Abstract
For the first time, bacterial spores have been evaluated as vaccine vehicles. Bacillus subtilis spores displaying the tetanus toxin fragment C (TTFC) antigen were used for oral and intranasal immunization and were shown to generate mucosal and systemic responses in a murine model. TTFC-specific immunoglobulin G titers in serum (determined by enzyme-linked immunosorbent assay) reached significant levels 33 days after oral dosing, while responses against the spore coat proteins were relatively low. Tetanus antitoxin levels were sufficient to protect against an otherwise lethal challenge of tetanus toxin (20 50% lethal doses). The robustness and long-term storage properties of bacterial spores, coupled with simplified genetic manipulation and cost-effective manufacturing, make them particularly attractive vehicles for oral and intranasal vaccination.
This paper reports the first steps in the development of Bacillus subtilis endospores as novel vaccine vehicles. The development of improved vaccination strategies has always been of the utmost importance for a number of reasons: to provide better levels of local immunity against pathogens which enter the body primarily through the mucosal surfaces, to provide needle-less routes of administration, to offer improved safety and minimal adverse side effects, and finally, to provide economical vaccines for developing countries where suboptimal storage and transportation facilities can prevent effective immunization programs. Considerable progress has been made with the development of improved mucosal vaccination strategies as well as novel delivery systems (19). Oral immunization with soluble antigens has long been known to generate poor immune responses due to antigen degradation in the stomach, limited absorption, and tolerance. To improve mucosal immune responses, a number of unique carrier systems have been developed which fall into two general categories, nonliving and living. Nonliving systems include liposomes, microparticles, immunity-stimulating complexes, and formulations based on cholera toxin and Escherichia coli heat-labile enterotoxins (LT toxins) (4, 16). Live carrier systems include plants, bacteria, and viruses (19). Bacterial systems for heterologous antigen presentation have attracted considerable interest, but because these rely largely on live attenuated pathogens such as Salmonella and mycobacteria, considerable safety concerns remain.
The gram-positive bacterium B. subtilis has been extensively studied as a model prokaryotic system with which to understand gene regulation and the transcriptional control of unicellular differentiation (6, 18). This organism is not regarded as a pathogen and is classified as a novel food that is currently being used as a probiotic for both human and animal consumption (15). The single, distinguishing feature of this microorganism is that it produces an endospore as part of its developmental life cycle when starved of nutrients. The mature spore, when released from its mother cell, can survive in a metabolically dormant form for hundreds if not thousands of years (5). The spore offers unique resistance properties and can survive extremes of temperature, desiccation, and exposure to solvents and other noxious chemicals. These unique attributes would make the spore an attractive vehicle for delivery of heterologous antigens or, indeed, any bioactive molecule to extreme environments such as the gastrointestinal tract.
We have chosen tetanus as a model disease for these studies. Tetanus is caused by tetanus toxin, a potent neurotoxin, synthesized by Clostridium tetani. The current tetanus vaccine, while producing significant levels of protection, has several shortfalls. Firstly, preparation involves the handling of large quantities of tetanus toxin, which is a hazardous procedure; secondly, the vaccine has to be kept cold; and finally, there is a need for repeated booster immunizations. The requirement for an improved tetanus vaccine has led to the development of a number of alternative vaccines including tetanus toxin derivatives (7, 13) as well as to the use of carrier systems in which a 47-kDa fragment of the toxin referred to as tetanus toxin fragment C (TTFC) is expressed. TTFC is nontoxic and immunogenic (14), and its expression in E. coli (14), Saccharomyces cerevisiae (21), Salmonella (3), and Lactococcus lactis (20) has been shown to provide protection against tetanus toxin challenge.
For this work we have asked whether B. subtilis spores expressing a chimeric protein, CotB-TTFC, by which TTFC is displayed on the surface of the B. subtilis endospore, can generate protective immunity. Our work confirms the “proof of principle” that spores can be engineered and developed as oral (and intranasal) vaccines. The ease of genetic manipulation, long-term storage properties, and ease of production could make the B. subtilis spore an attractive second-generation vaccine vehicle.
MATERIALS AND METHODS
Preparation of spores.
B. subtilis strain RH103 (amyE::cotB-tetC) was used for all immunizations together with its isogenic ancestor, PY79 (22). RH103 has been described elsewhere (12) and carries a fusion of TTFC (47 kDa) to the C terminus of the outer spore coat protein CotB (59 kDa). The chimeric cotB-tetC gene was carried at the amyE locus of B. subtilis and was therefore in trans to the endogenous cotB gene. Sporulation of either RH103 or PY79 was made in Difco sporulation medium by the exhaustion method as described elsewhere (17). Sporulating cultures were harvested 22 h after the initiation of sporulation. Purified suspensions of spores were made as described by Nicholson and Setlow (17), with lysozyme treatment to break any residual sporangial cells followed by washing in 1 M NaCl-1 M KCl and water (two times). Phenylmethylsulfonyl fluoride was included to inhibit proteolysis. After the final suspension in water, spores were treated at 68°C for 1 h to kill any residual cells. Next, the spore suspension was titrated immediately for CFU/ml before freezing at −20°C. Using this method, we could reliably produce 6 × 1010 spores per liter of Difco sporulation medium culture. Each batch of spores prepared in this way was checked for the presence of the 106-kDa hybrid CotB-TTFC protein in extracts of spore coat protein by Western blotting using a polyclonal TTFC antiserum.
Immunofluorescence microscopy.
B. subtilis strains (PY79, RH103) were induced to sporulate by the resuspension method (17). Samples were collected at defined times after the onset of sporulation and fixed directly in the resuspension medium using the procedure described by Harry et al. (9), with the following modifications. After suspension in GTE-lysozyme (50 mM glucose, 20 mM Tris-HCl [pH 7.5], 10 mM EDTA, 2 mg of lysozyme/ml), samples (10 μl) were immediately applied to microscope coverslips (BDH) that had been treated with 0.01% (wt/vol) poly-l-lysine (Sigma). After 4 min, the liquid was aspirated from the coverslip, which was then allowed to dry completely for 2 h at room temperature. The glass coverslip was washed three times in phosphate-buffered saline (PBS) (pH 7.4), blocked for 15 min with 2% bovine serum albumin (BSA) in PBS at room temperature, and then washed nine more times. Samples were incubated with rabbit anti-CotB and mouse anti-TTFC sera for 45 min at room temperature, washed three times, and then incubated further with anti-rabbit immunoglobulin G (IgG)-fluorescein isothiocyanate and anti-mouse IgG-tetramethyl rhodamine isothiocyanate conjugates (Sigma) for 45 min at room temperature. After three washings, the coverslip was mounted onto a microscope slide and viewed under a Nikon Eclipse E600 fluorescence microscope. Images were captured using a Nikon DMX1200 digital camera, processed with Lucia GF software, and saved in TIFF format.
TTFC protein.
Recombinant TTFC was produced in E. coli BL21(DE3)(pLys) from a pET28b expression vector (Novagen) that carried the tetC gene fused to a C-terminal polyhistidine tag. High levels of expression were obtained upon induction of the bacteria, and purification of TTFC was done by passage of a cell lysate through a nickel affinity column. Eluted TTFC-His protein was checked for integrity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the concentration was determined by use of the Bio-Rad DC protein assay kit.
Indirect ELISA for detection of antigen-specific serum and mucosal antibodies.
Plates were coated with 50 μl of the specific antigen (2 μg/ml in carbonate-bicarbonate buffer) per well and left at room temperature overnight. The antigen was either extracted spore coat protein or purified TTFC protein. After blocking with 0.5% BSA in PBS for 1 h at 37°C, serum samples were applied as a twofold dilution series, starting with a 1/40 dilution in enzyme-linked immunosorbent assay (ELISA) diluent buffer (0.1 M Tris-HCl [pH 7.4], 3% [wt/vol] NaCl, 0.5% [wt/vol] BSA, 10% [vol/vol] sheep serum [Sigma], 0.1% [vol/vol] Triton X-100, 0.05% [vol/vol] Tween 20). Every plate carried replicate wells of a negative control (a 1/40 diluted preimmune serum) and a positive control (sera from mice immunized parenterally with either TTFC protein or spores). Plates were incubated for 2 h at 37°C before the addition of anti-mouse horseradish peroxidase conjugates (all obtained from Sigma, with the exception of Serotec for the subclasses). Plates were incubated for one more hour at 37°C and then developed with the substrate TMB (3,3′,5,5′-tetramethyl-benzidine; Sigma). Reactions were stopped by 2 M H2SO4. Dilution curves were drawn for each sample and end-point titers were calculated as the dilutions producing the same optical density as the 1/40 dilution of a pooled preimmune serum. Statistical comparisons between groups were made by the Mann-Whitney U test. A P value of >0.05 was considered not significant. For ELISA analysis of fecal IgA, we followed the procedure of Robinson et al. (20), using approximately 0.1 g of fecal pellets that had been suspended in PBS with BSA (1%) and phenylmethylsulfonyl fluoride (1 mM), incubated at 4°C overnight, and then stored at −20°C prior to ELISA. For each sample, the end-point titer was calculated as the dilution producing the same optical density as the undiluted preimmune fecal extract.
Immunizations.
Groups of seven or eight mice (female, C57BL/6, 8 weeks old) were dosed orally, intranasally, or intraperitoneally with suspensions of either spores expressing CotB-TTFC (strain RH103) or control spores that did not express CotB-TTFC (strain PY79). For both oral and intranasal dosings, mice were lightly anesthetized with halothane. Oral and intranasal routes employed a multiple dosing regimen used previously for optimal mucosal immunizations (2, 20). A naïve, unimmunized control group was included. Oral dosings also included a group of seven mice receiving 4 μg of purified TTFC protein per dose. The different dosing regimens were as follows.
(i) Oral immunization doses contained 1.67 × 1010 spores in a volume of 0.15 ml and were administered by intragastric gavage on days 0, 2, 4, 18, 20, 22, 34, 35, and 36. Serum samples were collected on days −1, 17, 33, and 54 and fecal samples were collected on days −2, 17, 33, and 52.
(ii) Intranasal immunization doses contained 1.11 × 109 spores in a volume of 20 μl and were administered using a micropipette on days 0, 2, 16, 17, 30, and 31. Serum samples were collected on days −1, 15, 29, and 48 and fecal samples were collected on days −1, 15, 29, and 47.
(iii) Immunization doses via the intraperitoneal route contained 1.5 × 109 spores in a volume of 0.15 ml and were administered on days 0, 14, and 28. Serum samples were taken on days −1, 7, 22, 36, and 43.
Tetanus toxin challenge.
On day 60 after the primary oral immunization, RH103-immunized mice were injected subcutaneously with a challenge dose of tetanus toxin equivalent to 10 or 20 50% lethal doses (LD50). The purified toxin (20 μg of protein/flocculation unit [Lf]) was suspended in 0.9% sterile NaCl. The LD50 of tetanus toxin was first determined experimentally to be 0.0003 Lf (i.e., 1 LD50 = 6 ng of protein) and the injection volume was 200 μl/mouse. Animals were closely monitored for signs of tetanus, and mice that developed symptoms of paralysis were humanely euthanized. Individuals showing no symptoms after 14 days were considered immune. Mice that received oral immunization by TTFC purified protein were challenged with 10 LD50. Naïve mice or mice immunized with PY79 spores were challenged with 2 LD50.
Extraction of spore coat proteins.
Spore coat proteins were extracted from suspensions of spores at high densities (>1010 spores/ml) by use of an SDS-dithiothreitol extraction buffer as described in detail elsewhere (17). Extracted proteins were assessed for integrity by SDS-PAGE and for concentration by use of the Bio-Rad DC protein assay kit.
Dissemination experiments.
BALB/c mice (female, 5 weeks old) were dosed orally with 109 spores of strain SC2362 (rrnO-lacZ cat) per dose consecutively for 5 days. SC2362 provides a Lac phenotype that is recognizable by blue colonies on nutrient agar (containing X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside]) as well as by chloramphenicol resistance (5 μg/ml; encoded by the cat gene). At times thereafter, groups of four mice were sacrificed and sample organs and tissues were dissected in the following sequence. First, fresh fecal pellets were collected, after which the animal was killed by inhalation of CO2 and decontaminated with 70% alcohol. Peritoneal macrophages were collected by injecting 3 ml of sterile PBS into the abdominal cavity, followed by gentle massaging. The peritoneal exudate was then collected by use of a 21-gauge needle and syringe and was processed immediately. The abdominal cavity was then opened and the liver was excised. The intestine was unbundled and the mesentery was removed. Next, the spleen and kidneys were collected, after which the Peyer's patches were located and excised while contamination from the intestinal lumen contents was avoided (surrounding tissues were also excised as negative controls). Finally, cervical and submandibular glands were collected. Sterile dissecting instruments were changed between organs. Samples were homogenized by use of a vortex mixer in 1 ml of PBS with 3 ml of glass beads (a mixture of 2- and 4-mm diameters) and then were plated immediately (on nutrient agar containing X-Gal and chloramphenicol) to establish total viable CFU counts or heat treated (65°C, 1 h) prior to plating to determine spore counts.
RESULTS
Surface expression of a heterologous antigen on the spore surface.
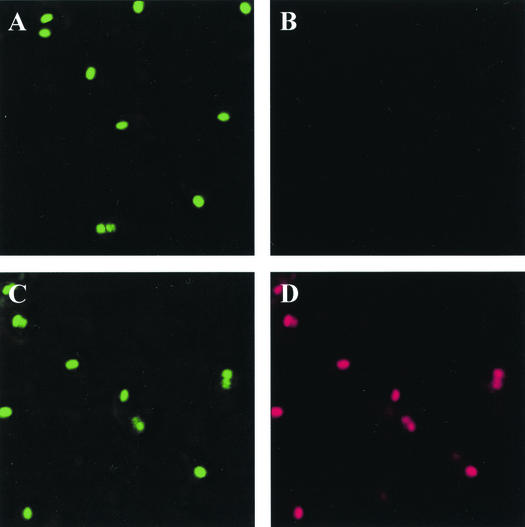
Recombinant spores (RH103) expressing TTFC fused as a chimera to the spore coat protein CotB have been described elsewhere (12). Before assessing the immune responses to spores expressing TTFC, we verified that TTFC was surface exposed by use of immunofluorescence as shown in Fig. 1. By using polyclonal sera against TTFC and CotB we could detect TTFC in sporulating cultures harvested at hour 5 following the initiation of spore formation. We could also detect CotB and TTFC at hours 4 and 6 (data not shown). Sporangial cells were used for labeling since other studies have shown that high levels of background labeling prohibit the use of released endospores (9). Our results showed intact ovoid forespores that were labeled by anti-TTFC serum. Labeling with CotB antiserum detected CotB in both recombinant and nonrecombinant spores (Fig. 1A and C). We could also detect some TTFC as well as CotB in the mother cells and not attached to the coat. We believe this may occur if CotB (and hence CotB-TTFC) must self-assemble or be assembled with other coat proteins before deposition onto the spore coat. This has been attributed to other spore coat proteins such as CotA (5) and may be a general feature of spore coat synthesis and assembly.
FIG. 1.
Detection of presence of CotB and TTFC by immunofluorescence. Sporulation of B. subtilis strains was induced by the resuspension method (17), and samples were taken 5 h after the onset of sporulation. (A and B) PY79 (wild type). (C and D) RH103 (CotB-TTFC-expressing strain). Samples were labeled with rabbit anti-CotB and mouse anti-TTFC antisera, followed by anti-rabbit IgG-fluorescein isothiocyanate (green) and anti-mouse IgG-tetramethyl rhodamine isothiocyanate (red) conjugates.
Serum anti-TTFC responses following intraperitoneal injection of recombinant spores expressing TTFC.
Before commencing oral and intranasal immunizations we used a pilot study to evaluate the immunogenicity of recombinant spores. Groups of eight C57 mice were injected (intraperitoneally) with recombinant or nonrecombinant spores. Our immunization schedule used a standard regimen of three injections (containing 1.5 × 109 spores/dose) of either recombinant RH103 spores (expressing hybrid CotB-TTFC) or nonrecombinant PY79 spores. In a previous study (12), RH103 spores were shown to carry approximately 9.75 × 10−5 pg of TTFC polypeptide/spore, so our immunizing dose would contain 0.15 μg of TTFC. Immunization with RH103 spores resulted in peak anti-TTFC IgG titers of 1.5 × 103 (as determined by indirect ELISA; data not shown), which were significantly different (P < 0.05) from those for control groups (1.1 × 102 for PY79 and 0.8 × 101 for naïve mice), demonstrating that TTFC was stably expressed and appropriately immunogenic when displayed on the spore surface.
Serum anti-TTFC responses following oral and intranasal immunization.
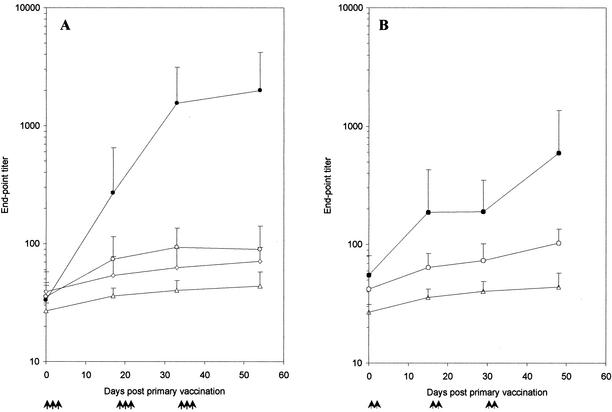
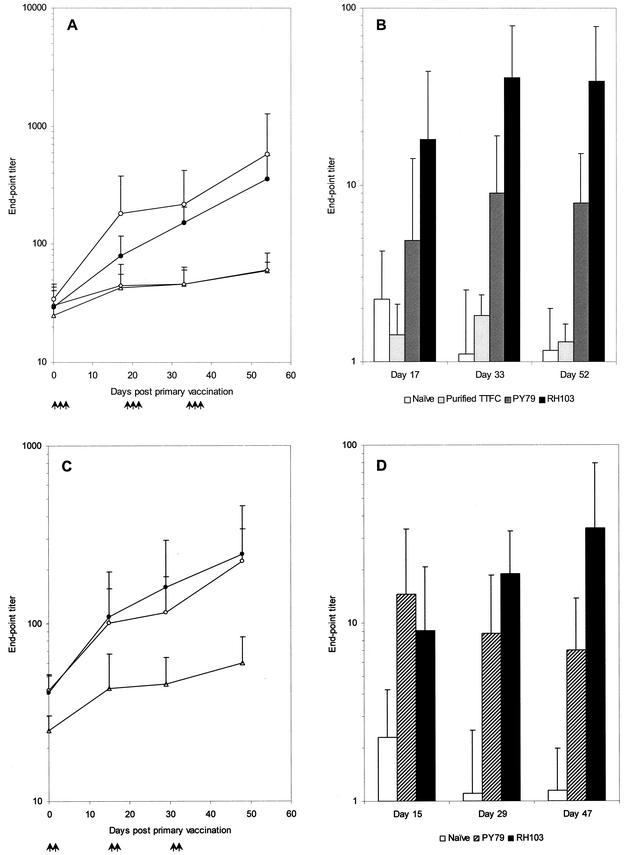
To test for induction of mucosal and systemic responses, groups of seven mice were immunized either orally (1.67 × 1010 spores/dose; 1.65 μg of TTFC/dose) or intranasally (1.11 × 109 spores/dose; 0.11 μg of TTFC/dose). Note that, technically, larger doses could not be given by the nasal route. As shown in Fig. 2A, oral immunization of mice with RH103 (CotB-TTFC) spores gave titers greater than 103 by day 33, significantly above (P < 0.05) those of mice dosed with nonrecombinant spores (PY79), mice given purified TTFC protein (4 μg/dose), or the naïve control group. TTFC protein was not used as a control for the intranasal route since previous work has shown that TTFC delivered nasally (with a low dose, i.e., less than 10 μg/dose) is not immunogenic (4). Somewhat lower levels of TTFC-specific IgG end-point titers were found at day 48 following intranasal immunization (Fig. 2B). Our data showed that for either route, the titers for the naïve and nonrecombinant immunizations were not significantly different (P > 0.05). Groups administered spores expressing TTFC fused to CotB responded with significantly higher TTFC-specific IgG titers than their corresponding control groups (P < 0.05) from day 33 onwards for oral groups and from day 29 for intranasal groups. By work not shown here, we have also found that RH103 spores given orally with or without cholera toxin (type Inaba 569B; 0.33 μg/dose) caused no significant difference in anti-TTFC IgG titers.
FIG. 2.
Systemic responses after mucosal immunizations. Serum anti-TTFC specific IgG responses after oral (A) or intranasal (B) immunization with recombinant B. subtilis spores expressing CotB-TTFC. Groups of seven mice were immunized (↑) orally or intranasally with spores expressing CotB-TTFC (RH103) (•) or nonrecombinant spores (PY79) (○). Doses of 1.67 × 1010 spores were used for the oral route and of 1.1 × 109 spores were used for the intranasal route, and individual serum samples from groups were tested by ELISA for TTFC-specific IgG. Sera from a naïve control group (▵) and a group orally immunized with 4 μg of purified TTFC protein per dose (◊) were also assayed. Data are presented as arithmetic means and error bars are standard deviations.
Serum anti-TTFC antibody isotypes.
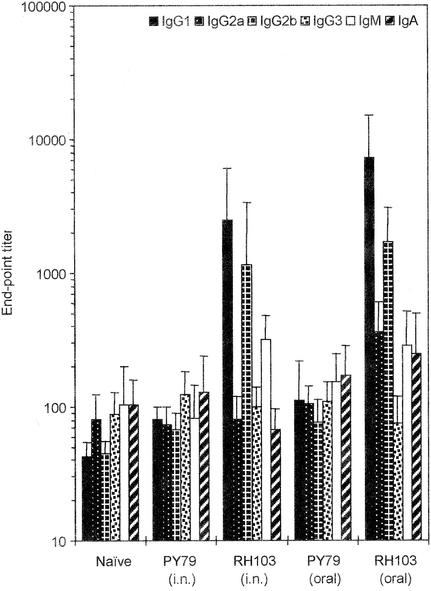
Sera from mice immunized mucosally were also examined for the presence of TTFC-specific IgG, IgA, and IgM antibody isotypes as well as the IgG1, IgG2a, IgG2b, and IgG3 subclasses (Fig. 3). Mice immunized orally with RH103 spores expressing CotB-TTFC showed high levels, at day 54, of the IgG1 and IgG2b isotypes. For the IgG1, IgG2a, and IgG2b subclasses, the mean titers were significantly different from baseline titers for the two control groups, (i) naïve mice and (ii) mice immunized with nonrecombinant spores (P < 0.05). Little change was observed with the IgG3, IgM, and IgA subclasses. For mice immunized intranasally, the sera at day 48 showed a predominance of the IgG1, IgG2b, and IgM subclasses. For these subclasses, titers were significantly higher than in the control groups (P < 0.05). In contrast, no significant variation (P > 0.05) in any of the isotypes was seen between groups administered nonrecombinant spores and the naïve group.
FIG. 3.
Antibody isotype profiles. Anti-TTFC antibody isotype profiles on day 54 post-oral immunization or day 48 post-intranasal (i.n.) immunization with recombinant spores expressing CotB-TTFC (RH103) or nonrecombinant B. subtilis spores (PY79) as described in the legend to Fig. 2. TTFC-specific IgG1, IG2a, IgG2b, IgG3, IgM, and IgA isotypes were determined by indirect ELISA. Sera from a naïve control group were also assayed. The end-point titer was calculated as the dilution of serum producing the same optical density as a 1/40 dilution of a pooled preimmune serum. Data are presented as arithmetic means and error bars are standard deviations.
Mucosal anti-TTFC IgA responses.
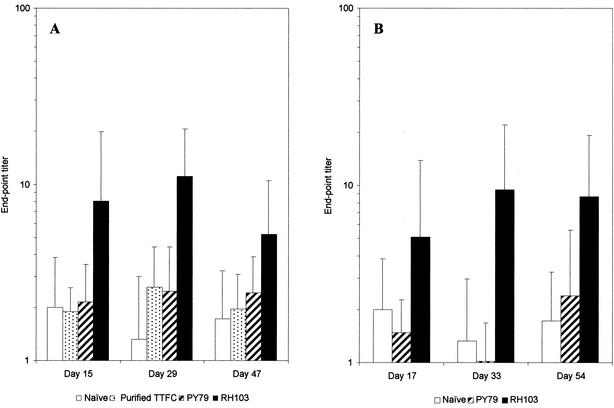
Fresh fecal pellets from mice immunized orally or intranasally were tested for the presence of TTFC-specific secretory IgA (sIgA) by ELISA (Fig. 4). Immunization with spores expressing CotB-TTFC by either route elicited clear TTFC-specific sIgA responses. In groups of mice immunized orally or intranasally, TTFC-specific sIgA titers peaked at day 33 (Fig. 4). The end-point titers of fecal TTFC-specific sIgA were shown to be significantly higher than those of the control groups (nonrecombinant spores and naïve groups) (P < 0.05), between which there was no significant difference (P > 0.05).
FIG. 4.
TTFC-specific fecal IgA responses. Groups of seven mice were immunized orally (A) or intranasally (B) with recombinant spores expressing CotB-TTFC (RH103) or nonrecombinant spores (PY79) as described in the legend to Fig. 2. Fresh fecal pellets were collected from these immunized mice as well as from a naïve group and were tested for the presence of TTFC-specific IgA as described in Materials and Methods. The end-point titer was calculated as the dilution of the fecal extract producing the same optical density as the undiluted preimmune fecal extract. Data are presented as arithmetic means and error bars are standard deviations.
Protection against tetanus toxin challenge after oral immunization.
The high IgG titers (>103) in serum observed after oral immunization were at potentially protective levels. In order to test the biological activity of the elicited antitoxin response and the associated level of protection, mice orally immunized with CotB-TTFC-expressing B. subtilis spores (RH103) were challenged with a lethal dose of tetanus toxin (10 or 20 LD50) given subcutaneously (Table 1). Mice were fully protected against the challenge of 10 LD50. Of eight mice challenged with 20 LD50, one had clear symptoms after 72 h. All naïve mice and mice immunized with wild-type B. subtilis spores (PY79) showed clear signs of tetanus within 72 h after a challenge of 2 LD50. Oral immunization with purified TTFC protein (4 μg/dose) gave no protection against 10 LD50, and all mice showed clear symptoms of tetanus within 24 h. The systemic antibody responses elicited via oral immunization with B. subtilis spores expressing CotB-TTFC were therefore protective.
TABLE 1.
Protection of mice against lethal systemic challenge with tetanus toxin after oral immunizationa
| Immunization group | Toxin challenge dose (LD50) | No. of survivors/total |
|---|---|---|
| Naïve | 2 | 0/5 |
| PY79 spores | 2 | 0/8 |
| TTFC purified protein | 10 | 0/8 |
| RH103 (CotB-TTFC) spores | 10 | 8/8 |
| 20 | 7/8 |
Groups of eight mice were immunized orally with 1.67 × 1010 spores of B. subtilis or 4 μg of TTFC purified protein on days 0, 2, 4, 18, 20, 22, 34, 35, and 36 before being injected subcutaneously with a challenge dose of tetanus toxin on day 60. Individuals developing no symptoms after 14 days were considered immune.
Antispore responses.
In addition to anti-TTFC responses, antispore IgG and sIgA responses following oral and intranasal immunization were determined (Fig. 5). Oral immunization with both CotB-TTFC-expressing spores (RH103) and nonrecombinant spores (PY79) produced systemic spore coat-specific IgG levels (Fig. 5A) that were significantly higher than those of the naïve group (P < 0.05). Lower, but still significant (P < 0.05), levels of spore coat-specific IgG titers were observed after intranasal immunization whether recombinant or nonrecombinant spores were used (Fig. 5C).
FIG. 5.
Antispore serum IgG and mucosal IgA responses. Groups of seven mice were immunized (↑) by the oral (A and B) or intranasal (C and D) route with recombinant spores expressing CotB-TTFC (•) or nonrecombinant spores (○) as described in the legend to Fig. 2. Individual samples were tested by indirect ELISA for B. subtilis spore coat-specific serum IgG (A and C) or spore coat-specific fecal IgA (B and D). Sera from a naïve group (▵) were also assayed. The end-point IgG titer was calculated as the dilution of serum producing the same optical density as a 1/40 dilution of a pooled preimmune serum. The end-point IgA titer was calculated as the dilution of the fecal extract producing the same optical density as the undiluted preimmune fecal extract. Data are presented as arithmetic means and error bars are standard deviations.
Spore coat-specific sIgA levels observed in the feces of orally immunized mice (Fig. 5B) showed substantial responses against recombinant spores. Interestingly, these levels were significantly higher (P < 0.05) than when nonrecombinant spores were used for immunization. When the intranasal route (Fig. 5D) was used for immunization, a similar profile of spore coat-specific sIgA levels was observed, with a reduction of IgA levels over time in mice dosed with nonrecombinant spores. Again, the levels of spore coat-specific sIgA were significantly higher than those in naïve mice (P < 0.05).
Dissemination of spores.
Inbred BALB/c mice were dosed daily with 109 spores/dose for 5 consecutive days. Pilot studies had shown that this consecutive dosing regimen was sufficient to establish recoverable and statistically relevant counts. At times points after the final dosing, groups of four mice were sacrificed and key lymphoid organs were dissected. In addition, feces were collected and homogenized and counts were determined. Total viable counts and heat-resistant counts were determined for homogenized tissues and feces. Recovered viable counts are given in Table 2 and show recovery of bacteria from intestinal Peyer's patches and mesenteric lymph nodes, suggesting interaction with the gut-associated lymphoid tissue. Most interesting was the recovery of viable counts from the submandibular glands and cervical lymph nodes, with no recovery of significant counts from the liver and spleen. Recovery of bacteria from head and neck tissues with little or no recovery from widely disseminated systemic sites suggests that spores may have crossed the rhinopharyngeal mucosa. Counts in feces declined steadily as bacteria were cleared from the gastrointestinal tract, although little difference between total and spore counts was observed. As has been shown previously (10), this does not indicate that spores do not germinate but rather that germinated spores (vegetative cells) cannot survive long-term in fecal material.
TABLE 2.
Recovery of B. subtilis from target organs after oral immunizationa
| Day | Recovery of B. subtilis (mean CFU ± SD) from:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fecesb
|
Peyer's patches/mesenteric lymph nodes
|
Submandibular gland/cervical lymph nodes
|
Spleen
|
Peritoneal macrophages
|
Liver
|
Kidneys
|
||||||||
| Total count | Spore count | Total count | Spore count | Total count | Spore count | Total count | Spore count | Total count | Spore count | Total count | Spore count | Total count | Spore count | |
| 1 | 1.68 × 106 ± 1.1 × 106 | 1.73 × 106 ± 1.0 × 106 | 227 ± 134 | 166 ± 124 | 105 ± 71 | 42 ± 29 | NS | 0 | 75 ± 40 | 45 ± 31 | NS | NS | NS | NS |
| 2 | 5.25 × 105 ± 4.5 × 105 | 7.96 × 105 ± 6.7 × 105 | 27 ± 18 | 17 ± 20 | 117 ± 9 | 65 ± 26 | NS | NS | 45 ± 17 | 45 ± 38 | ND | ND | ND | ND |
| 3 | 1.79 × 105 ± 1.1 × 105 | 1.36 × 105 ± 1.0 × 105 | NS | NS | 15 ± 10 | 22 ± 18 | 25 ± 19 | NS | 30 ± 27 | 36 ± 24 | ND | ND | ND | ND |
| 5 | 4.61 × 104 ± 0.7 × 104 | 4.41 × 104 ± 0.8 × 104 | NS | NS | 405 ± 59 | 110 ± 87 | 0 | NS | 56 ± 50 | 33 ± 30 | NS | ND | NS | NS |
| 7 | 2.99 × 104 ± 1.4 × 104 | 1.21 × 104 ± 1.1 × 104 | NS | NS | 126 ± 39 | 39 ± 15 | NS | NS | 30 ± 27 | NS | NS | ND | NS | NS |
| 9 | 1.44 × 103 ± 1.1 × 103 | 1.08 × 103 ± 1.0 × 103 | NS | NS | 29 ± 20 | 19 ± 15 | NS | NS | NS | NS | 0 | 0 | NS | NS |
Groups of four BALB/c mice were dosed orally with 1 × 109 spores of B. subtilis strains SC2362 (rrnO-lacZ) for five consecutive days (total dose, 5 × 109 spores). Total counts, no heat treatment; spore counts, samples treated at 65°C for 1 h. ND, not determined; NS, not significant (<10 viable CFU per sample).
Counts for feces are per gram.
DISCUSSION
This paper reports the first detailed analysis of the immune responses elicited following oral and intranasal immunization of mice with recombinant spores. Our results strongly suggest that spores have potential as carriers of heterologous antigens and, most importantly, for oral and possibly nasal vaccination. The recombinant spores used for this study carried most of TTFC exposed on the outermost layers of the spore. As a chimeric protein fused to the CotB spore coat protein, the TTFC moiety would be expected to be exposed on the surface of the spore. As such, it is both surprising and encouraging that the recombinant protein could survive transit across the stomach barrier. This would have been the hypothetical obstacle to oral immunization using bacterial spores. Although TTFC has been shown to be surface exposed when fused to CotB (12), the large size of this molecule may enable surface expression by disruption and protrusion through the coat layers while a smaller antigen or an epitope may be hidden. In other work we have shown that the TTFC moiety of CotB-TTFC spores is stable in water for significant periods of time (>10 weeks) and does not appear to degrade (data not shown). Any future use of spores as vaccine vehicles, though, would require careful analysis of the size limitations of antigen presentation, antigen stability, and optimal routes for expression on a case-by-case basis.
Our immunization strategy followed an initial characterization of immune responses after parenteral immunization to demonstrate that TTFC was suitably immunogenic when fused to CotB. Administration to the mucosa was performed via both the oral and intranasal routes. The oral route used three consecutive doses of spores which were adapted from other oral immunization strategies requiring multiple doses (2, 20). Although our dose for oral immunizations was quite high, employing nine doses of 1.67 × 1010 spores, we do not appear to be able to achieve measurable TTFC-specific systemic responses with 10-fold lower doses of spores expressing CotB-TTFC (data not shown). Similar studies using bacterial delivery systems also require large and consecutive doses of bacteria, for example, seven doses of 5 × 109 L. lactis organisms were required to confer protection against challenge with tetanus toxin (20).
It is clear that spores themselves elicit both systemic and mucosal immune responses. The systemic responses to spore components were generally lower than the TTFC-specific responses which would be beneficial for a vaccine vehicle. Interestingly, there were noticeable differences in the antispore responses of serum IgG and mucosal sIgA depending on whether recombinant spores (expressing CotB-TTFC) or nonrecombinant spores were used. Spore-specific IgG levels were somewhat higher when nonrecombinant spores were used for dosing. This suggests that when dosed with recombinant spores expressing CotB-TTFC, the systemic immune response was biased towards the foreign antigen rather than the spore. This was in contrast to the mucosal sIgA responses: spore-specific sIgA levels were higher when recombinant spores were used for immunization than when nonrecombinant spores were used. We interpret this as evidence that there must exist a level of tolerance against the spore in the mucosa.
Mucosal responses were evident from the significant levels of TTFC-specific fecal IgA responses. For tetanus, though, only systemic IgG responses are required for immunity, so it is both encouraging and interesting that administration of recombinant spores at the mucosa can provide protection at a distant site of induction. The observed stimulation of sIgA responses, although not important for protection against tetanus, would be important and necessary for immunity against a mucosal pathogen and demonstrates that spores have the potential to be developed as mucosal vaccines.
In other work it has been shown that the majority of B. subtilis spores transit the mouse gastrointestinal tract within 24 h (10). Although we have evidence that at least a small percentage of spores may germinate in the jejunum and ileum, it appears that most are excreted (1), as might be expected for this soil organism. Undoubtedly, animals are naturally exposed to Bacillus species, which may in part explain their relatively low immunogenicity, although this does not appear to impair their responses to spores expressing TTFC in this study. Our analysis of spore dissemination following oral delivery showed low numbers of viable bacteria in the Peyer's patches and mesenteric lymph nodes. This shows that spores are able to interact with the gut-associated lymphoid tissue and must have transited the mucosa. Spores are approximately 1.2 μm in length so are of sufficient size to be taken up by M cells and then transported into the Peyer's patches where they could interact with macrophages, dendritic cells, or B cells before being transported to the efferent lymph nodes. We have also shown that spores can cross the rhinopharyngeal mucosa. This is remarkably similar to the fate of Mycobacterium tuberculosis when given orally to mice (11). It is likely that these counts result from coprophagia, but in any event we can state that translocation across the mucosa can occur at more than one site. Finally, these dissemination experiments show that spores do not exhibit any significant increase in numbers in the tissues and organs we examined. This suggests that spores are not able to grow and colonize once they have transited the mucosa and that they are gradually cleared from internal organs. We cannot rule out germination of intact spores once they have crossed the mucosa, though, and this aspect is of course potentially important for cellular immunity.
Having established that spores can be used for vaccination, we must address the important question of whether cellular immunity is invoked since this is important for a good vaccine. Bacillus anthracis spores have been shown to germinate within alveolar macrophages (8); in the gastrointestinal tract, it is possible that spores might undergo a similar fate within phagocytic cells of the Peyer's patches. Analysis of the cytokine profiles of draining lymph node CD4+ T-helper cells following antigenic stimulation in vitro as well as lymphoproliferation studies will be required to determine the involvement of cell-mediated immunity, and these studies are in progress.
The most encouraging aspect of this work is that oral immunization with recombinant spores was able to confer protection against an otherwise lethal dose of tetanus toxin at levels comparable to those in other studies using noncolonizing bacteria (20). In principle, the level of protection observed in these experiments might be suitable for an oral booster vaccine. While further experiments to optimize dosing regimens for the oral and nasal routes may generate higher levels of immunity, what is important is that spores have potential as vaccine vehicles. It is worth emphasizing that the number of different proteins found on the spore coat could enable more than one antigen to be expressed. Coupled with the ease of genetic manipulation in B. subtilis, this versatility makes spore vaccines particularly attractive for exploitation. The long-term storage properties of spores and ease of production offer a number of additional advantages over comparable systems, while the generally-regarded-as-safe status assigned to this bacterial species and its current use as a probiotic could enable spore vaccines to be licensed. In conclusion, the discovery that these uniquely robust bioparticles can be used for oral vaccination provides a major step forward in the search for new and efficacious vaccines.
Acknowledgments
This work was supported by grants from the Wellcome Trust to S.M.C. and N.F. and the European Union to S.M.C. and E.R.
We thank Chris Hale for expert assistance in fluorescent microscopy and Philip Beesley and Layla Hughes for assistance with the challenge experiments.
Editor: J. D. Clements
REFERENCES
- 1.Casula, G., and S. M. Cutting. 2002. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 68:2344-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challacombe, S. J. 1983. Salivary antibodies and systemic tolerance in mice after oral immunisation with bacterial antigens. Ann. N. Y. Acad. Sci. 409:177-192. [DOI] [PubMed] [Google Scholar]
- 3.Chatfield, S. N., I. G. Charles, A. J. Makoff, M. D. Oxer, G. Dougan, D. Pickard, D. E. Slater, and N. F. Fairweather. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology 10:888-892. [DOI] [PubMed] [Google Scholar]
- 4.Douce, G., C. Turcotte, I. Cropley, M. Roberts, M. Pizza, M. Domenghini, R. Rappuoli, and G. Dougan. 1995. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc. Natl. Acad. Sci. USA 92:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueiredo, D., C. Turcotte, G. Frankel, Y. Li, O. Dolly, G. Wilkin, D. Marriott, N. Fairweather, and G. Dougan. 1995. Characterization of recombinant tetanus toxin derivatives suitable for vaccine development. Infect. Immun. 63:3218-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidi-Rontani, C., M. Weber-Levy, E. Labruyere, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9-17. [DOI] [PubMed] [Google Scholar]
- 9.Harry, E. J., K. Pogliano, and R. Losick. 1995. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J. Bacteriol. 177:3386-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoa, T. T., L. H. Duc, R. Isticato, L. Baccigalupi, E. Ricca, P. H. Van, and S. M. Cutting. 2001. The fate and dissemination of B. subtilis spores in a murine model. Appl. Environ. Microbiol. 67:3819-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoft, D. F., and M. Gheorghiu. 1996. Mucosal immunity induced by oral administration of bacille Calmette-Guerin, p. 269-279. In H. Kiyono, P. L. Ogra, and J. R. McGhee (ed.), Mucosal vaccines. Academic Press, San Diego, Calif.
- 12.Isticato, R., G. Cangiano, H. T. Tran, A. Ciabattini, D. Medaglini, M. R. Oggioni, M. De Felice, G. Pozzi, and E. Ricca. 2001. Surface display of recombinant proteins on Bacillus subtilis spores. J. Bacteriol. 183:6294-6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, Y., P. Foran, N. F. Fairweather, A. de Pavia, U. Weller, G. Dougan, and J. O. Dolly. 1994. A single mutation in the recombinant light chain of tetanus toxin abolishes its proteolytic activity and removes the toxicity seen after reconstitution with native heavy chain. Biochemistry 33:7014-7020. [DOI] [PubMed] [Google Scholar]
- 14.Makoff, A. J., S. P. Ballantine, A. E. Smallwood, and N. F. Fairweather. 1989. Expression of tetanus toxin fragment C in E. coli: its purification and potential as a vaccine. Bio/Technology 7:1043-1046. [Google Scholar]
- 15.Mazza, P. 1994. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll. Chim. Farm. 133:3-18. [PubMed] [Google Scholar]
- 16.Michalek, S. M., D. T. O'Hagan, S. Gould-Fogerite, G. F. Rimmelzwaan, and A. D. M. E. Osterhaus. 1999. Antigen delivery systems: nonliving microparticles, liposomes, cochleates, and ISCOMS, p. 759-778. In J. Mestecky, P. L. Ogra, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology, 2nd ed. Academic Press, San Diego, Calif.
- 17.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 18.Piggot, P. J., and J. G. Coote. 1976. Genetic aspects of bacterial endospore formation. Bacteriol. Rev. 40:908-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozzi, G., and J. M. Wells (ed.). 1997. Gram-positive bacteria. Vaccine vehicles for mucosal immunisation. Springer-Verlag, Berlin, Germany.
- 20.Robinson, K., L. M. Chamberlain, K. M. Schofield, J. M. Wells, and R. W. F. Le Page. 1997. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 15:653-657. [DOI] [PubMed] [Google Scholar]
- 21.Romanos, M. A., A. J. Makoff, N. F. Fairweather, K. M. Neesley, D. E. Slater, F. B. Rayment, M. M. Payne, and J. J. Clare. 1991. Expression of tetanus toxin fragment C in yeast: gene synthesis is required to eliminate fortuitous polyadenylation sites in AT-rich DNA. Nucleic Acids Res. 19:1461-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youngman, P., J. Perkins, and R. Losick. 1984. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 12:1-9. [DOI] [PubMed] [Google Scholar]