Abstract
Lung tissue removed from neonatal calves with acute Mannheimia haemolytica pneumonia showed that rapid up-regulation of the basal mRNA expression of tracheal antimicrobial peptide (TAP), NF-κB, and intercellular adhesion molecule 1 occurred after infection; TAP and interleukin 8 expression were highly correlated. This work suggests that the coordinated expression of β-defensin and inflammatory elements occurs during bacterial pneumonia.
Innate immunity is important for preventing microbial infections of the respiratory tract shortly after birth, when the mucosa is initially exposed to a variety of microbes. Antimicrobial peptides (AMP) are increasingly being recognized as an evolutionarily ancient, and important, component of innate host defense in the lung. Tracheal antimicrobial peptide (TAP) was the first epithelial-cell-derived AMP found in mammals (16) and was subsequently characterized as a β-defensin with inductive properties (14-16). TAP mRNA has been detected in the columnar epithelial cells of the trachea and bronchi of adult cattle; however, TAP expression is not present in fetal cattle (12). Whether TAP is expressed in neonatal lungs has not been determined.
The promoter region of TAP contains motifs of nuclear factor κB (NF-κB) (12), a transcription factor which mediates the induction of genes involved in immune and inflammatory responses (23). Experiments with cultured bovine tracheal epithelial cells have demonstrated that NF-κB up-regulates TAP expression in response to lipopolysaccharide (13). Members of the NF-κB family induce expression of the β-defensin 2 gene in humans (27) and AMP in insects (7, 20, 26), which indicates that there is a conserved signal transduction pathway for AMP up-regulation among varied species. In addition, NF-κB induces the expression of interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-8 in bovine alveolar macrophages exposed to Mannheimia haemolytica leukotoxin and endotoxin (18). To our knowledge, the in vivo nuclear translocation of NF-κB during acute inflammation has not been assessed in bovine respiratory epithelia.
M. haemolytica is an important respiratory pathogen of ruminants and a known inducer of a bovine β-defensin, lingual antimicrobial peptide (LAP), in cattle older than 3 weeks of age (38). M. haemolytica incites a severe inflammatory response in the lungs of ruminants, characterized by dense infiltrates of neutrophils (3, 41, 42). Neutrophil-mediated damage can be severe and can destroy the protective barrier and innate immunity provided by the respiratory mucosa (8, 9, 36, 40). Inhibition of neutrophil transmigration reduces the severity of the lesions characteristic of this disease (11) and may also preserve the activity of the innate immune system.
One potent inflammatory mediator of M. haemolytica pneumonia in ruminants is the cytokine IL-8 (4). IL-8 is produced by endothelial cells, epithelial cells, activated macrophages, and neutrophils and causes the activation and chemotaxis of neutrophils (4). IL-8 production is induced by other cytokines, IL-1 and TNF being two of the most potent (24). Neutrophils also release elastase, which induces IL-8 synthesis in human bronchial epithelial cells in vitro (5, 28), and α-defensins, which have been shown to stimulate the synthesis of IL-8 by human airway epithelial cells (39).
Adhesion molecules, including selectins (l-, e-, and p-selectin) and intercellular adhesion molecule 1 (ICAM-1), are also integral to the inflammatory response during M. haemolytica pneumonia. Selectins mediate a transient, tethering-like adherence of neutrophils to vascular endothelial cells, whereas the ICAM-1—β2-integrin interaction mediates a more stable type of adherence (2, 6, 17, 32, 35, 37). Neutrophil transmigration into lung tissue can be reduced in neonatal calves during M. haemolytica pneumonia with the selectin adhesion molecule inhibitor TBC1269 (11, 30). TBC1269 also decreases neutrophil-mediated pulmonary damage (11, 23, 31). TBC1269 is a nonoligosaccharide selectin antagonist which provides competitive inhibition of sialyl-Lewis x binding, in vitro, to selectins (21, 22). This mimetic analog has also been used in vivo in cattle (30), in an ovine model of asthma (1), and in clinical studies for human allergic asthma.
The purposes of this study were to test the hypotheses that mRNA expression of TAP occurs in neonatal calves and that its expression increases during experimental pneumonia caused by M. haemolytica. The expression of other molecules important in the acute inflammatory response of this disease, IL-8 and ICAM-1, was also expected to increase. The reduction of neutrophil infiltration with the selectin adhesion molecule inhibitor TBC1269 may alter TAP expression during pneumonia. Finally, as the nuclear translocation of NF-κB is associated with increased TAP and inflammatory gene expression, we expected to find NF-κB nuclear translocation in the respiratory epithelia of calves with acute bacterial pneumonia.
Colostrum-deprived, male, 1- to 3-day-old Holstein calves were maintained in accordance with guidelines approved by an institutional animal care and use committee. One to 3 days after arrival, the calves were anesthetized and then inoculated with either M. haemolytica (108 CFU/ml in 5 ml of pyrogen-free saline [11]) or 5 ml of pyrogen-free saline (0.14 M NaCl) by fiberoptic bronchoscopy (2, 3, 10) (Table 1). One group of calves inoculated with M. haemolytica received TBC1269 prepared in sterile pyrogen-free saline (pH 7.33) (25 mg/kg of body weight, given intravenously [i.v.] 30 min before and 2 h after bacterial inoculation; Texas Biotechnology Corp., Houston, Tex.). TBC1269 (22) is a synthetic sialyl-Lewis x nonoligosaccharide mimetic antagonist {1,6-bis[3-(3-carboxymeth-ylphenyl)-4-(2-α-d-mannopyranosyloxy)phenyl]hexane} thatinhibits (by 50%) the binding of human l-, e-, and p-selectins at concentrations of 87, 105, and 17 μM, respectively (1), and does not have cytotoxic effects on neutrophils (29). The calves were euthanized 2 or 6 h after inoculation by i.v. injection of an overdose of sodium pentobarbital, and lung tissue was collected from the initial site of inoculation.
TABLE 1.
Abundance of epithelial-cell nuclear staining for NF-κB within the bronchi and bronchioles examined in neonatal calves by immunohistochemistry
| Calf | Inoculum | Time postinoculation (h) | Scorea |
|---|---|---|---|
| 1 | Saline | 2 | 1 |
| 2 | Saline | 2 | 0 |
| 3 | Saline | 2 | 0.5 |
| 4 | Saline | 6 | 0.5 |
| 5 | Saline | 6 | 0 |
| 6 | Saline | 6 | 1 |
| 7 | M. haemolytica | 2 | 1 |
| 8 | M. haemolytica | 2 | 1.5 |
| 9 | M. haemolytica | 2 | 0.5 |
| 10 | M. haemolytica | 2 | 0.5 |
| 11 | M. haemolytica | 6 | 2 |
| 12 | M. haemolytica | 6 | 1 |
| 13 | M. haemolytica | 6 | 1 |
| 14 | M. haemolytica | 6 | 1 |
| 15 | M. haemolytica + TBC1269 | 6 | 0 |
| 16 | M. haemolytica + TBC1269 | 6 | 0.5 |
| 17 | M. haemolytica + TBC1269 | 6 | 0 |
| 18 | M. haemolytica + TBC1269 | 6 | 1.5 |
Scores: 0, no nuclear staining; 1, minimally detectable staining (two to four nuclei of bronchi or bronchioles stained); 2, up to 5% of nuclei stained positively. In cases where bronchi or bronchioles stained variously on the same slide, a half score was given. Significantly more nuclear staining was seen in the calves inoculated with M. haemolytica than in those inoculated with saline (controls) (P = 0.06).
The isolation of RNA from the lung tissues of most of the calves was performed with a phenol and guanidine isothiocyanate solution (TRIzol; Life Technologies, Inc., Grand Island, N.Y.). The RNA from one calf (calf 7) was isolated via column purification of RNA (RNAqueous-Midi; Ambion, Austin, Tex.). RNA from each calf was measured with a spectrophotometer (model DU 640B; Beckman Coulter, Fullerton, Calif.) to determine its quantity and purity ratio and assessed for integrity by the UV visualization of rRNA bands following denaturing gel electrophoresis and ethidium bromide staining.
cDNA was produced by reverse transcriptase of RNA (TaqMan reverse transcriptase reagents; PE Biosystems, Foster City, Calif.). Human control RNA (TaqMan rRNA control reagents; PE Biosystems) and diethyl pyrocarbonate-treated water were used as positive and negative RNA controls. DNase treatment was performed prior to reverse transcriptase treatment to remove potential genomic DNA contamination (RQ1 RNase-free DNase; Promega, Madison, Wis.).
Sequence-specific oligonucleotide primers and fluorescent probes for the detection and relative quantification of TAP, IL-8, and ICAM-1 cDNA were designed with software (ABI Prism Primer Express, version 1.5; PE Applied Biosystems) and were engineered to be within the coding sequence of each respective mRNA. Primer and probe sequences were as follows (where 6FAM is 6-carboxyfluorescein, the fluorescent reporter dye, and TAMRA is 6-carboxytetramethylrhodamine, the fluorescent quencher dye): for TAP, 5′GGCAGTAAAATGCTGTAGAAAGAAGTAAA3′, 5′CTCTGTCAAAGGGCGCAGTT3′, and 5′6FAM-ACACAGCCGGGATCAATGCCCAG-TAMRA3′; for IL-8, 5′TGGGCCACACTGTGAAAATTC3′, 5′CCTTCTGCACCCACTTTTCCT3′, and 5′6FAM-TAAGCTTACCAATGGAAACGAGGTCTGCCTAAAC-TAMRA3′; and for ICAM-1, 5′CGATCGACCCTCACCTTGAG3′, 5′CCATCCAGCGTGCAATTCA3′, and 5′6FAM-CCCGATTGTGGAAGTAGGCTCACGGT-TAMRA3′. The sequences were compared to those in all available sequence databases via a search tool (Basic Local Alignment Search Tool, version 1.4; National Center for Biotechnology Information, Bethesda, Md.), and only unique sequences were used. Sequence-specific oligonucleotide primers and a fluorescent probe for the detection of cDNA corresponding to the endogenous reference gene, 18S rRNA, were purchased commercially (PE Biosystems).
Based on the optimization and validation experiments (ABI Prism 7700; PE Applied Biosystems), the standard curve method was chosen for data analysis for both TAP and IL-8 and the CT method (the PCR cycle at which an increase in the reporter fluorescence [i.e., 6FAM] can be detected above baseline) was chosen for data analysis for ICAM-1. Primer and probe concentrations of 900 and 200 nM for TAP, 300 and 200 nM for IL-8, and 900 and 200 nM for ICAM-1 were used. Endogenous reference primer and probe concentrations of 50 and 200 nM were used. The assay composition was as follows: the 50-μl PCR mixture contained 25 μl of master mix (TaqMan universal PCR master mix,2× concentration; PE Biosystems), 5 μl of a 1:10 dilution of cDNA (or diethyl pyrocarbonate-treated water for the negative control), and the specified amounts of the forward and reverse primer and probe for each substance and for the endogenous reference gene. Microwell plates were run in the sequence detection system under the following conditions: 2 min at 50°C, 10 min at 95°C, and then 40 cycles of 15 s at 95°C followed by 1 min at 60°C. The resulting data were analyzed (ABI Prism sequence detection systems, version 1.7; Perkin-Elmer Corp.; and Excel, version 9.0; Microsoft). For each animal, the mRNA level of each substance was normalized to the level of the endogenous reference gene and expressed relative to the level of the corresponding substance from the calibrator animal, calf 1, the calf with the lowest message levels of all three substances being assessed.
Statistical analysis was performed using the means of the results from three replicate tubes for each calf to test the amount of the target normalized to that of the reference (TAP and IL-8) and the change in the CT (ICAM-1). A P value of ≤0.1 was considered significant. To determine significant treatment or time effects, a two-factor analysis of variance (SAS, version 8.1; SAS Institute, Cary, N.C.) was used to analyze the values from those calves that did not receive TBC1269. There was no significant interaction between time and treatment in the factorial analysis, so only the main effects of time and treatment were further examined. To determine if the selectin inhibitor had a significant effect, a t test was performed on the data from the calves tested 6 h after inoculation with M. haemolytica who did or did not receive TBC1269. To compare the results from all five groups to each other, a one-way analysis of variance including data from all of the calves was used initially to determine significant differences among the results for the five neonatal groups. As overall differences were detected only for ICAM-1, pairwise comparisons of data for this group were performed. A correlation procedure was used to compare individual relative mRNA expression levels of TAP, IL-8, and ICAM-1 to each other for evidence of a correlation of expression.
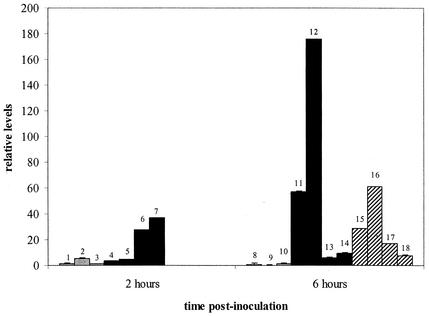
Pulmonary TAP mRNA was expressed at a basal level in neonatal calves (Fig. 1). TAP expression was 25-fold higher in the calves inoculated with M. haemolytica than in the controls. Although this increase is not significant (P = 0.14), it suggests that the lung is capable of induced expression at birth. Group mean TAP mRNA levels are higher at 6 h after inoculation with M. haemolytica than at 2 h. However, the levels of pulmonary TAP mRNA expression vary widely between individuals within each treatment group, which indicates that some individuals may have robust TAP expression but that others may have limited induction. This variation may be due to one or more factors. First, as TAP is not expressed in the fetus (12), its expression in the neonate may be less consistent than in a mature animal and in neonates that had long gestation times. However, even in mature ruminants, β-defensin expression varies between individuals (19). Finally, subtle differences in levels of TAP expression may be present due to sampling, as bronchi and bronchioles have higher levels of TAP expression than alveolar epithelia (12). In any case, the variability of TAP expression might explain differences in neonatal susceptibilities to pneumonia. In addition, we have shown that bronchoalveolar lavage fluid that contains the AMP anionic peptide from neonatal calves infected with M. haemolytica has little or no anti-M haemolytica activity in vitro, compared to the significant anti-M haemolytica activity of bronchoalveolar lavage fluid containing anionic peptide from adult cattle (11). It is unknown if TAP from neonates has full antimicrobial activity compared to that of TAP from adults.
FIG. 1.
Individual relative levels of TAP mRNA (means ± standard deviations). The values have been normalized to an endogenous reference gene, 18S rRNA, and are expressed relative to the values for the calibrator, calf 1. The calves were treated as follows: they were inoculated with 5 ml of pyrogen-free saline (0.14 M NaCl) solution (gray bars), inoculated with 5 ml of a solution that contained 108 CFU of M. haemolytica/ml (black bars), or treated with a selectin inhibitor (TBC1269, 25 mg/kg of body weight, given i.v.) 30 min before and 2 h after being inoculated with 5 ml of a solution that contained 108 CFU of M. haemolytica/ml (hatched bars). The small numbers identify individual calves.
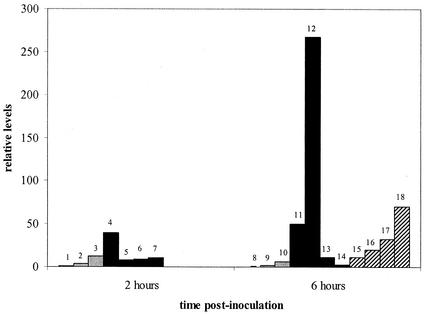
Although IL-8 mRNA was expressed at a 12-fold-higher level in the calves inoculated with M. haemolytica than in the controls, these differences are not significant (P = 0.25) (Fig. 2). As with TAP, levels of pulmonary IL-8 mRNA expression vary between the individuals within each treatment group and group mean IL-8 mRNA levels are higher at 6 h after inoculation with M. haemolytica than at 2 h. The graphs for the relative levels of TAP, IL-8, and ICAM-1 mRNA expression have similar shapes (Fig. 1 to 3). Individual relative levels of TAP and IL-8 mRNA are positively correlated with each other, with a correlation coefficient of 0.89 (P < 0.0001). This coordinate expression is similar to that found in previous studies of TAP and LAP genes (34), and it is unknown whether these substances affect the expression of each other. Although α-defensins released by human neutrophils simulate IL-8 synthesis by human airway epithelial cells (39), further studies are needed to assess the effects on IL-8 mRNA expression of β-defensins released by bovine neutrophils and those released by the epithelium (TAP and LAP) in bovine airways. Between IL-8 and ICAM-1, the correlation is 0.41 (P = 0.09), and between TAP and ICAM-1, it is 0.27 (P = 0.28), which is not statistically significant. For each substance (TAP, IL-8, and ICAM-1), mRNA expression is stimulated by some similar cytokines, including IL-1 and TNF (6, 13, 24-26, 33, 34).
FIG. 2.
Individual relative levels of IL-8 mRNA (means ± standard deviations). See the legend to Fig. 1 for a description of treatments and bar identifications.
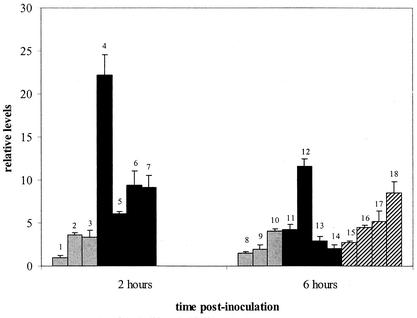
FIG. 3.
Individual levels of ICAM-1 mRNA (means plus ranges). See Fig. 1 for a description of treatments and bar identifications. Pulmonary ICAM-1 mRNA expression was significantly higher in the calves inoculated with M. haemolytica than in the controls inoculated with saline (P = 0.01).
Pulmonary ICAM-1 mRNA expression was threefold higher in calves inoculated with M. haemolytica than in the controls (P = 0.01) (Fig. 3), a finding similar to that for older calves inoculated with M. haemolytica (32). In contrast to those of TAP and IL-8, ICAM-1 mRNA levels are higher at 2 h after inoculation with M. haemolytica than at 6 h (P < 0.1), which likely reflects the importance of ICAM-1 in mediating the migration of neutrophils from the blood to the tissue site of inflammation in the immediate host response to infection.
Because M. haemolytica pneumonia is characterized by dense infiltrates of neutrophils and by necrosis (9, 36), it is reasonable to assume that treatment with an adhesion molecular inhibitor may spare epithelial cells, allowing increased TAP mRNA expression. However, TBC1269 did not increase TAP expression. For TAP, IL-8, and ICAM-1, there were no significant differences between mRNA expression levels in calves 6 h after inoculation with M. haemolytica and treatment with TBC1269 and in calves not treated (P values were 0.45, 0.47, and 0.74, respectively); however, for TAP and IL-8, the expression levels in TBC1269-treated calves are between those in calves tested 2 and 6 h after inoculation and not treated, thereby resembling levels earlier in the progression of the disease. For ICAM-1, the expression levels were very similar in treated and untreated calves 6 h after inoculation. Moreover, the expression levels were higher at 2 h after inoculation with M. haemolytica than at 6 h in the treated calves (P = 0.10).
NF-κB mediates the expression of TAP and other inflammatory mediators. NF-κB nuclear translocation, a marker of activation, was determined by immunohistochemistry of lung tissue sections (2, 3). Briefly, formalin-fixed and paraffin-embedded sections were treated with pronase E (0.1 g of protease XIV [Sigma Chemical Co., St. Louis, Mo.] and CaCl2) and incubated with 20% normal swine serum (Invitrogen, Grand Island, N.Y.) in PBS containing 0.1% Tween 20 and then for 48 h with the primary antibody (polyclonal rabbit anti-human NF-κB, which binds to bovine NF-κB; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) at a dilution of 1:5,000 or the control immunoglobulin G (normal rabbit immunoglobulin G, at 1 mg/ml; Upstate Biotechnology Inc., Lake Placid, N.Y.) at a dilution of 1:5,000 in common reagent diluent (BioGenex, San Ramon, Calif.) containing added 5% normal swine serum and 5% normal goat serum. Peroxidase activity was blocked with 2% H2O2, and the secondary antibody was applied (a 1:300 dilution of biotinylated goat anti-rabbit antibody; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.), followed by treatment with supersensitive streptavidin-conjugated horseradish peroxidase reagent (BioGenex) and the chromogen Nova Red (Vector, Burlingame, Calif.). Slides were read by one pathologist (J. M. Caverly) in random order (treatment groups were not identified). Epithelial cells from at least two bronchi and five bronchioles were examined on each slide, and the abundance of nuclear staining for NF-κB was scored (0, no nuclear staining; 1, minimally detectable staining, 2 to 4 nuclei of bronchi or bronchioles were stained; 2, <5% of the nuclei were stained; 3, 5 to 15% of the nuclei were stained; 4, >15% of the nuclei were stained). In cases where bronchi or bronchioles stained variously on the same slide, a half score was given.
Significantly more epithelial-cell nuclear translocation of NF-κB was present in tissue from the calves inoculated with M. haemolytica than in that from the calves inoculated with saline (P = 0.06) (Table 1), which is consistent with mRNA expression in cultured bovine tracheal epithelial cells (13). There were no significant differences over time (P = 0.5) or upon TBC1269 treatment (P = 0.13).
Based on this in vivo study, we concluded that TAP and ICAM-1 mRNA expression occurs in neonatal calves and rapidly increases during acute M. haemolytica pneumonia relative to the basal expression levels seen in control calves. However, between individual animals there is considerable variation in the mRNA expression of TAP, IL-8, and ICAM-1. Additionally, the reduction of pulmonary neutrophil transmigration and neutrophil-mediated damage through use of the selectin inhibitor TBC1269 results in TAP and IL-8 mRNA expression levels that are lower than the corresponding levels in untreated calves, although these results are not statistically significant. Within an individual, TAP and IL-8 mRNA expression correlate positively with each other. Finally, increased translocation of NF-κB to the nuclei of epithelial cells in calves with M. haemolytica pneumonia is associated with these findings in gene expression.
Acknowledgments
We gratefully acknowledge the statistical expertise of Tammy Benson and Kevan Flaming, the technical help of Kristi DeKruif and Eric Snook, and real-time PCR access by Doug Jones and Jeff Beetham.
This study was supported by USDA National Research Initiative, Cooperative State Research, Education, and Extension Service grant 970-2653.
Editor: F. C. Fang
REFERENCES
- 1.Abraham, W. M., A. Ahmed, J. R. Sabater, I. T. Lauredo, Y. Botvinnikova, R. J. Bjercke, X. Hu, B. M. Revelle, T. P. Kogan, I. L. Scott, R. A. Dixon, E. T. Yeh, and P. J. Beck. 1999. Selectin blockade prevents antigen-induced late bronchial responses and airway hyperresponsiveness in allergic sheep. Am. J. Respir. Crit. Care Med. 159:1205-1214. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann, M. R., K. A. Brogden, A. F. Florance, and M. E. Kehrli, Jr. 1999. Induction of CD18-mediated passage of neutrophils by Pasteurella haemolytica in pulmonary bronchi and bronchioles. Infect. Immun. 67:659-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann, M. R., M. E. Kehrli, Jr., and K. A. Brogden. 1996. Passage of CD18− and CD18+ bovine neutrophils into pulmonary alveoli during acute Pasteurella haemolytica pneumonia. Vet. Pathol. 33:639-646. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 5.Bédard, M., C. D. McClure, N. L. Schiller, C. Francoeur A. Cantin, and M. Denis. 1993. Release of interleukin-8, interleukin-6, and colony-stimulating factors by upper airway epithelial cells: implications for cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 9:455-462. [DOI] [PubMed] [Google Scholar]
- 6.Bevilacqua, M. P., R. M. Nelson, G. Mannori, and O. Cecconi. 1994. Endothelial-leukocyte adhesion molecules in human disease. Annu. Rev. Med. 45:361-378. [DOI] [PubMed] [Google Scholar]
- 7.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 8.Breider, M. A., S. Kumar, and R. E. Corstvet. 1991. Interaction of bovine neutrophils in Pasteurella haemolytica mediated damage to pulmonary endothelial cells. Vet. Immunol. Immunopathol. 27:337-350. [DOI] [PubMed] [Google Scholar]
- 9.Breider, M. A., R. D. Walker, F. M. Hopkins, T. W. Schultz, and T. L. Bowerstock. 1988. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can. J. Vet. Res. 52:205-209. [PMC free article] [PubMed] [Google Scholar]
- 10.Brogden, K. A., M. R. Ackermann, and B. M. DeBey. 1995. Pasteurella haemolytica lipopolysaccharide-associated protein induces pulmonary inflammation after bronchoscopic deposition in calves and sheep. Infect. Immun. 63:3595-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caverly, J. M., Z. A. Radi, C. B. Andreasen, R. A. F. Dixon, K. A. Brogden, and M. R. Ackermann. 2001. Comparison of bronchoalveolar lavage fluid obtained from Mannheimia haemolytica-inoculated calves with and without prior treatment with the selectin inhibitor TBC1269. Am. J. Vet. Res. 62:665-672. [DOI] [PubMed] [Google Scholar]
- 12.Diamond, G., D. E. Jones, and C. L. Bevins. 1993. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc. Natl. Acad. Sci. USA 90:4596-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond, G., V. Kaiser, J. Rhodes, J. P. Russell, and C. L. Bevins. 2000. Transcriptional regulation of β-defensin gene expression in tracheal epithelial cells. Infect. Immun. 68:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27-38. [DOI] [PubMed] [Google Scholar]
- 15.Diamond, G., J. P. Russell, and C. L. Bevins. 1996. Inducible expression of an antimicrobial peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc. Natl. Acad. Sci. USA 93:5156-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond, G., M. Zasloff, H. Eck, M. Brasseur, W. Lee Maloy, and C. Bevins. 1991. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc. Natl. Acad. Sci. USA 88:3952-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frenette, P. S., and D. D. Wagner. 1996. Adhesion molecules—part II: blood vessels and blood cells. N. Engl. J. Med. 335:43-45. [DOI] [PubMed] [Google Scholar]
- 18.Hsuan, S. L., M. S. Kannan, S. Jeyaseelan, Y. S. Prakash, C. Malazdrewich, M. S. Abrahamsen, G. C. Sieck, and S. K. Maheswaran. 1999. Pasteurella haemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-κB activation and calcium elevation. Microb. Pathog. 26:263-273. [DOI] [PubMed] [Google Scholar]
- 19.Huttner, K. M., D. J. Brezinski-Caliguri, M. M. Mahoney, and G. Diamond. 1998. Antimicrobial peptide expression is developmentally regulated in the ovine gastrointestinal tract. J. Nutr. 128:297S-299S. [DOI] [PubMed]
- 20.Ip, Y. T., M. Reach, Y. Engstrom, L. Kadalayil, H. Cai, S. Gonzalez-Crespo, K. Tatei, and M. Levine. 1993. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell 75:753-763. [DOI] [PubMed] [Google Scholar]
- 21.Kogan, T. P., B. Dupré, H. Bui, K. L. McAbee, J. M. Kassir, I. L. Scott, X. Hu, P. Vanderslice, P. J. Beck, and R. A. Dixon. 1998. Novel synthetic inhibitors of selectin-mediated cell adhesion: synthesis of 1,6-bis[3-(3-carboxymethylphenyl)-4-(2-α-d-mannopyranosyloxy)phenyl]hexane(TBC1269). J. Med. Chem. 41:1099-1111. [DOI] [PubMed] [Google Scholar]
- 22.Kogan, T. P., B. Dupre, and L. L. Scott. 1997. Di- and trivalent small molecule selectin inhibitors. Patent WO9701335.
- 23.Kopp, E. B., and S. Ghosh. 1995. NF-kappa B and Rel proteins in innate immunity. Adv. Immunol. 58:1-27. [DOI] [PubMed] [Google Scholar]
- 24.Kunkel, S. L., N. Lukacs, and R. M. Strieter. 1995. Chemokines and their role in human disease. Agents Actions Suppl. 46:11-22. [DOI] [PubMed] [Google Scholar]
- 25.Lassalle, P., P. Gosset, Y. Delneste, A. Tsicopoulos, A. Capron, M. Joseph, and A. B. Tonnel. 1993. Modulation of adhesion molecule expression on endothelial cells during the late asthmatic reaction: role of macrophage-derived tumor necrosis factor-alpha. Clin. Exp. Immunol. 94:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 27.Liu, L., L. Wang, H. P. Jia, C. Zhao, H. H. Heng, B. C. Schutte, P. B. McCray, Jr., and T. Ganz. 1998. Structure and mapping of the human β-defensin HBD-2 gene and its expression at sites of inflammation. Gene 222:237-244. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura, H., K. Yoshimura, N. G. McElvaney, and R. G. Crystal. 1992. Neutrophil elastase in respiratory epithelial lining fluid of individual with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J. Clin. Investig. 89:1478-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palma-Vargas, J. M., L. Toledo-Pereyra, R. E. Dean, J. M. Harkema, R. A. Dixon, and T. P. Kogan. 1997. Small-molecule selectin inhibitor protects against liver inflammatory response after ischemia and reperfusion. J. Am. Coll. Surg. 185:365-372. [PubMed] [Google Scholar]
- 30.Radi, Z., K. A. Brogden, R. A. Dixon, and M. R. Ackermann. 2002. Quantitation of neutrophil infiltration and intercellular adhesion molecule-1 (ICAM-1) mRNA expression with and without selectin inhibition during acute Mannheimia (Pasteurella) haemolytica pneumonia in neonatal calves. Vet. Pathol. 39:697-705. [DOI] [PubMed] [Google Scholar]
- 31.Radi, Z. A., J. M. Caverly, R. A. Dixon, K. A. Brogden, and M. R. Ackermann. 2001. Effects of the synthetic selectin inhibitor TBC1269 on tissue damage during acute Mannheimia haemolytica-induced pneumonia in neonatal calves. Am. J. Vet. Res. 62:17-22. [DOI] [PubMed] [Google Scholar]
- 32.Radi, Z. A., K. B. Register, E.-K. Lee, M. E. Kehrli, Jr., K. A. Brogden, J. M. Gallup, and M. R. Ackermann. 1999. In situ expression of intracellular adhesion molecule-1 (ICAM-1) mRNA in calves with acute Pasteurella haemolytica pneumonia. Vet. Pathol. 36:437-444. [DOI] [PubMed] [Google Scholar]
- 33.Rothlein, R., M. Czajkowski, M. M. O'Neill, S. D. Marlin, E. Mainolfi, and V. J. Merluzzi. 1988. Induction of intracellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J. Immunol. 141:1665-1669. [PubMed] [Google Scholar]
- 34.Russell, J. P., G. Diamond, A. P. Tarver, T. F. Scanlin, and C. L. Bevins. 1996. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect. Immun. 64:1565-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon, S. I., A. R. Burns, A. D. Taylor, P. K. Gopalan, E. B. Lynam, L. A. Sklar, and C. W. Smith. 1995. l-selectin (CD62L) cross-linking signals neutrophil adhesive functions via the mac-1 (CD11b/CD18) β2-integrin. J. Immunol. 155:1502-1514. [PubMed] [Google Scholar]
- 36.Slocombe, R. F., J. Malark, R. Ingersoll, F. J. Derksen, and N. E. Robinson. 1985. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am. J. Vet. Res. 46:2253-2258. [PubMed] [Google Scholar]
- 37.Spertini, O., F. W. Luscinskas, G. S. Kansas, J. M. Munro, J. D. Griffin, M. A. Gimbrone, Jr., and T. F. Tedder. 1991. Leukocyte adhesion molecule-1 (LAM-1, l-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J. Immunol. 147:2565-2573. [PubMed] [Google Scholar]
- 38.Stolzenberg, E. D., G. M. Anderson, M. R. Ackermann, R. H. Whitlock, and M. Zasloff. 1997. Epithelial antibiotic induced in states of disease. Proc. Natl. Acad. Sci. USA 94:8686-8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Wetering, S., S. P. G. Mannesse-Lazeroms, M. A. J. A. van Sterkenburg, M. R. Daha, J. H. Dijkman, and P. S. Hiemstra. 1997. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am. J. Physiol. 272:L888-L896. [DOI] [PubMed]
- 40.Weiss, D. J., M. C. Bauer, L. O. Whiteley, S. K. Maheswaran, and T. R. Ames. 1991. Changes in blood and bronchoalveolar lavage fluid components in calves with experimentally induced pneumonic pasteurellosis. Am. J. Vet. Res. 52:337-344. [PubMed] [Google Scholar]
- 41.Whiteley, L. O., S. K. Maheswaran, D. J. Weiss, and T. R. Ames. 1991. Alterations in pulmonary morphology and peripheral coagulation profiles caused by intratracheal inoculation of live and ultraviolet light-killed Pasteurella haemolytica A1 in calves. Vet. Pathol. 28:275-285. [DOI] [PubMed] [Google Scholar]
- 42.Whiteley, L. O., S. K. Maheswaran, and D. J. Weiss, and T. R. Ames. 1990. Immunohistochemical localization of Pasteurella haemolytica A1-derived endotoxin, leukotoxin, and capsular polysaccharide in experimental bovine pasteurella pneumonia. Vet. Pathol. 27:150-161. [DOI] [PubMed] [Google Scholar]