Abstract
Pyolysin (PLO), a cholesterol-dependent cytolysin expressed by Arcanobacterium pyogenes, is an important host-protective antigen. However, this molecule is toxic and requires inactivation prior to its use as a vaccine. Three genetically toxoided, nonhemolytic PLO molecules, HIS-PLO.F497, HIS-PLO.ΔP499, and HIS-PLO.A522, were found to be nontoxic, and vaccinated mice were protected from infection, indicating the potential of these toxoids as vaccines. Furthermore, in a mouse model of infection, A. pyogenes carrying the F497 mutation was as attenuated as a PLO-deficient strain, indicating that the cytolytic activity of PLO is important in virulence.
Arcanobacterium pyogenes, a widely distributed inhabitant of the mucous membranes of domestic animals, is found associated with the respiratory, gastrointestinal, and genital tracts (10, 18, 28, 40), and infection with this organism can occur following a precipitating injury or infection. Economically important diseases caused by this organism include mastitis and abortion in dairy cows (23) and liver abscesses in feedlot cattle (22, 26).
A. pyogenes expresses a cholesterol-dependent cytolysin (CDC), pyolysin (PLO) (6), which is a major virulence factor in infections by this organism. CDCs, expressed by many gram-positive pathogens, exert their effects by binding to cholesterol and forming large, oligomeric pores in eukaryotic-cell membranes (7). The CDCs can affect a wide variety of physiological processes in the host, including complement activation (33), up-regulation of cytokine production (15, 30, 39), and inhibition of the respiratory burst and bactericidal activity of polymorphonuclear leukocytes and monocytes (27, 32), and are directly cytotoxic for polymorphonuclear leukocytes and macrophages (19, 42). PLO is also an important host-protective antigen, as formalin-inactivated, recombinant, His-tagged PLO (HIS-PLO) was efficacious as a vaccine in mice (19). However, the toxicity of PLO limits its usefulness as a vaccine without prior inactivation.
The CDCs possess a characteristic, C-terminal undecapeptide sequence, which has been implicated in the initial interaction of the toxin with the host cell membrane (13, 14, 16). Mutational analysis of the undecapeptide in PLO and other CDCs has identified residues which are critical for cytolytic activity (8, 9, 21, 24, 34). Specifically, we have shown that replacement of tryptophan 497 with phenylalanine or deletion of a proline residue at position 499 significantly reduced the hemolytic and cholesterol binding activities of PLO (8). In addition, other residues, not found within the undecapeptide region, affect the pore-forming ability of CDCs, particularly residues involved in the formation of the transmembrane hairpins (36, 37) and residues located at the C terminus of the protein (31, 38). Knowledge of the residues critical for toxic activity allowed the design of genetic toxoids, i.e., recombinant toxins with mutations affecting activity, for use as immunoprophylactic agents.
Three genetically toxoided HIS-PLO proteins were evaluated for their potential as vaccines. The previously described HIS-PLO.F497 and HIS-PLO.ΔP499 proteins have mutations in the PLO undecapeptide region which significantly reduce the hemolytic and cholesterol binding activities of these molecules (8). As it was unknown whether antibodies to a native undecapeptide were required to neutralize PLO activity, a mutant HIS-PLO molecule which contained a mutation outside the undecapeptide region was also chosen. This mutant protein, HIS-PLO.A522, was selected from a number of mutations identified by error-prone PCR. Briefly, error-prone PCR was performed in the presence of 5 mM MgCl2, as previously described (12), by using primers 5′-acagcatcctcgagtgccggattgggaaac-3′ and 5′-tggaattccctaggatttgacattgt-3′, which amplify the A. pyogenes plo gene. PCR products were cloned into the six-His tag vector pTrcHis B (Invitrogen) by using the XhoI and EcoRI restriction sites incorporated into the primers (underlined), and nonhemolytic colonies were identified on Luria-Bertani agar containing 5% ovine blood and 100 μg of ampicillin/ml following electroporation of Escherichia coli DH5α. Plasmid DNA was extracted from nonhemolytic colonies (3) and subjected to automated DNA sequencing to identify mutations. One such plasmid, pJGS128, was found to contain two mutations. The first mapped to codon 43 of plo and resulted in no change in the amino acid sequence (CCG to CCA), while a second mutation in codon 522 resulted in a threonine (ACG) to alanine (GCG) change. HIS-PLO.A522 was purified by using TALON resin (Clontech) as previously described (8). The hemolytic and cholesterol binding activities of HIS-PLO.A522 were then assessed (8). HIS-PLO.A522 had significantly reduced hemolytic and cholesterol binding activities compared with HIS-PLO, with 0.8 and 2.6% of wild-type activities, respectively. The ability of HIS-PLO.A522 to bind to host cell membranes was also determined as previously described (11). HIS-PLO.A522 was able to bind to host cell membranes as well as HIS-PLO did (Fig. 1), despite the significant reduction in cholesterol binding of this mutant (2.6% of wild type). This result is consistent with the suggestion that PLO may bind to other host cell membrane receptors in addition to cholesterol (8).
FIG. 1.
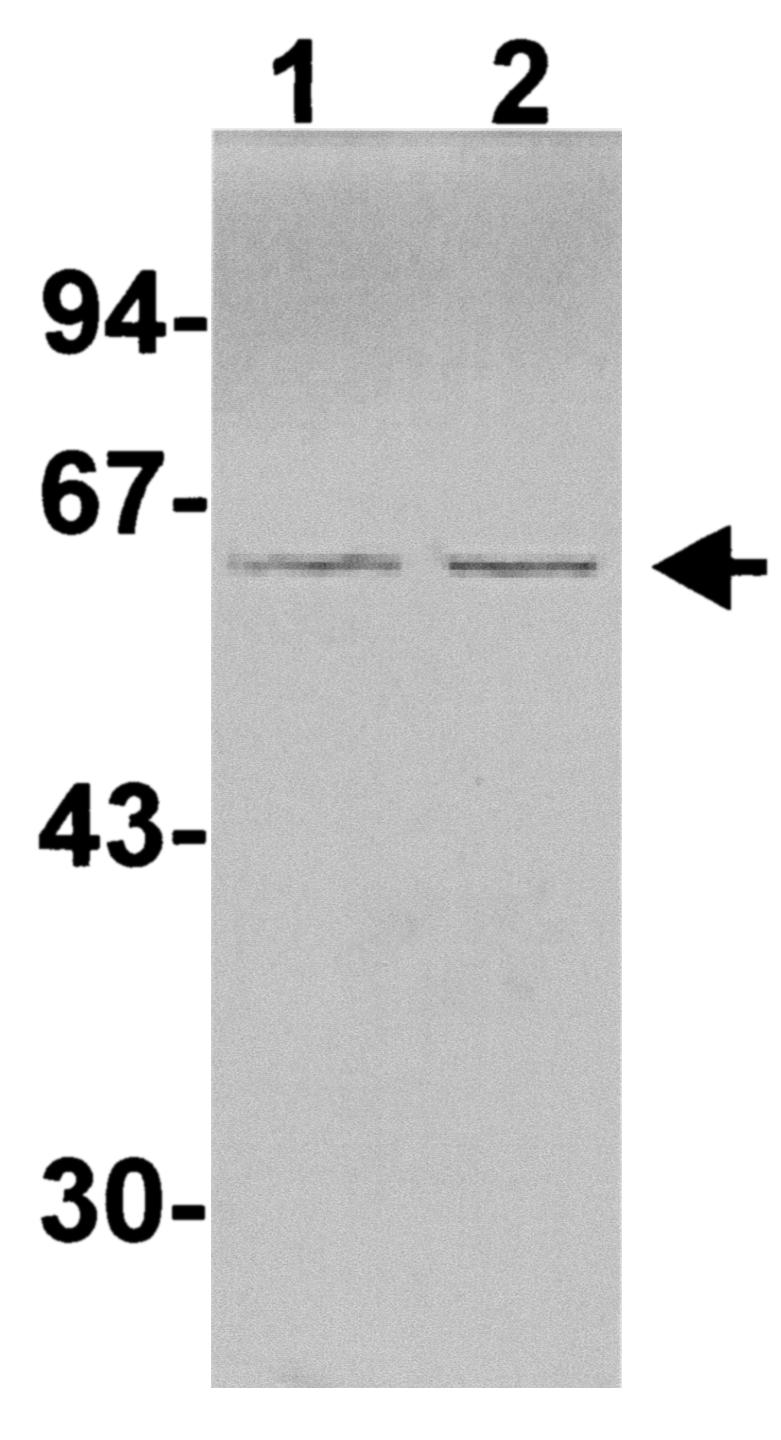
Membrane binding activity of HIS-PLO proteins. HIS-PLO proteins were diluted to a concentration of 2.5 μg/ml in bovine serum albumin (1 mg/ml), 0.5 ml was added to an equal volume of 10% ovine blood, and the mixture was incubated on ice for 20 min. The cells were subsequently harvested by centrifugation and lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Samples incubated with HIS-PLO (lane 1) or HIS-PLO.A522 (lane 2) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunostained with a 1/100 dilution of goat anti-HIS-PLO. The position of the 55-kDa PLO band is indicated by the arrow. Molecular mass markers, in kilodaltons, are indicated on the left.
To assess the toxicity of the HIS-PLO genetic toxoids, groups of five outbred ICR mice were injected intraperitoneally (i.p.) with 10 μg of HIS-PLO.F497, HIS-PLO.ΔP499, or HIS-PLO.A522 and were monitored over 7 days. The i.p. administration of 10 μg of untreated HIS-PLO was uniformly lethal in less than 24 h (19). In contrast, at no time did the mice treated with the genetic toxoids display any clinical signs, indicating that these recombinant proteins were nontoxic in the amounts given.
Groups of five outbred ICR mice were immunized i.p. with 10 μg of HIS-PLO toxoids in Ribi MPL+TDM emulsion (Corixa) on days 0 and 14. The mice were bled from the orbital sinus on day 27, and the serum antibody responses were determined using a PLO hemolysis neutralization assay (6). Formalin-inactivated HIS-PLO gave the best antibody response, followed by HIS-PLO.A522 and then HIS-PLO.ΔP499 and HIS-PLO.F497, which contained mutations in the undecapeptide (Table 1). The differences in antibody responses to the vaccines may be due to the formalin treatment allowing for better exposure of important epitopes, as in the case of formalin-inactivated HIS-PLO, or the presence of a wild-type undecapeptide resulting in the production of more neutralizing antibodies, as for HIS-PLO and HIS-PLO.A522. To determine whether these antibody titers were protective, the immunized mice and five unimmunized control mice were challenged i.p. with 4.7 × 108 CFU of A. pyogenes BBR1 (19) 28 days postvaccination. All five unimmunized and challenged mice displayed signs of illness by 24 to 48 h, with large numbers of bacteria recovered from the liver and peritoneal fluid (PF) (Table 1). In contrast, all the mice immunized with HIS-PLO proteins displayed no signs of illness at any time and no bacteria were isolated from the liver and PF at 7 days postchallenge (Table 1). Therefore, immunization with any of these toxoids resulted in complete protection against A. pyogenes in this animal model, indicating that these genetic toxoids may have utility as vaccines.
TABLE 1.
Average bacterial viable counts and serum antibody titers from immunized and control mice
| Mouse group | Avg serum hemolysis neutralization titera | Avg bacterial viable count (CFU) fromb:
|
|
|---|---|---|---|
| Liver | PF | ||
| HIS-PLO.F497 immunized | 111.4 | —c | — |
| HIS-PLO.ΔP499 immunized | 222.9 | — | — |
| HIS-PLO.A522 immunized | 608.9 | — | — |
| HIS-PLO toxoid immunized | 1,024 | — | — |
| Unimmunized | <16 | 4.1 × 108 | 5.6 × 107 |
Minimum titer detected was 16.
Minimum titers detected were 500 CFU/g for liver and 10 CFU/ml for PF.
—, below the limits of detection.
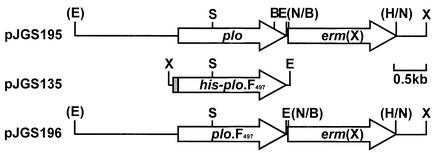
As cytolytic activity is only one of the potential effects of PLO on the host, the ability of one of these mutant PLO proteins to participate in the pathogenesis of A. pyogenes infections was assessed. A strain carrying plo.F497 was constructed as follows. A 3.6-kb EcoRI-XhoI fragment from the cosmid ApH1, containing plo (6), was cloned into pHSS21 (29), and the EcoRI site was destroyed with T4 DNA polymerase. EcoRI and NruI sites (underlined in the primer sequence) were incorporated immediately downstream of the plo stop codon by using a Transformer site-directed mutagenesis kit (Clontech) with the mutagenic primer 5′-ctcatcaccatcgcgaagaattcgttgcggtaac-3′. A 1.6-kb HindIII-BamHI fragment containing the erm(X) gene from pNG2 (35) was treated with T4 DNA polymerase, and this fragment was cloned into the NruI site to generate the recombinant plasmid pJGS195 (Fig. 2). The 1.0-kb SalI-EcoRI fragment of pJGS195 was replaced with the 1.0-kb SalI-EcoRI fragment of pJGS135, which carries the F497 mutation (8), to form pJGS196 (Fig. 2).
FIG. 2.
Maps of the plasmids used to construct A. pyogenes strains JGS350 and JGS351. Plasmid names are shown on the left. BamHI (B), EcoRI (E), HindIII (H), NruI (N), SalI (S), and XhoI (X) sites are shown. Restriction sites shown in parentheses were destroyed during the construction of these plasmids. The shaded area of pJGS135 indicates the six-His tag encoded by pTrcHis B. A bar indicating 0.5 kb is shown on the right.
pJGS195 and pJGS196 plasmid DNA were introduced into A. pyogenes BBR1 by electroporation (17), and recombinants were selected on brain heart infusion-blood agar containing 15 μg of erythromycin (Em)/ml. As these plasmids were ColE1 replicon based, they acted as suicide plasmids in A. pyogenes (17). Emr mutants, designated JGS350 and JGS351, transformed with pJGS195 and pJGS196, respectively, were chosen for further analysis. JGS350 was constructed to ensure that there were no effects of the erm(X) gene insertion on PLO expression.
Southern blotting of A. pyogenes genomic DNA digested with EcoRI-XhoI revealed a 3.6-kb band in BBR1 and 3.1-kb bands in JGS350 and JGS351 when probed with a plo-specific probe (data not shown). The presence of erm(X) in JGS350 and JGS351 was confirmed with an erm(X)-specific probe, while the lack of vector sequences was confirmed by using a pHSS21-specific (vector) probe (data not shown). Furthermore, the plo genes from BBR1, JGS350, and JGS351 were amplified by PCR and the products were digested with BamHI. Incorporation of the F497 mutation results in loss of the BamHI site in plo (Fig. 2). The products from BBR1 and JGS350, but not JGS351, were digested by BamHI (data not shown), confirming the allelic replacement of the wild-type plo gene in JGS351 by the integration of the F497 mutation and the erm(X) gene as the result of a double crossover event.
The activity of PLO in culture supernatant fluid (CSF) from BBR1, JGS350, or JGS351 was determined by hemolytic assay (8). CSF from JGS351 contained no measurable hemolytic activity (titer, <1). In contrast, the average titers of BBR1 and JGS350 CSF were 32 and 32, respectively, indicating that insertion of the erm(X) cassette had no effect on the expression of PLO in JGS350. Additionally, Western blotting with antiserum against PLO revealed that similar amounts of PLO protein were expressed by the three strains (data not shown).
The relative levels of virulence of BBR1 and JGS351 were assessed in a mouse i.p. challenge model. Groups of eight outbred ICR mice were challenged with 10-fold serial dilutions of A. pyogenes BBR1 or JGS351 as previously described (19), and the mice were monitored over 7 days. The infection rates and bacterial viable counts for strains BBR1 and JGS351 are shown in Table 2. In this model, infection with 3.7 × 109 CFU of BBR1 was uniformly lethal to mice within less than 16 h (Table 2). Challenge with 3.7 × 108 CFU of BBR1 resulted in infection of seven-eighths of the mice within 48 to 72 h, while 10-fold-fewer CFU of BBR1 were unable to establish an infection. In contrast, six-eighths of the mice challenged with 1.8 × 1010 and only three-eighths of the mice challenged with 1.8 × 109 CFU of JGS351 were infected. All the mice challenged with 1.8 × 108 CFU of JGS351 remained clinically normal, and at necropsy, no bacteria were isolated (Table 2). For JGS351, the 50% infectious dose, as calculated by the Reed-Muench method (41), was 5.6 × 109 CFU, 1.7 log10 higher than for BBR1 (9.9 × 107 CFU) and approximately the same as for a plo knockout strain, PLO-1 (6.5 × 109 CFU [19]).
TABLE 2.
Virulence of A. pyogenes BBR1 and JGS351
| Challenge strain and dose (CFU) | No. infected/total no. | Avg bacterial viable count (CFU) from infected mouse specimena:
|
|
|---|---|---|---|
| Liver | PF | ||
| BBR1 | |||
| 3.7 × 107 | 0/8 | —b | — |
| 3.7 × 108 | 7/8 | 1.9 × 108 | 8.7 × 107 |
| 3.7 × 109 | 8/8 | NDc | ND |
| JGS351 | |||
| 1.8 × 108 | 0/8 | — | — |
| 1.8 × 109 | 3/8 | 1.1 × 109 | 5.9 × 107 |
| 1.8 × 1010 | 6/8 | 2.4 × 109 | 3.1 × 109 |
Minimum titers detected were 500 CFU/g for liver and 10 CFU/ml for PF.
—, below the limits of detection.
ND, not determined.
CDCs can affect host physiological processes in a multifactorial manner. In fact, the regions of the CDC molecule responsible for cytokine up-regulation and complement activation are distinct from those required for hemolytic and cytolytic and cholesterol binding activities (4, 20, 25). Therefore, as JGS351, expressing PLO.F497, is reduced for virulence to a similar degree as a plo knockout strain, this suggests that cytolytic ability, rather than another CDC activity, may play the predominant role in the pathogenic effect of PLO, at least in a murine i.p. model of infection. Similarly, Streptococcus pneumoniae strains expressing pneumolysin with decreased complement binding activities had reduced virulence in a murine pneumonia model (2) but had essentially wild-type virulence in bacteremia models in mice (5) and rats (1).
Genetic toxoids of PLO provide a major advantage over native or recombinant PLO in that they do not require inactivation prior to their use as vaccines. The results obtained in this study indicate that these genetic toxoids may be efficacious veterinary vaccines. Vaccination experiments designed to prevent disease in economically important animals, such as the prevention of liver abscesses in feedlot cattle or mastitis in dairy cows, are an important step in determining the efficacy of PLO-based vaccines for A. pyogenes disease.
Acknowledgments
This work was supported by NRICGP/USDA award 99-35204-7818.
We thank Dawn M. Bueschel for excellent technical assistance.
Editor: A. D. O'Brien
REFERENCES
- 1.Alcantara, R. B., L. C. Preheim, and M. J. Gentry. 1999. Role of pneumolysin's complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect. Immun. 67:2862-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24:167-174. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology, vol. 1. Greene Publishing Associates and John Wiley and Sons, Inc., New York, N.Y.
- 4.Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2002. Induction of gamma interferon and nitric oxide by truncated pneumolysin that lacks pore-forming ability. Infect. Immun. 70:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23:201-209. [DOI] [PubMed] [Google Scholar]
- 6.Billington, S. J., B. H. Jost, W. A. Cuevas, K. R. Bright, and J. G. Songer. 1997. The Arcanobacterium (Actinomyces) pyogenes hemolysin, pyolysin, is a novel member of the thiol-activated cytolysin family. J. Bacteriol. 179:6100-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billington, S. J., B. H. Jost, and J. G. Songer. 2000. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol. Lett. 182:197-205. [DOI] [PubMed] [Google Scholar]
- 8.Billington, S. J., J. G. Songer, and B. H. Jost. 2002.. The variant undecapeptide sequence of pyolysin is required for full cytolytic activity. Microbiology 148:3947-3954. [DOI] [PubMed]
- 9.Boulnois, G. J., J. C. Paton, T. J. Mitchell, and P. W. Andrew. 1991. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol. Microbiol. 5:2611-2616. [DOI] [PubMed] [Google Scholar]
- 10.Carter, G. R., and M. M. Chengappa. 1991. Essentials of veterinary bacteriology and mycology, 4th ed. Lea and Febiger, Philadelphia, Pa.
- 11.de los Toyos, J. R., F. J. Mendez, J. F. Aparicio, F. Vázquez, M. del Mar García Suárez, A. Fleites, C. Hardisson, P. J. Morgan, P. W. Andrew, and T. J. Mitchell. 1996. Functional analysis of pneumolysin by use of monoclonal antibodies. Infect. Immun. 64:480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fromant, M., S. Blanquet, and P. Plateau. 1995. Direct random mutagenesis of gene-sized DNA fragments using polymerase chain reaction. Anal. Biochem. 224:347-353. [DOI] [PubMed] [Google Scholar]
- 13.Heuck, A. P., E. M. Hotze, R. K. Tweten, and A. E. Johnson. 2000. Mechanism of membrane insertion of a multimeric barrel protein: perfringolysin O creates a pore using ordered and coupled conformational changes. Mol. Cell 6:1233-1242. [DOI] [PubMed] [Google Scholar]
- 14.Hotze, E. M., A. P. Heuck, D. M. Czajkowsky, Z. Shao, A. E. Johnson, and R. K. Tweten. 2002. Monomer-monomer interactions drive the prepore to pore conversion of a β-barrel-forming cholesterol-dependent cytolysin. J. Biol. Chem. 277:11597-11605. [DOI] [PubMed] [Google Scholar]
- 15.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs, T., M. D. Cima-Cabal, A. Darji, F. J. Méndez, F. Vázquez, A. A. C. Jacobs, Y. Shimada, Y. Ohno-Iwashita, S. Weiss, and J. R. de los Toyos. 1999. The conserved undecapeptide shared by thiol-activated cytolysins is involved in membrane binding. FEBS Lett. 459:463-466. [DOI] [PubMed] [Google Scholar]
- 17.Jost, B. H., S. J. Billington, and J. G. Songer. 1997. Electroporation-mediated transformation of Arcanobacterium (Actinomyces) pyogenes. Plasmid 38:135-140. [DOI] [PubMed] [Google Scholar]
- 18.Jost, B. H., K. H. Post, J. G. Songer, and S. J. Billington. 2002. Isolation of Arcanobacterium pyogenes from the porcine gastric mucosa. Vet. Res. Commun. 26:419-425. [DOI] [PubMed] [Google Scholar]
- 19.Jost, B. H., J. G. Songer, and S. J. Billington. 1999. An Arcanobacterium (Actinomyces) pyogenes mutant deficient in production of the pore-forming cytolysin pyolysin has reduced virulence. Infect. Immun. 67:1723-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohda, C., I. Kawamura, H. Baba, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect. Immun. 70:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korchev, Y. E., C. L. Bashford, C. Pederzolli, C. A. Pasternak, P. J. Morgan, P. W. Andrew, and T. J. Mitchell. 1998. A conserved tryptophan in pneumolysin is a determinant of the characteristics of channels formed by pneumolysin in cells and planar lipid bilayers. Biochem. J. 329:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechtenberg, K. F., T. G. Nagaraja, H. W. Leipold, and M. M. Chengappa. 1988. Bacteriologic and histologic studies of hepatic abscesses in cattle. Am. J. Vet. Res. 49:58-62. [PubMed] [Google Scholar]
- 23.Lewis, G. S. 1997. Uterine health and disorders. J. Dairy Sci. 80:984-994. [DOI] [PubMed] [Google Scholar]
- 24.Michel, E., K. A. Reich, R. Favier, P. Berche, and P. Cossart. 1990. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitution in listeriolysin O. Mol. Microbiol. 4:2167-2178. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell, T. J., P. W. Andrew, F. K. Saunders, A. N. Smith, and G. J. Boulnois. 1991. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol. Microbiol. 5:1883-1888. [DOI] [PubMed] [Google Scholar]
- 26.Nagaraja, T. G., S. B. Laudert, and J. C. Parrott. 1996. Liver abscesses in feedlot cattle. Part I. Causes, pathogenesis, pathology, and diagnosis. Compend. Contin. Educ. Pract. Vet. 18:S230-S241, S256.
- 27.Nandoskar, M., A. Ferrante, E. J. Bates, N. Hurst, and J. C. Paton. 1986. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bacteriocidal activity by pneumolysin. Immunology 59:515-520. [PMC free article] [PubMed] [Google Scholar]
- 28.Narayanan, S., T. G. Nagaraja, N. Wallace, J. Staats, M. M. Chengappa, and R. D. Oberst. 1998. Biochemical and ribotypic comparison of Actinomyces pyogenes and A. pyogenes-like organisms from liver abscesses, ruminal wall, and ruminal contents of cattle. Am. J. Vet. Res. 59:271-276. [PubMed] [Google Scholar]
- 29.Nickoloff, J. A., and R. J. Reynolds. 1991. Subcloning with new ampicillin- and kanamycin-resistant analogs of pUC19. BioTechniques 10:469-472. [PubMed] [Google Scholar]
- 30.Nishibori, T., H. Xiong, I. Kawamura, M. Arakawa, and M. Mitsuyama. 1996. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect. Immun. 64:3188-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owen, R. H. G., G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1994. A role in the cell-binding for the C-terminus of pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae. FEMS Microbiol. Lett. 121:217-222. [DOI] [PubMed] [Google Scholar]
- 32.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bacteriocidal activity, and migration by pneumolysin. Infect. Immun. 41:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paton, J. C., B. Rowan-Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43:1085-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekino-Suzuki, N., M. Nakamura, K.-I. Mitsui, and Y. Ohno-Iwashita. 1996. Contribution of individual tryptophan residues to the structure and activity of θ-toxin (perfringolysin O), a cholesterol-binding cytolysin. Eur. J. Biochem. 241:941-947. [DOI] [PubMed] [Google Scholar]
- 35.Serwold-Davis, T. M., and N. B. Groman. 1986. Mapping and cloning of Corynebacterium diphtheriae plasmid pNG2 and characterization of its relatedness to plasmids from skin coryneforms. Antimicrob. Agents Chemother. 30:69-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shatursky, O., A. P. Heuck, L. A. Shepard, J. Rossjohn, M. W. Parker, A. E. Johnson, and R. K. Tweten. 1999. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99:293-299. [DOI] [PubMed] [Google Scholar]
- 37.Shepard, L. A., A. P. Heuck, B. D. Hamman, J. Rossjohn, M. W. Parker, K. R. Ryan, A. E. Johnson, and R. K. Tweten. 1998. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an α-helical to β-sheet transition identified by fluorescence spectroscopy. Biochemistry 37:14563-14574. [DOI] [PubMed] [Google Scholar]
- 38.Shimada, Y., M. Nakamura, Y. Naito, K. Nomura, and Y. Ohno-Iwashita. 1999. C-terminal amino acid residues are required for the folding and cholesterol binding property of perfringolysin O, a pore-forming cytolysin. J. Biol. Chem. 274:18536-18542. [DOI] [PubMed] [Google Scholar]
- 39.Stevens, D. L., and A. E. Bryant. 1997. Streptolysin O modulates cytokine synthesis in human peripheral blood mononuclear cells. Adv. Exp. Med. Biol. 418:925-927. [DOI] [PubMed] [Google Scholar]
- 40.Timoney, J. F., J. H. Gillespie, F. W. Scott, and J. E. Barlough. 1988. Hagan and Bruner's microbiology and infectious diseases of domestic animals, 8th ed. Cornell University Press, Ithaca, N.Y.
- 41.Welkos, S., and A. O'Brien. 1994. Determination of median lethal and infectious doses in animal model systems. Methods Enzymol. 235:29-39. [DOI] [PubMed] [Google Scholar]
- 42.Yoshikawa, H., I. Kawamura, M. Fujita, H. Tsukada, M. Arakawa, and M. Mitsuyama. 1993. Membrane damage and interleukin-1 production in murine macrophages exposed to listeriolysin O. Infect. Immun. 61:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]