Abstract
Immunization with a pneumococcal conjugate vaccine (PNC) containing serotype 19F induces cross-reactive antibodies to 19A in mice and human infants. Active immunization with PNC and passive immunization with serum samples from infants vaccinated with PNC containing serotype 19F, but not serotype 19A, protected against lung infection caused by both serotypes in a murine model.
Infection caused by Streptococcus pneumoniae (pneumococcus) is a major cause of morbidity and mortality worldwide, especially among elderly people and young children, and it is a leading cause of bacterial pneumonia, bacteremia, meningitis, and otitis media (8, 17, 20). More than 90 different pneumococcal serotypes can be distinguished by their polysaccharide (PS) capsule, but approximately 90% of clinical episodes of invasive pneumococcal infections in humans are caused by 23 pneumococcal serotypes. A 23-valent pneumococcal PS (PPS) vaccine has been available in the most recent decades, and studies have confirmed the clinical efficacy of the PPS vaccine against pneumococcal infections in adults (26). Nonetheless, immunization with native PPS is ineffective for the group at highest risk, children under 2 years of age (5). In contrast, PPS protein conjugate vaccines (PNC) have been shown to be immunogenic in infants and children (2, 4, 27, 28) and to induce immunologic memory (1, 22). Induction of protective immunity against invasive pneumococcal infections and otitis media has been reported in young infants (3, 6). Recently, a 7-valent PNC was licensed in the United States and Europe. These seven serotypes are responsible for 50 to 70% of all invasive pneumococcal infections, depending on geographic location (9, 10). It is of some concern that infections and carriage due to serotypes not included in the vaccine may increase after introduction of a 7-, 9-, or 11-valent PNC (19). Nevertheless, several serotypes are structurally similar and thus cross-reactive. It has been demonstrated that pneumococcal serotype 6B induces functional antibodies to the related serotype 6A (21, 24, 29, 31). Because of its higher chemical stability, serotype 6B was included as a representative for serogroup 6 in the PNC (23). Similarly, serotype 19F was chosen as the representative of serogroup 19, which may induce cross-reactive antibodies to serotype 19A (18). The protective capacity of immunization with serotype 19F against invasive infections caused by serotype 19A is unclear.
In the present study, the cross-reactivity of PPSs of serotypes 19F and 19A was assessed in mice by active immunization with a tetanus protein (TT)-serotype 19F PNC (19F-TT) or by passive immunization with serum samples obtained from infants vaccinated with an 11-valent PNC containing serotype 19F but not 19A. To assess vaccine-induced protection against pneumococcal pneumonia caused by the homologous serotype 19F or the cross-reactive serotype 19A, a well-established murine model of intranasal (i.n.) pneumococcal infection was used and efficacy against lung infection was evaluated (25).
The infant serum samples used in this study were obtained with informed consent from the parents, and the study was approved by the National Bioethics Committee of Iceland. The animal experiments were authorized by the Experimental Animal Committee of Iceland and complied with Animal Welfare Act 15/94.
Antibody response to serotypes 19F and 19A after active immunization of mice with 19F-TT.
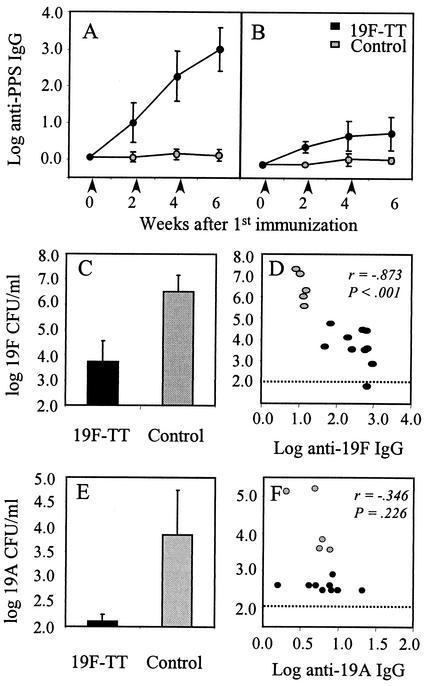
Adult NMRI mice (M&B AS, Ry, Denmark) were immunized subcutaneously with a predefined dose of 0.5 μg of 19F-TT (Aventis Pasteur, Marcy l'Etoile, France) in 200 μl of saline injected into the scapular girdle region three times at 2-week intervals. Mice injected with sterile saline were used as controls. The mice were bled from the tail vein before each immunization and 2 weeks after the last immunization for the measurement of PPS-specific immunoglobulin G (IgG) antibodies in serum by enzyme-linked immunosorbent assay (ELISA) as previously described (11). Low 19F-specific IgG titers were induced after the first immunization (Fig. 1A), but a significant increase in titers compared to those of the saline-injected control mice (P < 0.001) was observed after the second and third doses of 19F-TT. Furthermore, the immunization of mice with 19F-TT elicited IgG antibodies to serotype 19A (Fig. 1B), as previously demonstrated in humans and experimental animals (7, 21). Despite significant production of 19A-specific IgG after 19F-TT immunization, the titers were significantly lower than those against serotype 19F. To demonstrate the specificity of antibodies binding to serotype 19A PS, selected sera were tested in a competitive ELISA. Incubation of serum samples with purified 19A PS or 19F PS (1,000 μg/ml of undiluted serum) reduced the binding of IgG antibodies to 19A by 66 to 93% or 81 to 92%, respectively, which demonstrated that a proportion of the antibodies elicited by 19F-TT immunization truly cross-react with serotype 19A.
FIG. 1.
(A and B) Immunization of mice with 19F-TT induces antibodies to both serotype 19F (A) and serotype 19A (B). Mice were immunized with three doses of 19F-TT at times indicated by arrows. Data are presented as log mean ELISA units per milliliter, and the error bars show the standard deviations of the means. (C to F) The protective efficacy of 19F-TT immunization against pneumococcal lung infection caused by serotype 19F (C) or serotype 19A (E) is presented as the mean CFU in the lungs per each group 24 h after i.n. challenge. A relationship was observed between the number of CFU in the lungs and the levels of serotype-specific IgG antibodies in serum for serotype 19F (D) but not for serotype 19A (F). Black symbols, mice immunized with 19F-TT; gray symbols, unimmunized controls; dotted lines, detection limits for CFU.
Efficacy of 19F-TT immunization against pneumococcal lung infection caused by serotype 19F or 19A.
For the evaluation of vaccine-induced protection against pneumococcal infection by serotype 19F or 19A, we used a previously established immunization protocol (13, 14). Mice were immunized twice with 19F-TT and challenged i.n. with S. pneumoniae of either serotype 19F or 19A 2 weeks after the second immunization. Mice injected with sterile saline were used as controls. The mice were sacrificed 24 h after challenge. Their lungs were removed, and the number of pneumococcal CFU in the lung homogenate was evaluated as previously described (25). The time interval between infection and examination was chosen based on observations from previous studies which showed that 24 h is the optimal interval for the evaluation of vaccine-induced protection against pneumococcal lung infection in this mouse model (12-14). Challenge i.n. with ∼2.5 × 106 CFU of S. pneumoniae serotype 19F induced severe lung infection in the saline-injected controls (Fig. 1C). The mice immunized with 19F-TT had a significantly lower number of 19F pneumococci in their lungs than the saline-injected controls (P < 0.001) (Fig. 1C), and the pneumococcal density in the lungs correlated with the level of anti-19F IgG antibodies in the serum (r = −0.873; P < 0.001) (Fig. 1D). Although 19F-TT immunization elicited a vigorous 19F-specific IgG response, it was not sufficient to completely clear serotype 19F pneumococci from the lungs 24 h after challenge in this murine pneumococcal infection model. This is in contrast to what has been observed for other pneumococcal serotypes in this mouse model, in which relatively low PPS-specific IgG levels were sufficient to completely clear the lung infection (12-14). Thus, it is clear, as has previously been demonstrated in efficacy trials (3, 6) and in opsonophagocytic assays in vitro (21), that the protective levels of PPS-specific IgG antibodies differ between pneumococcal serotypes.
In another set of experiments, 19F-TT-immunized mice were challenged i.n with ∼8.5 × 106 CFU of S. pneumoniae of the related serotype 19A in order to evaluate vaccine-induced cross-protection. Challenge with serotype 19A caused significant lung infection in unimmunized controls (Fig. 1E), although serotype 19A was less virulent and the bacterial density was lower (P < 0.001) than that in control mice challenged with serotype 19F (Fig. 1C). Furthermore, the density of serotype 19A pneumococci in the lungs of the mice immunized with 19F-TT was lower than that of the unimmunized controls (P < 0.001) (Fig. 1E), which indicates that 19F-TT immunization induced protective antibodies against the related serotype 19A. However, no relationship was found between the level of 19A-specific IgG antibodies in serum and the density of S. pneumoniae serotype 19A pneumococci in the lungs (r = −0.346; P = 0.226) (Fig. 1F), which is probably explained by the low, although significant, antibody response to serotype 19A. Furthermore, the lack of significant correlation might be due to the influence of antibodies other than IgG, such as IgM and IgA, which are known to contribute to protection against pneumococcal infections (15).
Antibody responses to serotypes 19F and 19A in infants vaccinated with an 11-valent PNC.
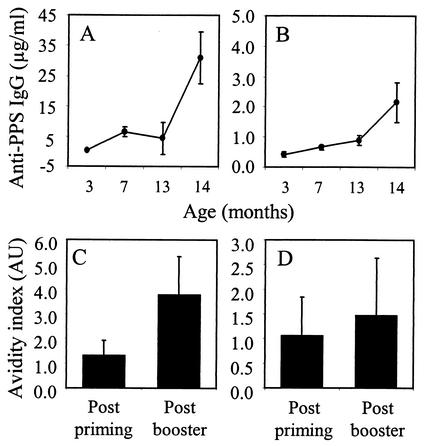
Fifty serum samples were obtained from a clinical study in which infants were given a primary series of vaccinations with an 11-valent PNC (Aventis Pasteur) at 3, 4, and 6 months of age and a booster vaccination at 13 months of age (S. T. Sigurdardottir, T. Gudnason, S. Kjartansson, K. Davidsdottir, K. G. Kristinsson, G. Ingolfsdottir, M. Yaich, O. Leroy, and I. Jonsdottir, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-50, 2000). The PNC contained serotype 19F but not serotype 19A. IgG antibodies to 19F and 19A were measured by ELISA as described previously (16). Vaccinated infants showed a significant IgG response to serotype 19F after a primary immunization series (P < 0.001) and after a booster immunization (P < 0.001) (Fig. 2A). Furthermore, a significant response was observed against serotype 19A after both the prime (P = 0.007) and booster immunizations (P < 0.001) (Fig. 2B). As observed after active immunization of mice with 19F-TT, significantly higher levels of PPS-specific IgG antibodies were elicited against serotype 19F than against serotype 19A (P < 0.001) and the 19F- and 19A-specific IgG levels correlated significantly (r = 0.449; P = 0.001). Vaccination with PNC induced an increase in the avidity of IgG antibodies both to serotype 19F (P < 0.001) (Fig. 2C) and to serotype 19A (P = 0.046) (Fig. 2D), indicating the generation of memory B cells specific for both PS serotypes upon immunization with a PNC containing only serotype 19F. The opsonophagocytic activity (OA) of the serum samples against serotype 19F pneumococci was measured as previously described (30). After the booster immunization, there was an increase in OA compared to that observed postprimary immunization (mean, 42.52 versus 91.97 arbitrary units; P < 0.001) and the OA correlated with anti-19F IgG titers (r = 0.834; P < 0.001). OA against serotype 19A was not measured due to sample limitations.
FIG. 2.
Vaccination of infants with an 11-valent tetanus protein-diphtheria toxoid PNC induces significant IgG antibody responses to both serotype 19F (A) and serotype 19A (B), with increased avidity to both serotype 19F (C) and serotype 19A (D). In panels A and B, the results are expressed as geometric mean concentrations and the error bars show 95% confidence intervals. The ages of the infants in the study are indicated on the x axis. In panels C and D, the results are expressed as mean avidity indexes and the error bars show the standard deviation of the mean.
Protective efficacy of serum samples from vaccinated infants against serotype 19F and 19A infections.
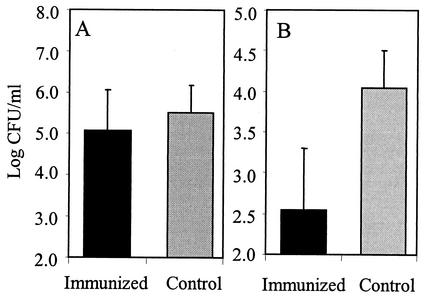
The infant serum samples obtained 1 month after the booster immunization were used to passively immunize mice (with 150 μl of undiluted serum) intraperitoneally 3 h prior to i.n. challenge with S. pneumoniae of serotype 19F. Portions of each serum sample were injected into two mice, and mice injected with sterile saline were used as controls. Again the mice were sacrificed 24 h after challenge, and their lungs were removed for evaluation of pneumococcal density (measured in CFU). As in previous experiments (Fig. 1C), challenge with serotype 19F pneumococci caused severe lung infections in the saline-injected control mice (Fig. 3A). Passive immunization with serum samples from PNC-vaccinated infants reduced the number of CFU in the lungs (P = 0.057). In agreement with results obtained from the active-immunization experiments, a negative correlation was found between the number of 19F CFU and the 19F-specific IgG titers in the infant serum samples (r = −0.356; P < 0.001). In addition, the number of 19F CFU correlated significantly with OA (r = −0.373; P < 0.001).
FIG. 3.
Passive immunization with serum samples obtained from 14-month-old human infants protects mice against lung infection caused by i.n. challenge with serotype 19F (A) or 19A (B) pneumococci. Results are expressed as mean log CFU per milliliter of lung homogenate (n = 100), and the error bars show the standard deviations of the means.
The same serum samples were used to passively immunize mice (150 μl per mouse) intraperitoneally 3 h prior to i.n. challenge with S. pneumoniae of serotype 19A. Control mice were injected with sterile saline. Whereas all of the control mice had pneumococci of serotype 19A in their lungs, 60 of 100 mice immunized with the serum samples had no detectable pneumococci in their lungs and the number of 19A CFU in the lungs of the passively immunized mice was significantly lower than that of the saline-injected controls (P < 0.001) (Fig. 3B). However, no relationship was found between the number of 19A CFU and the 19A-specific IgG titers in the infant serum samples (r = −0.086; P = 0.394). As in the active immunization experiments, this lack of correlation may be due to antibodies not measured in the ELISA, such as IgM and IgA, which may contribute to the level of protection (15). In addition, we have previously shown that for serotype 6A at low IgG antibody levels, protection was associated with opsonic activity to 6A (24).
In conclusion, we have shown here that immunization with TT-conjugated serotype 19F PPS vaccine induces protective 19F-specific IgG antibodies in both human infants and mice and that some of these antibodies cross-react and protect against serotype 19A infections in this murine pneumococcal pneumonia model. However, the response against serotype 19A remained significantly lower than the response against 19F both in vaccinated infants and in mice. It remains to be demonstrated whether the levels and functional activity of the cross-reactive antibodies induced in infants by this conjugate will be sufficient to provide protection against diseases caused by the cross-reactive serotype 19A.
Acknowledgments
We thank Stefania Bjarnarson, Alda Birgisdottir, and Fifa Konradsdottir (Department of Immunology, Landspitali—University Hospital) for excellent technical assistance. The work of the investigators Thorolfur Gudnason, Katrin Davidsdottir, Sveinn Kjartansson, and Karl G. Kristinsson is duly acknowledged. We appreciate the facilities provided by the Department of Microbiology, Landspitali—University Hospital.
This work was supported by Aventis Pasteur and the Student Innovation Fund of the University of Iceland, Reykjavik.
Editor: J. N. Weiser
REFERENCES
- 1.Åhman, H., H. Kayhty, H. Lehtonen, O. Leroy, J. Froeschle, and J. Eskola. 1998. Streptococcus pneumoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr. Infect. Dis. J. 17:211-216. [DOI] [PubMed] [Google Scholar]
- 2.Åhman, H., H. Käyhty, P. Tamminen, A. Vuorela, F. Malinoski, and J. Eskola. 1996. Pentavalent pneumococcal oligosaccharide conjugate vaccine PncCRM is well-tolerated and able to induce an antibody response in infants. Pediatr. Infect. Dis. J. 15:134-139. [DOI] [PubMed] [Google Scholar]
- 3.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. R. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Dagan, R., R. Melamed, O. Zamir, and O. Leroy. 1997. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr. Infect. Dis. J. 16:1053-1059. [DOI] [PubMed] [Google Scholar]
- 5.Douglas, R. M., J. C. Paton, S. J. Duncan, and D. J. Hansman. 1983. Antibody response to pneumococcal vaccination in children younger than five years of age. J. Infect. Dis. 148:131-137. [DOI] [PubMed] [Google Scholar]
- 6.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Käyhty, P. Karma, R. Kohberger, G. R. Siber, and P. H. Mäkela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 7.Giebink, G. S., J. D. Meier, M. K. Quartey, C. L. Liebeler, and C. T. Le. 1996. Immunogenicity and efficacy of Streptococcus pneumoniae polysaccharide-protein conjugate vaccines against homologous and heterologous serotypes in the chinchilla otitis media model. J. Infect. Dis. 173:119-127. [DOI] [PubMed] [Google Scholar]
- 8.Gray, B. M., and H. C. Dillon. 1986. Clinical and epidemiologic studies of pneumococcal infection in children. Pediatr. Infect. Dis. J. 5:201-207. [DOI] [PubMed] [Google Scholar]
- 9.Hausdorff, W. P., J. Bryant, C. Kloek, P. R. Paradiso, and G. R. Siber. 2000. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin. Infect. Dis. 30:122-140. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsen, H., B. C. Adarna, D. Schulz, R. Rappuoli, and I. Jonsdottir. 2001. Characterization of the antibody response to pneumococcal glycoconjugates and the effect of heat-labile enterotoxin on IgG subclasses after intranasal immunization. J. Infect. Dis. 183:1494-1500. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsen, H., S. Bjarnarson, G. Del Giudice, M. Moreau, C.-A. Siegrist, and I. Jonsdottir. 2002. Intranasal immunization with pneumococcal conjugate vaccines with LT-K63, a nontoxic mutant of heat-labile enterotoxin, as adjuvant rapidly induces protective immunity against lethal pneumococcal infections in neonatal mice. Infect. Immun. 70:1443-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakobsen, H., E. Saeland, S. Gizurarson, D. Schulz, and I. Jónsdóttir. 1999. Intranasal immunization with pneumococcal polysaccharide conjugate vaccines protects mice against invasive pneumococcal infections. Infect. Immun. 67:4128-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakobsen, H., D. Schulz, M. Pizza, R. Rappuoli, and I. Jónsdóttir. 1999. Intranasal immunization with pneumococcal polysaccharide conjugate vaccines with nontoxic mutants of Escherichia coli heat-labile enterotoxins as adjuvants protects mice against invasive pneumococcal infections. Infect. Immun. 67:5892-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janoff, E. N., C. Fasching, J. M. Orenstein, J. B. Rubins, N. L. Opstad, and A. P. Dalmasso. 1999. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Investig. 104:1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Käyhty, H., H. Ahman, P. R. Rönnberg, R. Tillikainen, and J. Eskola. 1995. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J. Infect. Dis. 172:1273-1278. [DOI] [PubMed] [Google Scholar]
- 17.Klein, J. O. 1981. The epidemiology of pneumococcal disease in infants and children. Rev. Infect. Dis. 3:246-253. [DOI] [PubMed] [Google Scholar]
- 18.Lee, C.-J., and B. A. Fraser. 1980. The structures of cross-reactive types 19 (19F) and 57 (19A) pneumococcal capsular polysaccharides. J. Biol. Chem. 225:6847-6853. [PubMed] [Google Scholar]
- 19.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171-1176. [DOI] [PubMed] [Google Scholar]
- 20.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-809. [DOI] [PubMed] [Google Scholar]
- 21.Nahm, M. H., J. V. Olander, and M. Magyarlaki. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176:698-703. [DOI] [PubMed] [Google Scholar]
- 22.Obaro, S. K., Z. Huo, W. A. Banya, D. C. Henderson, M. A. Monteil, A. Leach, and B. M. Greenwood. 1997. A glycoprotein pneumococcal conjugate vaccine primes for antibody responses to a pneumococcal polysaccharide vaccine in Gambian children. Pediatr. Infect. Dis. J. 16:1135-1140. [DOI] [PubMed] [Google Scholar]
- 23.Robbins, J. B., R. Austrian, C.-J. Lee, S. C. Rastogi, G. Schiffman, J. Henrichsen, P. H. Mäkela, C. V. Broome, R. R. Facklam, R. H. Tiesjema, and J. C. Parke. 1983. Considerations for formulating the second-generation pneumococcal polysaccharide vaccine with emphasis on the cross-reactive types within groups. J. Infect. Dis. 148:1136-1159. [DOI] [PubMed] [Google Scholar]
- 24.Saeland, E., H. Jakobsen, G. Ingolfsdottir, S. T. Sigurdardottir, and I. Jonsdottir. 2001. Serum samples from infants vaccinated with a pneumococcal conjugate vaccine, PncT, protect mice against invasive infection caused by Streptococcus pneumoniae serotypes 6A and 6B. J. Infect. Dis. 183:253-260. [DOI] [PubMed] [Google Scholar]
- 25.Saeland, E., G. Vidarsson, and I. Jonsdottir. 2000. Pneumococcal pneumonia and bacteremia model in mice for the analysis of protective antibodies. Microb. Pathog. 29:81-91. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro, E. D., A. T. Berg, R. Austrian, D. Schroeder, V. Parcells, A. Margolis, R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 27.Shinefield, H. R., S. Black, P. Ray, I. Chang, E. Lewis, B. Fireman, J. Hackell, P. R. Paradiso, G. R. Siber, R. Kohberger, D. Madore, F. Malinowski, A. Kimura, C. Le, I. Landaw, J. Aguilar, and J. Hansen. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757-763. [DOI] [PubMed] [Google Scholar]
- 28.Sigurdardottir, S. T., G. Ingolfsdottir, K. Davidsdottir, T. Gudnason, S. Kjartansson, K. G. Kristinsson, F. Bailleux, O. Leroy, and I. Jonsdottir. 2002. Immune response to octavalent diphtheria- and tetanus-conjugated pneumococcal vaccines is serotype- and carrier-specific: the choice for a mixed carrier vaccine. Pediatr. Infect. Dis. J. 21:548-554. [DOI] [PubMed] [Google Scholar]
- 29.Vakevainen, M., C. Eklund, J. Eskola, and H. Käyhty. 2001. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines in infants. J. Infect. Dis. 184:789-793. [DOI] [PubMed] [Google Scholar]
- 30.Vidarsson, G., I. Jonsdottir, S. Jonsson, and H. Valdimarsson. 1994. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J. Infect. Dis. 170:592-599. [DOI] [PubMed] [Google Scholar]
- 31.Yu, X., B. M. Gray, S. Chang, J. I. Ward, K. Edwards, and M. H. Nahm. 1999. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J. Infect. Dis. 180:1569-1576. [DOI] [PubMed] [Google Scholar]