Abstract
The cysteine proteinases CPA and CPB from Leishmania major induced Th1 responses in patients with leishmaniasis due to Leishmania guyanensis. Furthermore, cysteine proteinases induced neither interleukin 4 (IL-4) nor IL-13 and low levels of IL-10 in controls and patients. The results suggest that CPs would be quite good candidates for a vaccine against different Leishmania species.
Leishmaniasis is a worldwide endemic disease, with 1 to 2 million new cases per year and 300 million people estimated to be at risk for the disease. Since preventive treatment is not available and chemotherapy is the only well-tolerated treatment, vaccination against leishmaniasis is an attractive option. However, vaccines using killed Leishmania against New and Old World leishmaniasis in visceral leishmaniasis (6) and localized cutaneous leishmaniasis (LCL) (14) confer low rates of protection. Thus, characterization and testing of new recombinant antigens for producing second-generation vaccines is crucial. A number of antigens from Leishmania have been purified and analyzed on the basis of either the development of Th1 protective responses in human peripheral blood mononuclear cells (PBMC) from controls and patients suffering from leishmaniasis or the induction of protective immunity in murine models of infection with Leishmania (www.who.int/tdr/prd/leish/vac). Among these antigens, type I and II cysteine proteases (CPA and CPB, respectively) of Leishmania major have been reported to be quite good candidate antigens for vaccination against infection with L. major (11, 12).
However, different Leishmania species can induce different immune responses in murine models and in humans (3, 5, 13). Thus, mechanisms responsible for an effective protective immune response to a particular Leishmania species should be investigated. In this context, we decided to analyze reactivity to CPA and CPB by PBMC from LCL patients infected with Leishmania guyanensis, the Leishmania species most frequently isolated in French Guiana, to determine their antigenicity.
First, genomic DNA from L. guyanensis was isolated, and the cysteine proteinase genes were amplified using primers specific to both cysteine proteinase types I and II. The PCR products were cloned into a Topo TA cloning vector as described by the manufacturer (Invitrogen, Groningen, The Netherlands) and sequenced with an ABI-Perkin-Elmer automated sequencing system. cpa and cpb sequences for L. major LV39 and Iran strain 175 (AJ130942 and U43706, respectively) and L. guyanensis were aligned by using the ED editor of the MUST package (10). The homologies for CPA were 80.5 and 72.4% and those for CPB were 81 and 76.0% at the level of nucleotide and amino acid sequences, respectively. Thus, once the similarity of cps genes between L. major and L. guyanensis had been shown, CPA and CPB purified from L. major were used for in vitro analysis of immune responses of patients infected with L. guyanensis.
PBMC were isolated as previously described (2, 3) from 15 patients with LCL due to L. guyanensis and from 13 healthy controls. Seven of 13 healthy controls had not been exposed to Leishmania and had negative in vitro reactivity against soluble Leishmania antigen (SLA), and 6 of 13 were asymptomatic healthy subjects who had been previously exposed to Leishmania, since they developed gamma interferon (IFN-γ) reactivity and specific antibodies against Leishmania antigens. Cultures for cytokine production (106 cells in 1 ml of culture medium) were plated on flat-bottom 24-well plates with or without antigen (SLA, recombinant CPA and CPB, or Leishmania-activated C kinase [LACK]) as previously described (2, 3). The supernatants were harvested after 2 days for IL-2 and after 7 days for IFN-γ, IL-4, IL-10, and IL-13 production and analyzed with an enzyme-linked immunosorbent assay (Pharmingen, San Diego, Calif.) with a sensitivity of 10 pg/ml.
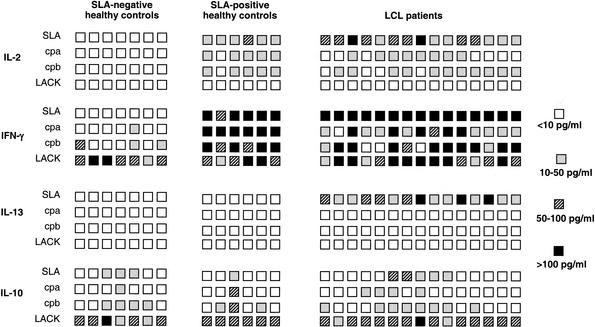
Individual production of cytokines in response to purified antigens is given in Fig. 1. All LCL patients produced IL-2 in response to SLA, and 8 and 11 of the 15 LCL patients produced IL-2 in response to CPA and CPB, respectively. Interestingly, six of eight patients reacted to both CPA and CPB (Fig. 1). All SLA-positive healthy controls reacted with IL-2 secretion in response to SLA, and five of these six controls reacted either with CPA or CPB. As expected, SLA-negative healthy controls did not produce IL-2 in response to the antigens. Furthermore, we noticed that LACK was unable to induce IL-2 secretion in PBMC from either healthy subjects or LCL patients (Fig. 1).
FIG. 1.
Individual cytokines response to purified CPA and CPB antigens in healthy controls and patients suffering from leishmaniasis due to L. guyanensis. Cytokine production by PBMC in culture for 2 (IL-2) or 7 days (IFN-γ, IL-10, and IL-13) in the presence of antigens was measured by ELISA. Each square represents one subject.
All LCL patients (15 of 15) secreted large amounts of IFN-γ in response to SLA, and 14 and 12 of these 15 patients reacted to CPA and CPB, respectively. All six healthy subjects who reacted to SLA also reacted to CPA and CPB. In contrast, among the seven SLA-negative healthy controls, only one produced IFN-γ in response to CPA and three produced IFN-γ in response to CPB. As expected and as previously described, production of IFN-γ in response to LACK was detected in all groups (2, 3, 7).
IL-4 production was analyzed in all groups of patients, but IL-4 was not detected in the supernatants of antigen-stimulated PBMC from all groups (data not shown). None of the SLA-positive and -negative healthy controls produced IL-13 in response to SLA, CPA, CPB, and LACK. In contrast, all LCL patients reacted with IL-13 secretion to SLA. However, none of these patients produced IL-13 in response to purified antigens (Fig. 1.).
Three of the seven SLA-negative healthy subjects produced IL-10 in response to SLA, and one and four reacted to CPA and CPB, respectively, but levels of IL-10 were always low (less than 20 pg/ml) in all subjects. Among the six SLA-positive healthy controls, one responded to SLA by producing IL-10 (18 pg/ml) and one and three reacted to CPA and CPB, respectively (15 pg/ml for CPA; 16 to 18 and 32 pg/ml for CPB). Only 5 of the 15 LCL patients produced IL-10 in response to SLA. Four and eight LCL patients reacted to CPA and CPB, respectively. As expected and as previously described (2, 3, 7), both healthy subjects and LCL patients produced IL-10 in response to LACK (Fig. 1).
A summary of the percentages of subjects in each group that produced Th1 cytokines (IL-2 and IFN-γ) and Th2 cytokines (IL-13 and IL-10) in response to purified antigens is given in Table 1. It clearly shows that the CPA and CPB were powerful antigens eliciting a Th1 response in SLA-positive healthy controls and LCL patients, since IL-2 and IFN-γ were produced in response to these two antigens in more than 50% of subjects. Although the IL-10 production was low in all tested groups, it was shown that more subjects secreted IL-10 in response to CPB than CPA. In fact, 50% of SLA-negative and -positive healthy subjects produced IL-10 in response to CPB, but only 14.3% reacted with IL-10 secretion to CPA. In contrast, and as previously described (2, 3, 7), LACK, a control antigen, is able to induce a mixture of Th1 and Th2 cell responses, since all controls and LCL patients produced large amounts of IFN-γ and IL-10 in response to antigen stimulation.
TABLE 1.
Reactivity againt purified antigens in healthy controls and LCL patients infected with L. guyanensis
| Antigen | % Reactivity ina:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLA-negative healthy controls
|
SLA-positive healthy controls
|
LCL patients
|
||||||||||
| Th1 cytokines
|
Th2 cytokines
|
Th1 cytokines
|
Th2 cytokines
|
Th1 cytokines
|
Th2 cytokines
|
|||||||
| IL-2 | IFN-γ | IL-10 | IL-13 | IL-2 | IFN-γ | IL-10 | IL-13 | IL-2 | IFN-γ | IL-10 | IL-13 | |
| SLA | 0 | 0 | 42.8 | 0 | 100 | 100 | 16.6 | 0 | 100 | 100 | 33.3 | 100 |
| CPA | 0 | 14.3 | 14.3 | 0 | 83.3 | 100 | 16.6 | 0 | 60 | 93.3 | 26.6 | 0 |
| CPB | 0 | 42.8 | 57.1 | 0 | 83.3 | 100 | 50 | 0 | 73.3 | 80 | 53.3 | 0 |
| LACK | 0 | 100 | 100 | 0 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | 0 |
Boldfaceindicates that at least 50% of the subjects produced cytokines in response to antigen stimulation.
The present results show that CPA and CPB purified from L. major can induce Th1 responses (IL-2 and IFN-γ production) in patients infected with L. guyanensis, which is not surprising since CPA and CPB genes are highly homologous in L. major and L. guyanensis. In fact, the ability of CPA and CPB to induce IFN-γ production in patients infected with either L. major or L. guyanensis suggests that these proteins would be good candidates for a subunit vaccine against the majority of Leishmania infection. The previously described homologies between CPA and CPB genes from L. major and the corresponding genes from Leishmania mexicana (4) (EMBL, admission no. AJ319727 for cpb), which induced atypical cutaneous leishmaniasis, and Leishmania infantum (EMBL admission no. AJ420285 for cpa and AJ420286 for cpb), one of the Leishmania species inducing visceral leishmaniasis, support this speculation.
Our present report also shows clearly that CPB is able to induce IL-10 production—even in small amounts—in more than 50% of SLA-positive healthy subjects and LCL patients. CPB has been described as a virulence factor in L. mexicana (9), and this might explain the IL-10 production in response to CPB, since IL-10 is a down-regulator of IFN-γ activity (1, 8). Thus, the use of cysteine proteinases as antigen vaccines is questionable. However, effective protection might be induced with an adequate adjuvant by bypassing IL-10 production. Furthermore, the delineation of epitopes responsible for IL-10 production may be important for designing the vaccine.
Nucleotide sequence accession numbers.
The L. guyanensis cpa and cpb sequences have been deposited in the EMBL database under accession numbers AJ512652 and AJ512653, respectively.
Acknowledgments
This study was supported by grants from the Pasteur Institute and the French Ministry of Research (Programme de Recherche Fondamentale, Microbiologie, Maladies Infectieuses et Parasitaires).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Bourreau, E., M. Collet, G. Prévot, G. Milon, D. Ashimoff, H. Hasagewa, C. Parra-Lopez, and P. Launois. 2002. IFN-γ producing CD45RA+ CD8+ and IL-10 producing CD45RA− CD4+ T cells generated in response to LACK in naive subjects never exposed to Leishmania. Eur. J. Immunol. 32:510-520. [DOI] [PubMed] [Google Scholar]
- 3.Bourreau, E., G. Prévot, J. Gardon, R. Pradinaud, H. Hasagewa, G. Milon, and P. Launois. 2002. LACK-specific CD4+ T cells that induce gamma interferon production in patients with localized cutaneous leishmaniasis during the early stage of infection. Infect. Immun. 70:3122-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks, D. R., H. Denise, G. D. Westrop, G. H. Coombs, and J. C. Mottram. 2001. The stage-regulated expression of Leishmania mexicana CPB cysteine proteases is mediated by an intercistronic sequence element. J. Biol. Chem. 276:47061-47069. [DOI] [PubMed] [Google Scholar]
- 5.Colmenares, M., S. Kar, K. Goldsmith-Pestana, and D. McMahon-Pratt. 2002. Mechanisms of pathogenesis: differences amongst Leishmania species. Trans. R. Soc. Trop. Med. Hyg. 19:S1-S3. [DOI] [PubMed]
- 6.Khalil, E. A., A. M. El Hassan, E. E. Zijlstraa, M. M. Mukhtar, H. W. Ghalib, B. Musa, M. E. Ibrahim, A. A. Kamil, M. Elsheick, A. Babiker, and F. Modabber. 2000. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-bind, BCG-controlled trial in Sudan. Lancet 356:1565-1569. [DOI] [PubMed] [Google Scholar]
- 7.Maasho, K., I. Satti, S. Nylén, G. Guzman, F. Koning, and H. Akuffo. 2000. A Leishmania homologue of receptors for activated C-kinase (LACK) induces both interferon-γ and interleukin-10 in natural killer cells of healthy blood donors. J. Infect. Dis. 182:570-578. [DOI] [PubMed] [Google Scholar]
- 8.Moore, K. W., A. O'Garra, R. de Waal Malefyt, P. Vieira, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 9.Mottram, J. C., A. E. Souza, J. E. Hutchison, R. Carter, M. J. Frame, and G. H. Coombs. 1996. Evidence from disruption of the lmcpb gene array of Leishmania mexicana that cysteine proteinases are virulence factors. Proc. Natl. Acad. Sci. USA 93:6008-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philippe, H. 1993. MUST, a computer package of management utilities for sequences and trees. Nucleic Acids Res. 21:5264-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafati, S., A. H. Salmanian, K. Hashemi, C. Schaff, S. Belli, and N. Fasel. 2001. Identification of Leishmania major cysteine proteinases as targets of the immune response in humans. Mol. Biochem. Parasitol. 113:35-43. [DOI] [PubMed] [Google Scholar]
- 12.Rafati, S., A. H. Salmanian, T. Taheri, M. Vafa, and N. Fasel. 2001. A protective cocktail vaccine against murine cutaneous leishmaniasis with DNA encoding cysteine proteinases of Leishmania major. Vaccine 19:3369-3375. [DOI] [PubMed] [Google Scholar]
- 13.Rocha, P. N., R. P. Almeida, O. Bacellar, A. R. de Jesus, D. C. Filho, A. C. Filho, R. L. Barral, R. L. Coffmann, and E. M. Carcalho. 1999. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J. Infect. Dis. 180:1731-1734. [DOI] [PubMed] [Google Scholar]
- 14.Sharifi, I., A. R. FeKri, M. R. Aflatonian, A. Khamasipour, A. Nadim, M. R. Mousavi, A. Z. Momemi, Y. Dowmati, T. Godal, F. E. Zicker, P. G. Smith, and F. Modabber. 1998. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 351:1540-1543. [DOI] [PubMed] [Google Scholar]