Abstract
Campylobacter jejuni can cause an enteritis that is associated with an acute inflammatory response at the gut epithelial surface. The signals inducing inflammation are unknown. C. jejuni can penetrate the intestinal epithelial barrier and may then interact with leucocytes, potentially inducing proinflammatory responses. To investigate this, we studied the interaction of C. jejuni with the human monocytic cell line THP-1 and show that a range of proinflammatory cytokines and chemokines is induced. These include interleukin-1α (IL-1α), IL-1β, IL-6, IL-8, and tumor necrosis factor alpha. Responses can be induced by killed Campylobacter as well as live bacteria and do not depend on the cytolethal distending toxin. C. jejuni infection of THP-1 cells triggers both nuclear translocation of functional NF-κB and stimulation of IL-1α, indicating that NF-κB-dependent and -independent stimulation is occurring. The extent of proinflammatory cytokine stimulation suggests that monocytes might significantly contribute to intestinal inflammation and disease pathology.
Campylobacter jejuni causes severe gastroenteritis in humans. The pathology includes severe inflammation of the intestinal mucosa with an influx of professional phagocytes (2, 11, 23, 24). C. jejuni can invade model epithelial cell monolayers in vitro, causing disruption to the epithelium and gaining access to its basal side (3, 4, 8, 18). Histological analysis of biopsy samples from patients with C. jejuni colitis has shown that the bacteria invade the colonic mucosa (22). In vitro experiments on a range of human-derived epithelial cell lines have shown that C. jejuni can induce secretion of interleukin-8 (IL-8) (9, 10, 15), which has also been reported in stools of patients with campylobacteriosis (20). IL-8 is a potent chemokine that attracts phagocytic cells, and its release by intestinal epithelial cells may be an important signal that leads to the accumulation of these cells at the gut surface during C. jejuni infection (25). One C. jejuni-derived factor that can induce IL-8 production from epithelial cells is the cytolethal distending toxin (CDT) (10), but CDT is not required for induction of IL-8 by live C. jejuni infection, indicating that IL-8 may be induced by other stimuli (10). Enterotoxin and cytotoxin are other possible inducers that have been documented in Campylobacter isolates, but the role of these toxins in initiating inflammation is unclear (5, 11, 24).
The roles of cytokines and cell types other than IL-8 and epithelial cells in C. jejuni infection have not been described. An important cell type with which C. jejuni may interact to induce responses is the monocyte or macrophage. These may be particularly important in perpetuating the inflammatory disease, and it has been shown that C. jejuni can persist within peripheral blood monocytes for up to 7 days, but no data have been provided to show whether these cell types release signals that could lead to the further stimulation of inflammatory responses (12). In this report, we describe the stimulation of the human monocytic cell line THP-1 by C. jejuni. We show that C. jejuni stimulates rapid production of a range of proinflammatory signals from both the promonocytic and monocytic states of these cells but that these responses are enhanced in monocytic THP-1 cells. Compared with the promonocytic THP-1 cells, the monocytic lines express a range of cell receptors, for example, CD14 and CD11a, that are involved in cell signaling in response to a range of bacterial pathogen-associated molecular patterns (6). These data suggest that monocytic cells could be an important contributor to the induction and maintenance of gut inflammation.
MATERIALS AND METHODS
C. jejuni NCTC11168 and C. jejuni NCTC11168 cdtB have been described previously (16, 17). C. jejuni NCTC11168 cdtB has an insertion mutation in the cdtB gene and lacks CDT-dependent cytotoxicity (17). C. jejuni G1 was isolated from a patient who went on to develop Guillain-Barré syndrome (14). C. jejuni 81-176 was isolated from an outbreak associated with unpasteurized milk (13). All strains were cultured for 2 days in Mueller-Hinton broth, from which they were diluted 1 in 50 into fresh prewarmed Mueller-Hinton medium and grown for 12 h prior to experimentation. The optical density was measured at 600 nm; then the bacteria were centrifuged and resuspended in prewarmed phosphate-buffered saline (PBS) to the desired cell density for inoculation of eukaryotic cell cultures at a multiplicity of infection (MOI) of 100 bacteria per cell. All cultures were grown at 37°C in a modified gas atmosphere of 10% carbon dioxide, 5% oxygen, and 85% nitrogen. Heat-killed bacteria were prepared in the same way, but after suspension in PBS, they were heated to 70°C for 20 min. All heat-killed cultures were assessed for nonviability by plating on sheep blood agar plates.
Cells and culture conditions.
THP-1 cells (21) were grown in antibiotic-free RPMI 1640 medium supplemented with 10% fetal calf serum. First stage differentiation was achieved by culturing cells in the presence of 0.1 μM 1α,25-dihydroxyvitamin D3 (vitamin D3; Sigma, Poole, United Kingdom). All cells were seeded at 105 cells per ml in 96-well plates 24 h prior to use. All infections were carried out by inoculating bacteria as suspensions in PBS at an MOI of 100, unless stated otherwise. Controls consisted of mock infections using PBS alone or positive controls of either 1 μg of phorbol-12-myristate 13-acetate (PMA; Sigma)/ml or Escherichia coli O55:B5 lipopolysaccharide (LPS) (Sigma) at a final concentration of 50 μg per ml. Cell culture was carried out in 96-well plates for cytokine assays. Cultures were infected by bacteria; then, at set times postinfection, cells and supernatants were removed and centrifuged at high speed for 30 s, and supernatant was taken for enzyme-linked immunosorbent assays. Viable counts of cell pellets were determined to assay for intracellular bacteria.
Intracellular bacterial counts.
The number of intracellular bacteria per eukaryotic cell culture was assessed with a gentamicin protection assay. At a set time postinfection, the culture supernatant was supplemented with medium containing gentamicin such that the final concentration of gentamicin would be 100 μg/ml. The cells were replaced in the incubator, and then 1 h later, the culture was centrifuged (10,000 × g). The resultant cell pellet was washed in PBS, the cells were lysed in 0.5% (vol/vol) Triton X-100, and the viable bacterial count was determined by plating serial dilutions of the lysate on sheep blood agar plates. Viable counts are expressed as CFU per milliliter, where the volume of a well is taken as 100 μl.
Cytokine assays.
Cytokines were assayed by capture Duo-ELISA with kits supplied by R & D Research (Abingdon, United Kingdom). IL-1α and IL-1β were assayed from undiluted cell supernatant fluid. Tumor necrosis factor alpha (TNF-α) and IL-6 were assayed from supernatants diluted 10-fold, and IL-8 was assayed from supernatants diluted 100-fold in PBS. All values are expressed as picograms per milliliter. All five cytokines assayed were determined from the same supernatant samples, which were split in duplicate; all experiments were repeated in triplicate.
Electrophoretic mobility shift assay (EMSA).
Nuclear pellets were prepared from C. jejuni-infected and control THP-1 cells. Cells (3 × 105) were washed twice with ice-cold PBS and then pelleted by centrifugation for 30 s. The cell pellet was resuspended in 400 μl of buffer 1 (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], and 10 μg each of leupeptin, pepstatin, and aprotinin/ml) and put on ice to swell for 15 min. After addition of 25 μl of 10% (wt/vol) NP-40, the samples were vortexed for 10 s and then centrifuged at 16,000 × g for 20 s. The cell pellets were resuspended in 50 μl of buffer 2 (20 mM HEPES [pH 7.9], 25% [wt/vol] glycerol, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.5 mM PMSF, and 10 μg each of leupeptin and pepstatin/ml) and, after light vortexing, were extracted on ice for 15 min. The extract was sonicated on ice for 30 s and then centrifuged at maximum speed (16,000 × g) for 15 min at 4°C. The supernatant containing the nuclear fraction was stored at −70°C.
Transcription factor NF-κB binds to the consensus oligonucleotide sequence 5′ AGTTGAGGGGACTTTCCCAGCC 3′. This oligonucleotide was synthesized and end labeled with [γ-32P]ATP and T4 polynucleotide kinase (Promega Corporation, Southampton, United Kingdom). EMSAs were performed in 10 μl of reaction mixture containing 5 μg of nuclear extract, 5% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 50 mM NaCl, 10 mM Tris-HCl [pH 7.5], 0.05 mg of poly(dI-dC) · poly(dI-dC)/ml, and 0.2 ng of oligonucleotide probe. The reaction mixtures were incubated for 20 min at room temperature. After addition of 1 μl of gel-loading buffer (250 mM Tris-HCl [pH 7.5], 0.2% bromophenol blue, 40% glycerol) the reaction products were resolved on 4% acrylamide gels with 2.5% (vol/vol) glycerol preelectrophoresed with 0.5% Tris-borate-EDTA at 100 V for 30 min. The radioactive bands were visualized by autoradiography.
Immunofluorescence microscopy.
THP-1 cells were cultured on poly-l-lysine coverslips, seeded in 24-well plates at 105 cells per ml. The cells were infected with bacteria 24 h later at an MOI of 100 or were inoculated with an O55:B5 LPS control (50 μg/ml). At time points postinoculation, the supernatant was removed and the coverslips were overlaid with 10% formaldehyde fixative. Samples were stored at 4°C until used. Fixed cells were stained for the p50 subunit of NF-κB and counterstained with propidium iodide as follows. Cells were permeabilized with 0.5% (vol/vol) Triton X-100 in PBS for 25 min, and nonspecific binding sites were blocked with 1% (wt/vol) bovine serum albumin in PBS. NF-κB p50 was detected with the rabbit polyclonal antiserum H-119 (Santa Cruz Biotech; supplied by Autogen Bioclear, Calne, United Kingdom), used at a working concentration of 1 μg/ml in PBS. Cells were washed with PBS to remove unbound label, and bound rabbit immunoglobulin G (IgG) was detected with anti-rabbit IgG-fluorescein isothiocyanate (FITC) conjugate (Sigma). Unbound conjugate antibody was removed by washing in PBS. The labeled cells were counterstained with a 30-s exposure to aqueous propidium iodide (10 ng/ml). Stained cells were washed in PBS, mounted with Vectashield (Vector Laboratories, Burlingame, Calif.), and analyzed with a Leica TCS NT confocal laser scanning microscope with version 1.6.587 of the manufacturer’s software.
RESULTS
Intracellular bacterial counts.
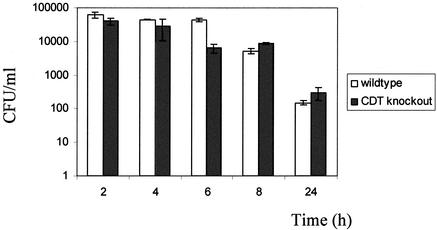
Live C. jejuni NCTC11168 and C. jejuni cdtB could be found inside both promonocytic and monocytic forms of THP-1 cells. There was no difference between the parental and the CDT mutant bacteria for either uptake or the profile of intracellular killing by monocytic THP-1 cells (Fig. 1).
FIG. 1.
Survival of intracellular C. jejuni in monocytic THP-1 cells. Data points are the averages of three values from the same experiment, with standard deviation. Open bars represent data for C. jejuni NCTC11168, and closed bars represent data for C. jejuni cdtB. The data shown are representative of three independent experiments.
Production of cytokines by promonocytic THP-1 cells.
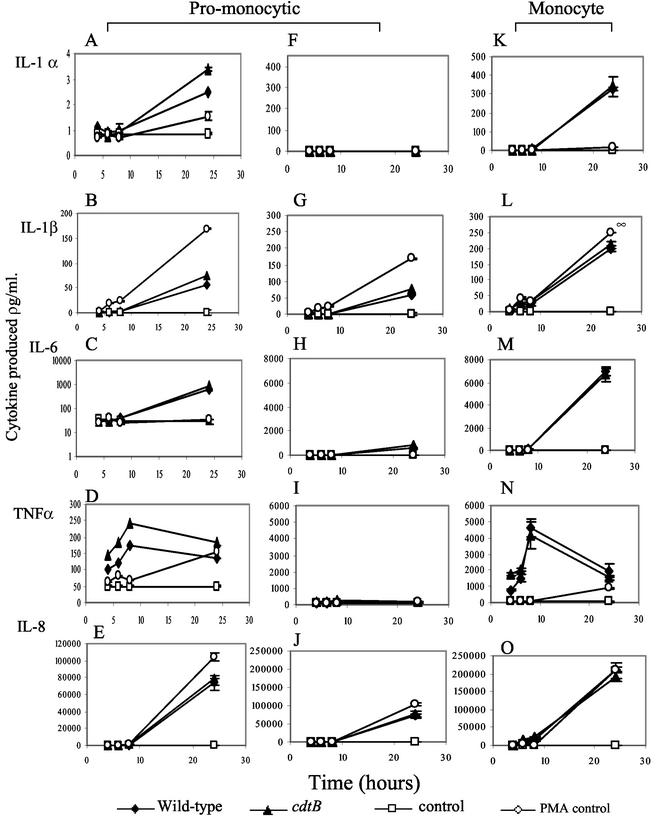
THP-1 cells grown in basic RPMI medium grow as promonocytes. Infections of these cells with C. jejuni NCTC11168 and C. jejuni NCTC11168 cdtB at MOIs of 100 were carried out, and levels of cytokines produced were assayed compared to those of uninfected cells (Fig. 2A to J). Positive controls with 1 μg of PMA/ml were used throughout. C. jejuni NCTC11168 stimulated a low level of IL-1α peaking at less than 10 pg/ml at 24 h postinfection. IL-1β, IL-6, and IL-8 appeared to accumulate over the time course (Fig. 2A to C and E). TNF-α levels initially increased with time but then decreased by 24 h postinfection (Fig. 2D).
FIG. 2.
Campylobacter-induced cytokine production by promonocytic and monocytic THP-1 cells. (A to E) Data for promonocytic THP-1 cells, shown on a variable-scale y axis. (F to J) Data for promonocytic THP-1 cells, shown on a y axis with the same scale as that used for monocytic cells. (K to O) Data for monocytic THP-1 cells. Each data point is the average of results for three samples. The data shown are representative of three replicate experiments.
Production of cytokines by monocytic THP-1 cells.
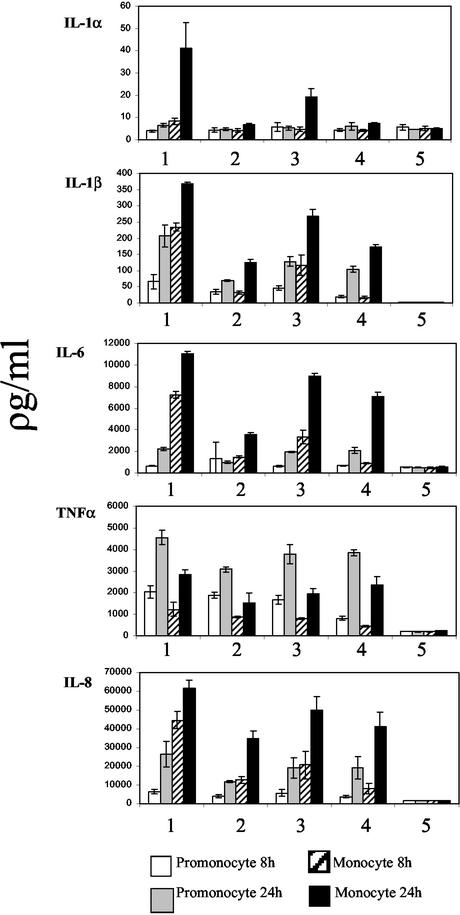
Monocytic THP-1 cells were infected at MOIs of 100 with C. jejuni NCTC11168 and C. jejuni NCTC11168 cdtB. Controls show that vitamin D3 alone has no effect on the production of the cytokines tested (Fig. 2A to E and K to O). Responses stimulated from differentiated monocytic cells were both more rapid and greater in magnitude than those seen from promonocytic cells. Once again, IL-1α, IL-1β, IL-6, and IL-8 accumulated over the time course (Fig. 2K to M and O), while TNF-α levels initially increased with time but then decreased by 24 h postinfection (Fig. 2N). Stimulation of cytokine production was dependent on the MOI, as serial dilutions of the C. jejuni inoculum (MOI of 1,000 to 10) stimulated a proportional reduction in cytokine release (data not shown). The stimulation showed some strain dependence between equivalent MOIs of C. jejuni NCTC11168 and C. jejuni G1 but not between NCTC11168 and C. jejuni 81-176. No significant difference was observed for cytokine production between C. jejuni NCTC11168- and C. jejuni 81-176-stimulated cells across experiments (P > 0.05 over four experiments) (Fig. 3 and data not shown).
FIG. 3.
Production of cytokines by different Campylobacter species at 4 h postinfection. Columns: 1, C. jejuni NCTC11168; 2, C. jejuni G1; 3 and 4, C. jejuni 81-176 and LPS positive control (50 μg/ml), respectively; 5, mock infected control. Samples were taken at 4 and 24 h postinfection. Values are means with standard deviations of results from three independent samples. The data shown are representative of four independent experiments.
Stimulation of cytokine production by killed bacteria.
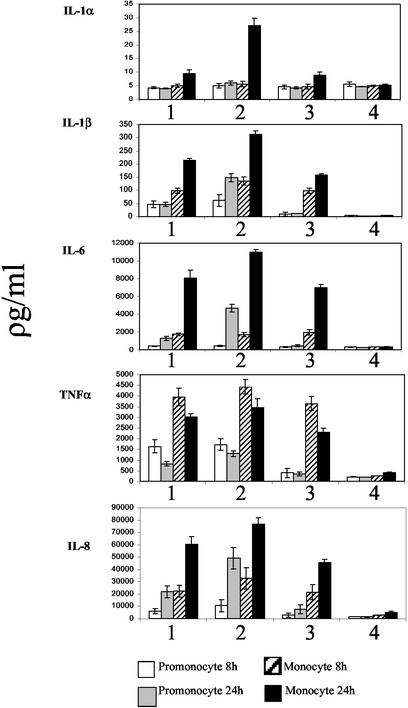
To determine whether cytokine production could be stimulated by killed bacteria, heat-killed C. jejuni was used to inoculate cell cultures at MOIs equivalent to the live infections. Heat-killed bacteria stimulated a range of inflammatory cytokine responses similar to those seen when live bacteria were used (Fig. 4). However, while no significant difference was observed for the production of TNF-α, the final cumulative levels of IL-1 α, IL-1β, IL-6, and IL-8 were lower from cells stimulated with heat-killed bacteria (Fig. 4).
FIG. 4.
Production of cytokines by heat-killed bacteria. Columns: 1, heat-killed C. jejuni NCTC11168; 2, live C. jejuni NCTC11168; 3, LPS positive control (50 μg/ml); 4, mock infected control. Samples were taken at 4 and 24 h postinfection. Values are means with standard deviations of results from three independent samples. The data shown are representative of three independent experiments.
Lack of requirement of CDT for stimulation of cytokine production.
To determine whether CDT activity was involved in the induction of proinflammatory responses from THP-1 cells, the experiments were repeated, comparing wild-type and cdtB-mutant bacteria as stimuli. No significant difference was observed between parental NCTC11168 and the cdtB mutant in their abilities to stimulation promonocytic or monocytic THP-1 cells (Fig. 2). Hence, functional CDT is not required for induction of IL-1α, IL-β, IL-6, TNF-α, or IL-8 (Fig. 2).
Activation of NF-κB.
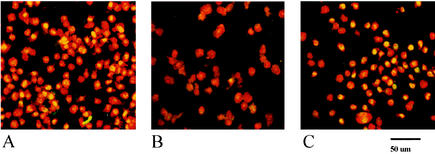
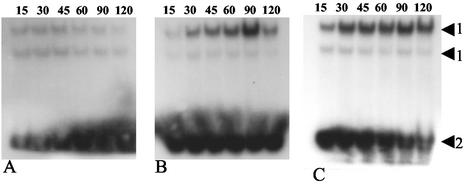
Nuclear proteins were isolated from THP-1 cells during infection. Confocal microscopy of monocytic THP-1 cells showed that the p50 subunit of NF-κB is translocated to the nucleus of the cells after 1 h of either infection with C. jejuni NCTC11168 or stimulation with O55:B5 LPS (Fig. 5). EMSAs showed that the translocated NF-κB accumulated in the nucleus of infected cells and had DNA binding activity (Fig. 6).
FIG. 5.
Confocal microscopy for translocation of p50 subunit of NF-κB. Infected cells were probed with antibodies to NF-κB p50 and secondary FITC conjugate and then counterstained with propidium iodide. Shown are the LPS positive control (50 μg/ml) (A), the mock-infected control (B), and C. jejuni NCTC11168 (C). Scale bar, 50 μm. The samples shown were taken at 6 h postinfection. The data shown are representative of three independent experiments.
FIG. 6.
EMSA for binding of NF-κB in the mock-infected control (A), C. jejuni NCTC11168 infected at an MOI of 100 (B), and heat-killed C. jejuni NCTC11168 (C). The samples were taken at the times shown (in minutes). Arrows: 1, protein-DNA complex formation incorporating NF-κB-specific probe; 2, unbound probe. The data are representative of two independent experiments.
DISCUSSION
Previous studies of cytokine stimulation by C. jejuni have concentrated on the interaction of the bacterium with epithelial cell lines (9, 10, 15). However, during infection there is an influx of neutrophils through the epithelial barrier, and in addition, C. jejuni can traverse the epithelial barrier and come into contact with a range of leukocytes (22). In this study, we have shown that both promonocytic and monocytic THP-1 cells can be stimulated by C. jejuni to produce a range of proinflammatory cytokines and chemokines. Significantly increased amounts of all the cytokines tested were produced by monocytic cells compared to promonocytic cells (Fig. 2). It is known that the cell markers CD14 and CD11a are both increased upon vitamin D3 treatment of these cells (6), and their increased expression in our experiments might help explain the increased sensitivity of the cells to C. jejuni infection. Differentiation of THP-1 cells from monocytes to macrophages is known to prime the cells for LPS stimulation via increased NF-κB accumulation (19), and there may be a difference in cell priming between promonocytic and monocytic cells. The promonocytic THP-1 cells are also more sensitive to PMA, suggesting that they are more sensitive to stimulation by other routes (Fig. 2).
We tested three different Campylobacter isolates, C. jejuni NCTC11168, C. jejuni G1, and C. jejuni 81-176. C. jejuni G1 produced significantly reduced levels of all cytokines at an MOI of 100 compared to C. jejuni NCTC11168 and C. jejuni 81-176. We also tested a mutant of C. jejuni NCTC11168 lacking CDT activity for its ability to induce these responses. Previous reports have shown that crude CDT preparations can induce the production of IL-8 but that live CDT-negative strains are also capable of inducing IL-8 production by epithelial cells (10). The mutant C. jejuni was able to induce production of IL-8 and the cytokines IL-1α, IL-1β, IL-6, and TNF-α as well as the wild-type bacteria. Therefore, CDT is not required for the induction of these cytokines during infection of THP-1 cells by C. jejuni. No differences were seen for the uptake or survival of the CDT mutant within THP-1 cells over a 24-h period (Fig. 1).
The results of the experiments with heat-killed bacteria showed that induction of cytokine production by THP-1 cells does not require live bacterial infection. The accumulated levels of IL-1β, IL-6, TNF-α, and IL-8 induced by killed bacteria were slightly reduced at 24 h compared with those induced by live infection, but this might simply reflect an increased interaction with cells by the live bacteria during infection.
NF-κB is a central mediator in the induction of several proinflammatory responses, in particular after LPS stimulation (7). C. jejuni stimulates the translocation of NF-κB during infection of epithelial cells (15), and we have now shown, using confocal microscopy, that the p50 subunit of NF-κB is translocated to the nuclear compartment of THP-1 cells during infection (Fig. 5). This can be visualized in about 25% of cells at 45 min and 90% of cells at 1 h postinfection (data not shown). This is equivalent to the activation stimulated by E. coli O55:B5 LPS. To assess the rate of NF-κB translocation, EMSAs were carried out (Fig. 6). The data show that NF-κB translocation occurs from 30 min postinfection onwards. It has previously been reported that LPS stimulation of NF-κB translocation can induce differentiation in THP-1 cells (19). Increased adherence of infected THP-1 cells to the surface of the microtiter plates was observed over a 24-h period, suggesting differentiation of the cells (data not shown). Taken together, the data suggest that stimulation of THP-1 cells by C. jejuni is similar to the activation that occurs in response to stimulation by LPS. Whether LPS is the main stimulus remains to be seen. There are many other potential candidate molecules that could contribute to the stimulus, as several bacterium-associated molecules are capable of inducing NF-κB-dependent inflammatory signals.
Mellits et al. (15) have suggested that stimulation of epithelial cells is mediated by a small (<3 kDa) protease-resistant molecule in studies using boiled filtered lysates. We present evidence that suggests that there is more than one route of stimulation of THP-1 cells and therefore the possibility that more than one stimulus exists.
IL-1α is induced through an NF-κB-independent manner (1). Our data therefore suggest that there are at least two transcriptional systems being triggered by the Campylobacter. There are several bacterial components, such as LPS, flagella, outer-membrane proteins, and chaperones, which can stimulate proinflammatory cytokines. The results here suggest that there might be more than one signal inducing proinflammatory cytokine responses. Previous reports have indicated that CDT toxin can induce IL-8 (10); however, the work described here indicates that CDT is not required to stimulate either the NF-κB-dependent or -independent cytokines. The stimulation of the proinflammatory cytokines tested is rapid and significant. Together, the results suggest that the stimulation of phagocytes during infection could play an important role in the development of inflammatory disease.
Acknowledgments
We thank P. Guerry, B. Wren, and N. Gregson for the supply of parental strains used in this study. We also thank D. Purdy, CAMR, for supplying NCTC11168 ctdB.
We acknowledge funding from DEFRA and BBSRC. C.E.B. is a Wellcome Trust Advanced Fellow.
Editor: B. B. Finlay
REFERENCES
- 1.Acres, R. B., A. Larsen, and P. J. Conlon. 1987. IL-1 expression in a clone of human T cells. J. Immunol. 138:2132-2136. [PubMed] [Google Scholar]
- 2.Blaser, M. J., R. B. Parsons, and W. L. Wang. 1980. Acute colitis caused by Campylobacter fetus ss. jejuni. Gastroenterology 78:448-453. [PubMed] [Google Scholar]
- 3.Brás, A. M., and J. M. Ketley. 1999. Transcellular translocation of Campylobacter jejuni across human polarized epithelial monolayers. FEMS Microbiol. Lett. 179:209-215. [DOI] [PubMed] [Google Scholar]
- 4.Everest, P. H., H. Goosens, J.-P. Butzler, D. Lloyd, S. Knutton, J. M. Ketley, and P. Williams. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319-325. [DOI] [PubMed] [Google Scholar]
- 5.Florin, I., and F. Antillon. 1992. Production of enterotoxin and cytotoxin in Campylobacter jejuni strains isolated from Costa Rica. J. Med. Microbiol. 37:22-29. [DOI] [PubMed] [Google Scholar]
- 6.Galdiero, M., M. D'Isanto, M. Vitello, E. Finamore, L. Peluso, and M. Galdiero. 2001. Porins from Salmonella enterica serovar Typhimurium induce TNF-α, IL-6 and IL-8 release by CD14 independent and CD11a/CD18-dependent mechanisms. Microbiology 147:2697-2704. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 8.Harvey, P., T. Battle, and S. Leach. 1999. Different invasion phenotypes of Campylobacter isolates in Caco-2 cell monolayers. J. Med. Microbiol. 48:461-469. [DOI] [PubMed] [Google Scholar]
- 9.Hickey, T. E., S. Baqar, L. Bourgeois, C. P. Ewing, and P. Guerry. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect. Immun. 67:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ketley, J. M. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 143:5-21. [DOI] [PubMed] [Google Scholar]
- 12.Kielhbauch, J. A., R. A. Albach, L. L. Baum, and K. -P. Chang. 1985. Phagocytosis of Campylobacter jejuni and its intracellular survival in mononuclear phagocytes. Infect. Immun. 48:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korlath, J. A., M. T. Osterholm, A. Judy, J. C. Forgang, and R. A. Robinson. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 152:592-596. [DOI] [PubMed] [Google Scholar]
- 14.Linton, D., A. V. Karlyshev, P. G. Hitchen, H. R. Morris, A. Dell, N. A. Gregson, and B. W. Wren. 2000. Multiple N-acetyl neuraminic acid synthetase (neuB) genes in Campylobacter jejuni: identification and characterization of the gene involved in sialylation of lipo-oligosaccharide. Mol. Microbiol. 35:1120-1134. [DOI] [PubMed] [Google Scholar]
- 15.Mellits, K. H., J. Mullen, M. Wand, G. Armbruster, A. Patel, P. L. Connerton, M. Skelly, and I. F. Connerton. 2002. Activation of the transcription factor NF-κB by Campylobacter jejuni. Microbiology 148:2753-2763. [DOI] [PubMed] [Google Scholar]
- 16.Parkhill. J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 17.Purdy, D., C. M. Buswell, A. E. Hodgson, K. McAlpine, I. Henderson, and S. A. Leach. 2000. Characterization of cytolethal distending toxin (CDT) mutants of Campylobacter jejuni. J. Med. Microbiol. 49:473-479. [DOI] [PubMed] [Google Scholar]
- 18.Russell, R. G., and D. C. Blake. 1994. Cell association and invasion of Caco-2 cells by Campylobacter jejuni. Infect. Immun. 62:3773-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takashiba, S., T. E. Van Dyke, S. Amar, Y. Murayama, A. W. Soskolne, and L. Shapira. 1999. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor κB. Infect. Immun. 67:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornley, J. P., T. Wright, K. Neal, D. Jenkins, and R. Spiller. 1998. A prospective cohort study of Campylobacter diarrhoea with the use of faecal inflammatory and leukocyte markers to investigate the resolution of disease. J. Med. Microbiol. 47:468. [Google Scholar]
- 21.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line. Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 22.vanSpreeuwel, J. P., G. C. Duursma, C. J. L. M. Meijer, R. Bax, P. C. M. Rosekrans, and J. Lindeman. 1985. Campylobacter colitis: histological immuno-histochemical and ultrastructural findings. Gut 26:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker, R. I., M. B. Caldwell, E. C. Lee, P. Guerry, T. J. Trust, and G. M. Ruiz-Palacios. 1986. Pathophysiology of Campylobacter enteritis. Microbiol. Rev. 50:81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wassenaar, T. M., and M. J. Blaser. 1999. Pathophysiology of Campylobacter jejuni infection in humans. Microbes Infect. 1:1023-1033. [DOI] [PubMed] [Google Scholar]
- 25.Wuyts, A., P. Proost, and J. van Damme. 1998. Interleukin-8 and other CXC chemokines, p. 271-311. In A. Thompson (ed.), The cytokine handbook, 3rd ed. Academic Press, San Diego, Calif.