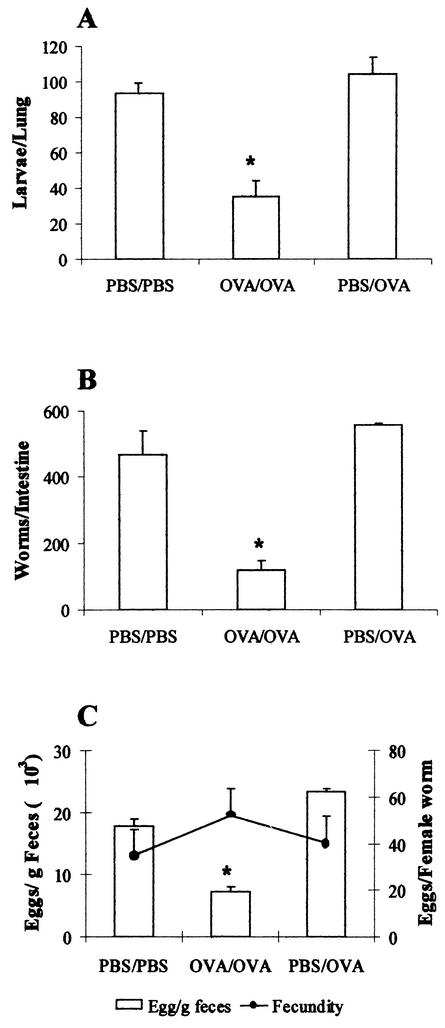
In rats that were immunized and challenged with OVA (OVA/OVA infected group), the number of migrating S. venezuelensis larvae recovered from lung tissue at 48 h after an OVA challenge and parasite infection was significantly lower (35 ± 9 larvae/lung) than the number of larvae recovered from any other infected group (94 to 105 larvae/lung; Fig. 5A). Similarly, the number of worms in the small intestine (117 ± 30 worms/intestine) and the number of parasite eggs in the feces (7,223 ± 722 eggs/g of feces) were lower in OVA/OVA infected rats at 7 days postinfection than in the other infected groups, in which an average of 460 worms were recovered from the small intestine and 17,000 to 22,000 eggs/g of feces were counted (Fig. 5B and C). Interestingly, even though egg elimination in the feces diminished in the OVA/OVA infected rats, the reduction was not a consequence of worm fecundity because the remaining females produced a similar number of eggs per adult female (Fig. 5C).
FIG. 5.
Parasite burdens of rats infected with S. venezuelensis concomitant with induction of allergic airway inflammation. Animals were immunized with a solidified egg white implant and challenged 14 and 21 days later with aerosolized OVA (OVA/OVA) or subjected to a sham operation and challenged with saline (PBS/PBS) or OVA (PBS/OVA). Rats were infected with S. venezuelensis (one subcutaneous inoculation of 1,500 L3 larvae) at the time of the second challenge. The total numbers of larvae in the lung (A), adult worms in the intestine (B), and eggs in the feces (C) were evaluated. The number of eggs per adult worm (fecundity) was determined (C). Each value represents the mean ± the SEM of 8 to 10 rats in each group. *, P < 0.05 compared to uninfected rats; #, P < 0.05 for PBS/PBS or PBS/OVA versus OVA/OVA rats.
S. venezuelensis infection affects AHR induced by experimental asthma.
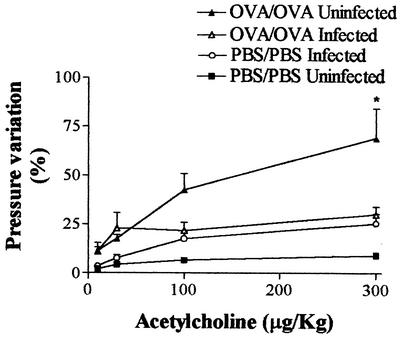
The significant variation in intratracheal pressure observed at 48 h after the last OVA challenge in the OVA/OVA immunized group (OVA/OVA uninfected) was drastically reduced in rats in which the experimental asthma protocol was combined with S. venezuelensis infection (OVA/OVA infected) (Fig. 6). It is important to note that, at this time (48 h postinfection), larvae were migrating through the lung tissue but either the infection combined with the sham immunization (PBS/PBS infected) or sham immunization alone (PBS/PBS uninfected) did not induce AHR (Fig. 6).
FIG. 6.
Infection with S. venezuelensis reduces airway hyperactivity in a model of allergic airway inflammation. Changes in airway function were measured 48 h after an aerosol challenge of immunized rats (OVA/OVA) or a saline challenge of rats subjected to a sham operation (PBS/PBS). Concomitantly with the aerosol challenge, animals were infected with 1,500 S. venezuelensis L3 larvae or not infected. Each value represents the mean ± the SEM of six to eight rats in each group. *, P < 0.05 compared to all other groups of animals.
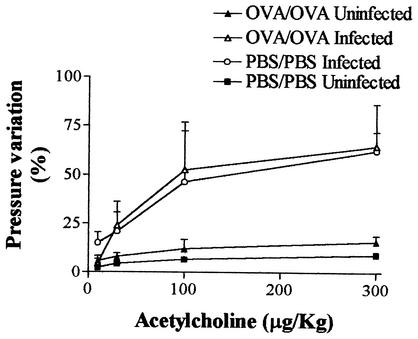
We have previously reported a significant increase in AHR 5 days after S. venezuelensis infection (26). Similarly, infection of nonimmunized rats (PBS/PBS infected) induced a significant leftward shift of the acetylcholine dose-response curve (Fig. 7). In the experimental asthma model (OVA/OVA uninfected), no AHR was present 5 days after an antigen challenge (Fig. 7). Moreover, the OVA immunization-challenge procedure had no significant effect on the AHR observed 5 days after the nematode infection (Fig. 7, OVA/OVA infected group).
FIG. 7.
Antigen challenge of immunized rats does not affect the airway hyperactivity observed 5 days after infection with S. venezuelensis. Changes in airway function were measured 5 days after an aerosol challenge of immunized rats (OVA/OVA) or a saline challenge of rats subjected to a sham operation (PBS/PBS). Concomitantly with the aerosol challenge, animals were infected with 1,500 S. venezuelensis L3 larvae or not infected. Each value represents the mean ± the SEM of six to eight rats in each group. *, P < 0.05 compared to uninfected groups.
DISCUSSION
Helminth infections (9) and so-called allergic diseases, such as asthma (23, 35), are associated with a predominant induction of a Th2 type of immune response, which regulates eosinophilia, mastocytosis, and the increased level of IgE observed in both phenomena. Because of these immunopathologic similarities, it has been hypothesized that an ongoing response against a helminth infection could influence the allergic response and vice versa (11, 22). However, studies of human populations have provided contradictory results—there are data suggesting that a strong Th2 response induced by the parasitic infection would predispose to an allergic reaction (18, 19), while others have suggested that a high prevalence of helminth infection in humans has a protective effect against allergic reactions (2, 17, 30, 37). To evaluate this issue in further detail, we developed an experimental rat model with which to study the association between allergic reactions and helminthic infections by combining a protocol by which to produce experimental asthma with a helminth infection with S. venezuelensis in the rat, a natural host for the parasite.
Our previous studies have shown that infection of rats with S. venezuelensis induced a predominant local Th2 response that resulted in lung eosinophilic inflammation, local IgE concentration increases, and marked mucus production (26). These pathological and immune alterations were accompanied by pulmonary functional changes detected via an increase in airway responsiveness to a bronchoconstricting agent between 5 and 7 days after the infection. As the S. venezuelensis infection model in rats was characterized by pathological and immune alterations typically seen in animal models of allergic asthma, we thought it would be a valuable model with which to investigate the association between helminth infection and allergic airway inflammation. It is worth noting that S. venezuelensis larvae undergo an obligatory phase of migration through the rat lung before they establish themselves in the intestine. Passage through the lungs is similarly observed after infection of humans with Ascaris lumbricoides, hookworm, and S. stercoralis.
Initial experiments were aimed at characterization of a model of allergic airway inflammation in rats that was accompanied by AHR. To this end, a protocol of immunization with a solidified egg white implant, followed by a challenge with aerosolized OVA, was used that is similar to what has been described for mice (21). In rats, two aerosolizations with antigen 1 week apart were necessary to enhance pulmonary inflammation and to induce significant changes in airway responsiveness to acetylcholine. Maximal changes in airway pathology and function were observed 48 h after the second aerosol challenge. Although the amount of eosinophils in the BAL fluid rarely reached 10 to 20% of the total migrating leukocytes, these cells were much more frequent around the trachea and smaller airways (data not shown). This was clearly demonstrated when tissue eosinophils were evaluated by measuring the tissue EPO content. Moreover, these findings are consistent with other rat models of experimental asthma (e.g., see reference 13).
The development of an asthmatic reaction concomitantly with the migration of S. venezuelensis larvae through the rat lung resulted in a significant reduction of the parasite burden, as assessed by the numbers of larvae in the lung, worms in the intestine, and eggs per gram of feces of the infected rats. There was a 65% reduction in the number of living larvae recovered from the lungs of OVA/OVA infected rats compared to the number of larvae recovered from PBS/PBS infected rats. After the migration stage, there was no further decrease in the number of adult worms recovered from the intestine (75% reduction) or in the total egg output (60% reduction). Indeed, the fecundity rates of existing females were similar in the allergic and nonallergic groups. Overall, these results suggest that most of the parasite attrition was compartmentalized in the lung and are in agreement with the increase in lung eosinophilic inflammation induced by an antigen challenge in immunized rats.
At the time of larval migration through the lungs, there was a significant increase in the number of eosinophils in the BAL fluid and bone marrow of the allergic and infected rats compared with rats that were only infected. In contrast to BAL fluid, we failed to find a significant difference in tissue eosinophilia, as assessed by EPO levels, between allergic and nonallergic infected rats. The association of tissue and blood eosinophilia and helminth infection is very frequent, but the relevance of eosinophils to a protective response of the host is still a matter of great controversy. Experiments have failed to show a role for eosinophils in protection against Schistosoma sp. or Trichinella spiralis infection in mice but have demonstrated a protective effect against Angiostrongylus cantonensis, Strongyloides ratti, or S. venezuelensis infection (reviewed in reference 6). A role for eosinophils has also been shown in the clearance of S. stercoralis larvae from muscles in primary infection (12) and after the protective immune response observed in immunized mice (1). In the first model, eosinophils were directly involved in larval killing during innate immunity, a process that was dependent on interleukin-5 (IL-5) and eosinophil levels but independent of IgM production (14). Host-adapted S. stercoralis L3 larvae were killed in vitro by two human eosinophil granule proteins: the major basic protein and the cationic protein (24). The results above suggest that Strongyloides larvae are susceptible to destruction by eosinophils in murine models. As in our system, the significant reduction of S. venezuelensis larvae coincided with the peak of allergy-induced eosinophilic inflammation. It is thus possible that eosinophils play a role in parasite attrition in our model, but this possibility awaits further experimentation once rat eosinophil-specific tools (e.g., anti-rat IL-5) become available.
Despite having enhanced accumulation of eosinophils in BAL fluid, allergic rats showed a significant reduction in AHR when infected concomitantly with S. venezuelensis. It is worth noting that the inhibition of allergy-induced AHR coincided with the migration of S. venezuelensis larvae through the lungs. We have previously shown that S. venezuelensis infection induces a late (5 days after infection) increase in AHR (26). Here, AHR was also observed 5 days after infection but there was no significant difference between the allergic and nonallergic groups. Thus, infection of rats with S. venezuelensis concomitantly with an allergic reaction induced prolonged airway eosinophilic inflammation in the BAL fluid, reduced the number of viable parasites, and inhibited allergy-induced changes in lung function. Inhibition of AHR occurred only during the phase of parasite migration through the lungs, suggesting that the parasites and/or products released in response to the parasite suppress AHR.
Previous reports have described a reduction of allergic manifestations, as assessed by skin prick tests, in heavily helminth-infected populations (2, 17, 30). The protective effect of helminth infection against allergic reactivity has frequently been attributed to a possible blocking effect of helminth-induced polyclonal IgE production on mast cell degranulation (17); i.e., nonspecific IgE saturates FcɛRI on mast cells, blocking the binding of allergen-specific IgE, inhibiting the cross-linkage of bound IgE by the allergens and, consequently, mast cell degranulation and the immediate hypersensitivity response to allergens. Wang et al. (31) demonstrated that mice infected with S. stercoralis prior to immunization and an intratracheal challenge with OVA showed increases in IL-4- and IL-5-producing cells in the BAL fluid but a significant decrease in the OVA-specific IgE response and eotaxin level in the BAL fluid. The authors suggested that a reduction of OVA-specific IgE level, but not of the total IgE level, induced by previous infection with the helminth would have an important role in alleviating the response to allergens. The possibility that parasite-induced IgE interfered with the allergic response evaluated here is unlikely; our previous work showed that the increase in the total and parasite-specific IgE levels in lung tissue occurred only 5 days after a single infection (26). Therefore, an increase in polyclonal IgE does not explain the inhibitory effect of S. venezuelensis infection on OVA-induced AHR.
Recently, a study of chronically Schistosoma haematobium-infected patients failed to confirm the relevance of the polyclonal IgE-blocking mechanism for modulation of the allergic reaction (30). In the latter study, the IL-10 production by peripheral blood mononuclear cells upon a challenge with schistosome antigen was correlated with a lowered risk of development of skin reactivity to mite antigens (30). An inhibitory effect of exogenously administered or endogenously produced IL-10 on airway inflammation was also demonstrated by Zuany-Amorim et al. (39) in mice treated with a suspension of killed Mycobacterium vaccae (SRP299). SRP299 treatment induced allergen-specific CD4+ CD45RBLo regulatory T cells, which inhibited the OVA-induced eosinophilic inflammation and bronchial hyperresponsiveness in an IL-10- and transforming growth factor β-dependent manner (39). The mechanisms underlying the inhibition of AHR in our model were not investigated in greater detail here. However, our previous studies have shown enhanced production of IL-10 during the migratory phase of S. venezuelensis in rats (26), suggesting that this cytokine plays a role in inhibiting AHR in our system.
In conclusion, our results show that infection of rats with S. venezuelensis interfered with the onset of AHR following an antigen challenge in immunized animals. It is worth noting that this interference was acute; i.e., there was no need for chronic helminth infection for inhibition of AHR to occur, and transient AHR was present on day 5 after infection. Moreover, inhibition occurred during the effector phase of the pulmonary allergic response, not during sensitization. The ability of parasites to switch off functional airway responses is therapeutically relevant because we may learn from parasites how to modulate lung function and, hence, the AHR characteristic of asthmatic patients.
Acknowledgments
We are grateful to the Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG (Minas Gerais State Foundation for Scientifical Research), the Conselho Nacional de Desenvolvimento Científico e Tecnológico, the CNPq (National Council for the Scientific and Technological Development) at the Programa de Apoio ao Desenvolvimento Científico e Tecnológico, the PADCT (Program for the Support of Scientific and Technological Development), and the Wellcome Trust for financial support.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abraham, D., H. L. Rotman, H. F. Haberstroh, W. Yutanawiboonchai, R. A. Brigandi, O. Leon, T. J. Nolan, and G. A. Schad. 1995. Strongyloides stercoralis: protective immunity to third-stage larvae in BALB/cByj mice. Exp. Parasitol. 80:297-307. [DOI] [PubMed] [Google Scholar]
- 2.Araujo, M. I., A. A. Lopes, M. Medeiros, A. A. Cruz, L. Sousa-Atta, D. Sole, and E. M. Carvalho. 2000. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 123:145-148. [DOI] [PubMed] [Google Scholar]
- 3.Beasley, R. 2002. The burden of asthma with specific reference to the United States. J. Allergy Clin. Immunol. 109:S482-S489. [DOI] [PubMed] [Google Scholar]
- 4.Beasley, R., J. Crane, C. K. Lai, and N. Pearce. 2000. Prevalence and etiology of asthma. J. Allergy Clin. Immunol. 105:S466-S472. [DOI] [PubMed] [Google Scholar]
- 5.Brener, Z., and G. Chaia. 1960. Isolamento e manutenção do Strongyloides ratti (Sandground, 1925) em condições de laboratório. Rev. Bras. Biol. 20:447-451. [Google Scholar]
- 6.Cara, D. C., D. Negrão-Corrêa, and M. M. Teixeira. 2000. Mechanisms underlying eosinophil trafficking and their relevance in vivo. Histol. Histopathol. 15:899-920. [DOI] [PubMed] [Google Scholar]
- 7.Collins, P. D., S. Marleau, D. A. Griffiths-Johnson, P. J. Jose, and T. J. Williams. 1995. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 182:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cookson, W. O., and M. F. Moffatt. 1997. Asthma: an epidemic in the absence of infection? Science 275:41-42. [DOI] [PubMed] [Google Scholar]
- 9.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 10.Forastiere, F., N. Agabiti, G. M. Corbo, V. Dell'Orco, D. Porta, R. Pistelli, S. Levenstein, and C. A. Perucci. 1997. Socioeconomic status, number of siblings, and respiratory infections in early life as determinants of atopy in children. Epidemiology 8:566-570. [DOI] [PubMed] [Google Scholar]
- 11.Grove, D. I. 1982. What is the relationship between asthma and worms? Allergy 37:139-148. [DOI] [PubMed] [Google Scholar]
- 12.Grove, D. I., C. Northern, and P. J. Heernan. 1986. Strongyloides stercoralis infections in the muscle of mice: a model for investigating the systemic phase of strongyloidiasis. Pathology 18:72-76. [DOI] [PubMed] [Google Scholar]
- 13.Haczku, A., P. Macary, E.-B. Haddad, T. J. Huang, D. M. Kemeny, R. Moqbel, and K. F. Chung. 1996. Expression of Th-2 cytokines interleukin-4 and -5 and of Th-1 cytokine interferon-gamma in ovalbumin-exposed sensitized brown-Norway rats. Immunology 88:247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbert, D. R., J. J. Lee, N. A. Lee, T. J. Nolan, G. A. Schad, and D. Abraham. 2000. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 165:4544-4551. [DOI] [PubMed] [Google Scholar]
- 15.Hopkin, J. M. 1998. The rise of asthma and atopy. Q. J. Med. 91:169-170. [DOI] [PubMed] [Google Scholar]
- 16.International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. 1998. Worldwide variation prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema. Lancet 351:1225-1232. [PubMed] [Google Scholar]
- 17.Lynch, N. R., I. Hagel, M. Perez, M. C. Di Prisco, R. Lopez, and N. Alvarez. 1993. Effect of anhelminthic treatment on the allergic reactivity of children in a tropical slum. J. Allergy Clin. Immunol. 92:404-411. [DOI] [PubMed] [Google Scholar]
- 18.Lynch, N. R., M. Palenque, I. Hagel, and M. C. DiPrisco. 1997. Clinical improvement of asthma after anhelminthic treatment in a tropical situation. Am. J. Respir. Crit. Care Med. 156:50-54. [DOI] [PubMed] [Google Scholar]
- 19.Mao, X. Q., D. J. Sun, A. Miyoshi, Z. Feng, Z. T. Handzel, J. M. Hopkin, and T. Shirakawa. 2000. The link between helminthic infection and atopy. Parasitol. Today 16:186-188. [DOI] [PubMed] [Google Scholar]
- 20.Matricardi, P. M., F. Rosmini, S. Riondino, M. Fortini, L. Ferrigno, M. Rapicetta, and S. Bonini. 2000. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. Br. Med. J. 320:412-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira de Siqueira, A. L., M. Russo, A. A. Steil, M. Mariano, and S. Jancar. 1997. New murine model of pulmonary eosinophilic hypersensitivity: contribution to experimental asthma. J. Allergy Clin. Immunol. 100:382-388. [DOI] [PubMed] [Google Scholar]
- 22.Pritchard, D. I. 1993. Points in question: atopy and helminth parasites. Int. J. Parasitol. 23:167-168. [DOI] [PubMed] [Google Scholar]
- 23.Robinson, D. S., Q. Hamid, S. Ying, A. Tsicopoulos, J. Barkans, A. M. Bentley, C. Corrigan, S. R. Durham, and A. B. Kay. 1992. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326:298-304. [DOI] [PubMed] [Google Scholar]
- 24.Rotman, H. L., W. Yutanawiboonchai, R. A. Brigandi, O. Leon, G. J. Gleich, T. J. Nolan, G. A. Schad, and D. Abraham. 1996. Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in Balb/cByJ mice. Exp. Parasitol. 82:267-278. [DOI] [PubMed] [Google Scholar]
- 25.Shirakawa, T., T. Enomoto, S. Shimazu, and J. M. Hopkin. 1997. The inverse association between tuberculin response and atopic disorder. Science 276:77-79. [DOI] [PubMed] [Google Scholar]
- 26.Silveira, M. R., K. P. Nunes, D. C. Cara, D. G. Souza, A. Corrêa, Jr., M. M. Teixeira, and D. Negrão-Corrêa. 2002. Infection with Strongyloides venezuelensis induces transient airway eosinophilic inflammation, an increase in immunoglobulin E, and hyperresponsiveness in rats. Infect. Immun. 70:6263-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stene, L. C., and P. Nafstad. 2001. Relation between occurrences of type 1 diabetes and asthma. Lancet 357:607-608. [DOI] [PubMed] [Google Scholar]
- 28.Strachan, D. P. 1989. Hay fever, hygiene, and household size. Br. Med. J. 299:1259-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Upton, M. N., A. McConnachie, C. McSharry, C. L. Hart, G. D. Smith, C. R. Gillis, and G. C. Watt. 2000. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the midspan family study surveys of parents and offspring. Br. Med. J. 321:88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van den Biggelaar, A. H., R. van Ree, L. C. Rodrigues, B. Lell, A. M. Deelder, P. G. Kremsner, and M. Yazdanbakhsh. 2000. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet 356:1723-1727. [DOI] [PubMed] [Google Scholar]
- 31.Wang, C.-C., T. J. Nolan, G. A. Schad, and D. Abraham. 2001. Infection of mice with the helminth Strongyloides stercoralis suppresses pulmonary allergic responses to ovalbumin. Clin. Exp. Allergy 31:495-503. [DOI] [PubMed] [Google Scholar]
- 32.Weiss, K. B., S. D. Sullivan, and C. S. Lyttle. 2000. Trends in the cost of illness for asthma in the United States, 1985-1994. J. Allergy Clin. Immunol. 106:493-499. [DOI] [PubMed] [Google Scholar]
- 33.Weiss, S. T. 1998. Environmental risk factors in childhood asthma. Clin. Exp. Allergy 28:29-34. [DOI] [PubMed] [Google Scholar]
- 34.Wiesch, D. G., D. A. Meyers, and E. R. Bleecker. 1999. Genetics of asthma. J. Allergy Clin. Immunol. 104:895-901. [DOI] [PubMed] [Google Scholar]
- 35.Wills-Karp, M. 1999. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17:255-281. [DOI] [PubMed] [Google Scholar]
- 36.Wills-Karp, M., J. Santeliz, and C. L. Karp. 2001. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1:69-75. [DOI] [PubMed] [Google Scholar]
- 37.Yazdanbakhsh, M. P., G. Kremsner, and R. van Ree. 2002. Allergy, parasites, and hygiene hypothesis. Science 296:490-494. [DOI] [PubMed] [Google Scholar]
- 38.Yazdanbakhsh, M., A. van den Biggelaar, and R. M. Maizels. 2001. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 22:372-377. [DOI] [PubMed] [Google Scholar]
- 39.Zuany-Amorim, C., E. Sawicka, C. Manlius, A. LeMoine, L. R. Brunet, D. M. Kemeny, G. Bowen, G. Rook, and C. Walker. 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 8:625-629. [DOI] [PubMed] [Google Scholar]