Abstract
Vibrio cholerae normally inhabits aquatic habitats but can cause a severe diarrheal illness in humans. Its arsenal of virulence factors includes a secreted hemagglutinin (HA) protease. An HA protease-deficient mutant of V. cholerae was isolated and designated E7946 mpc. E7946 mpc was found to contain a point mutation in the luxO quorum-sensing regulator. In accordance with this finding, E7946 mpc exhibits a defect in quorum sensing. The mutant luxO allele [luxO(Con)] produces a protein with a leucine-to-glutamine substitution at amino acid 104. Transfer of the luxO(Con) allele to an otherwise wild-type background was sufficient to eliminate HA protease expression; conversely, deletion of luxO(Con) from E7946 mpc restored protease activity. We demonstrate that LuxO(Con) constitutively represses the transcription of hapR, an essential positive regulator of HA protease. Interestingly, strains harboring luxO(Con) form enhanced biofilms, and enhanced biofilm formation does not appear to be dependent on reduced HA protease expression. Taken together, the results confirm the role of LuxO as a central “switch” that coordinately regulates virulence-related phenotypes such as protease production and biofilm formation.
Toxigenic Vibrio cholerae is the infectious agent of cholera, a severe diarrheal disease with 293,113 reported cases and 10,586 deaths worldwide in a typical year (1998), mostly in developing countries (28). The primary virulence factor of toxigenic V. cholerae is cholera toxin (CT), encoded by the ctxAB operon, which resides on a filamentous phage, CTXΦ (26). The toxin-coregulated pilus (TCP) has also been shown to be an essential colonization factor (12). V. cholerae strains lacking functional CT have been engineered for use as vaccines; however, some of these strains still exhibit residual reactogenicity in volunteers (25), suggesting that V. cholerae harbors toxic factors in addition to CT. In vitro studies (19, 29) have suggested that the secreted hemagglutinin (HA) protease of V. cholerae, encoded by the hapA gene (11), might be one such factor, as might a hemolysin, HlyA (23), and the recently described RTX toxin (8, 10, 18).
The environmental signals that promote expression of CT, HA protease, and other virulence factors remain largely undefined. Two membrane-localized complexes (TcpP/H and ToxR/S) have been shown to promote the transcription of toxT, a potent activator of CT and TCP transcription (5). Transcription of tcpP/H is itself activated by the synergistic action of AphA and AphB (24).
Recent work has also established that the LuxO protein, a σ54 dependent activator (15), is required for CT production in vitro and for colonization in an in vivo infant-mouse model (21, 30). LuxO was first characterized in Vibrio harveyi (1), where it has been shown to play a crucial role in “quorum sensing,” the ability of bacteria to regulate gene expression in response to changes in cell density. LuxO in V. cholerae is thought to be phosphorlyated and active at low cell density, where it represses (directly or indirectly) the transcription of hapR (30) (Fig. 1). At high cell density, LuxO is dephosphorylated and inactive and hapR is transcribed. HapR is an essential activator of the hapA protease gene (14) and also downregulates the expression of tcpPH. Recent evidence suggests that HapR decreases tcpPH transcription indirectly, by repressing the transcription of aphA (17).
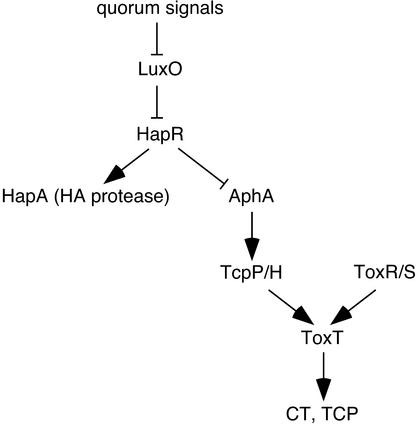
FIG. 1.
Simplified scheme of quorum-sensing signaling in V. cholerae. During bacterial growth, cell-permeable chemical signals (autoinducers) are produced and accumulate to threshold levels at high cell densities. These signals lead to the dephosphorylation of LuxO, rendering LuxO inactive. At low cell densities, LuxO is phosphorylated and inhibits hapR transcription by an unknown mechanism (possibly indirectly, through stimulating the production of a hapR transcriptional repressor). HapR is required for the transcription of hapA, which encodes the secreted extracellular hemagglutinin (HA) protease. In addition, HapR represses the transcription of aphA, an upstream activator of tcpP/H transcription. TcpP/H and ToxR/S together activate toxT transcription, which in turn positively regulates virulence gene (e.g., CT and TCP) expression.
Previously, a protease-defective mutant of V. cholerae El Tor strain E7946 was generated by Tn5 mutagenesis (3, 19). The protease mutant was designated E7946 mpc (for “multiple protease control”) since preliminary experiments suggested that mpc was defective in the secretion of multiple proteases (3). Here we report that mpc harbors a point mutation in luxO that constitutively activates LuxO function, represses protease production, and enhances biofilm formation by V. cholerae.
MATERIALS AND METHODS
Strains and plasmids.
Streptomycin-resistant isolates of V. cholerae El Tor strains E7946, C6706, and N16961 were used. For selection of V. cholerae, antibiotics were used at the following concentrations: streptomycin at 100 μg/ml, tetracycline at 1.5 μg/ml, kanamycin at 50 μg/ml, and ampicillin at 100 μg/ml. For selection of Escherichia coli, tetracycline was used at 10 μg/ml and ampicillin was used at 100 μg/ml. V. cholerae and E. coli were grown in Luria-Bertani (LB) medium at 37 or 30°C as indicated. E7946 mpc was isolated from a Tn5 mutagenesis experiment (3) and has been partially characterized (19). C6706 ΔluxO and C6706 ΔhapR have been described previously (30). The plasmid used to generate an in-frame deletion of luxO in E7956 mpc was the gift of M. Miller and B. Bassler. N16961 ΔhapA was the gift of K. Fullner.
A construct to transfer the point mutation in luxO from E7946 mpc into E7946 and C6706 wild-type strains was generated by amplifying a 2,017-bp segment of luxO (containing the point mutation) from E7946 mpc by using primers 5′1414Sac (ATAGAGCTCGGCTTACCAAGTGTTGACTCC) and 3′3431Xho (ATACTCGAGTTCTTCACCGATCTCCTTCG). The PCR product was digested with SacI and XhoI and cloned into pKEK229 (4), a derivative of pCVD442 (6) that has the polylinker of pWSK30 (27). The resulting vector was transferred by homologous recombination onto the E7946 and C6706 chromosome by mating and selection for ampicillin and streptomycin resistance. Vector sequences were removed by standard sucrose counterselection, and sequencing of PCR products amplified from the chromosome was used to confirm that the single nucleotide change had been introduced.
The suicide plasmid encoding a transcriptional fusion of lacZ to the hapR promoter has been described (30) and was introduced into the chromosome of the indicated strains by homologous recombination (single crossover). The chromosomal lacZ gene was deleted from the strains by standard methods using pDLT (9), a derivative of pCVD442. The pACYC184-toxS plasmid has been described (22). A control plasmid (pACYC184) was obtained from New England Biolabs (Beverly, Mass.). The pBBR-hapR plasmid for expressing hapR has been described previously (30) and is a derivative of pBBR1-MCS4 (16). A control plasmid, identical to pBBR-hapR except that it expressed the nonfunctional hapR allele from V. cholerae N16961, was also generated. The hapA open reading frame (VCA0865, were encoding HA protease) and 302 bp of upstream sequence were amplified with the 5′ primer ATAGAATTCAAATAGTTGATACATCCTAGAAACTG and the 3′ primer ATACTCGAGGTACGTCAATCCCCTGTTGATACTG, digested with XhoI and EcoRI, and cloned into pBBR1-MCS4. The isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible hapR expression plasmid pJZ146 has been described previously (30), and the corresponding control plasmid pMALc2x was obtained from New England Biolabs. All constructs were confirmed by sequencing.
Zymograms, milk plates, and azocasein protease assay.
Zymograms were performed with cell-free culture supernatants as described previously (30). Briefly, 0.2% gelatin (Difco, Detroit, Mich.) was incorporated into a 7% polyacrylamide gel for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which was used to separate unboiled supernatant proteins. After the SDS was rinsed away, the in-gel protease activity, which resulted in clearing of the gelatin substrate, was detected by staining the gel with Coomassie blue. For milk plates, single colonies were patched onto LB agar plates containing 1% (wt/vol) nonfat powdered milk and allowed to grow overnight at 37°C. Protease activity was detected by clearing of the opaque milk proteins incorporated into the plate. The azocasein protease assay was performed as previously described (2). One azocasein unit is equal to the amount of enzyme releasing 0.01 optical density at 442 nm (OD442) unit of colored substrate per h.
Luminescence.
Overnight cultures (grown at 30°C in LB medium) of strains harboring the V. harveyi luxCDABE luciferase operon (carried on the pBB1 cosmid [21]) were diluted 1:1,000 and allowed to grow with aeration at 30°C. Luminescence was detected with a Berthold LB9507 luminometer.
Biofilm assays.
Overnight cultures of V. cholerae were diluted into 1 ml of LB medium in autoclaved 12- by 75-mm borosilicate glass tubes and incubated at 22°C without agitation. After the indicated time, planktonic cells were washed away with distilled water and the remaining biofilm-associated cells were stained with 1% crystal violet. The tubes were rinsed three times and photographed. To quantify biofilm formation, stained biofilms were solubilized with 1 ml of dimethyl sulfoxide and OD at 570 nm determined.
RESULTS AND DISCUSSION
We sought to characterize a protease-deficient mutant of V. cholerae that had previously been isolated and designated E7946 mpc. Zymogram analysis demonstrates that E7946 mpc lacks expression of the secreted HA protease but still expresses several less active proteases (Fig. 2A). Southern blot analysis of E7946 mpc genomic DNA indicated that E7946 mpc harbors two Tn5 insertions (data not shown). Arbitrary PCR suggested that one Tn5 insertion is in the gene encoding ToxS, a well-characterized signaling protein essential for CT production by V. cholerae (22). Inverse PCR (13) was used to show that the second Tn5 insertion is in VC2699 (dcuA), a putative transporter of C4 dicarboxylic acids. Interestingly, however, extensive deletion and complementation analysis demonstrated that neither of the Tn5 insertions (individually or together) were responsible for the protease defect of E7946 mpc (data not shown).
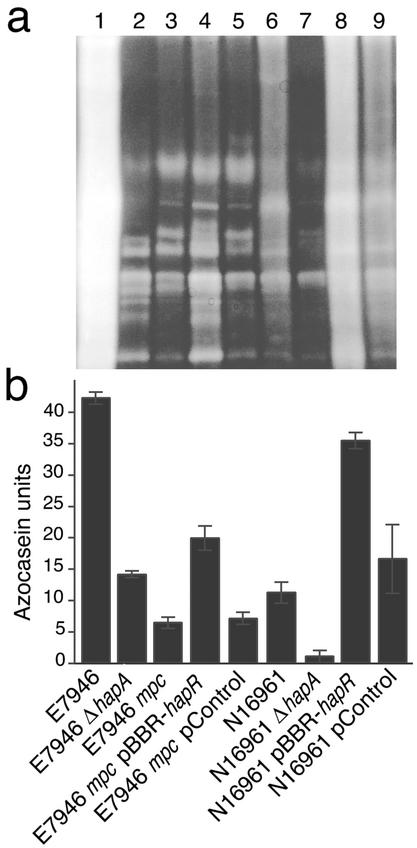
FIG. 2.
Reduced extracellular protease activity in E7946 mpc. (A) Zymogram in-gel protease assay. Cell-free supernatants from overnight cultures of various strains were run on gelatin-impregnated SDS-PAGE gels. After separation of supernatant proteins, protease-mediated digestion of the gelatin was allowed to proceed and was detected by staining of the gel with Coomassie blue. Strains 1 to 9 are arranged in the same order as the strains in panel B: lane 1, E7946; lane 2, E7946 ΔhapA; lane 3, E7946 mpc; lane 4, E7946 mpc carrying pBBR-hapR; lane 5, E7946 mpc carrying pBBR-Control; lane 6, N16961; lane 7, N16961 ΔhapA; lane 8, N16961 carrying pBBR-hapR; lane 9, N16961 carrying pBBR-Control. In this assay, the HA protease (HapA) runs as a smear (compare E7946 and E7946 ΔhapA). pBBR-hapR is a plasmid expressing hapR from E7946. pBBR-Control is a similar plasmid but expressing the nonfunctional (frame-shifted) hapR from N16961. (B) Cell-free supernatants from overnight cultures of indicated strains were assayed for digestion of azocasein in triplicate. Error bars indicate standard deviation.
Previous work established that protease production in V. cholerae is under the control of a quorum-sensing signaling circuit (21, 30) (Fig. 1). We therefore hypothesized that E7946 mpc might harbor a novel mutation in a quorum-sensing gene. This hypothesis was supported by the observation that E7946 mpc exhibited a >1,000-fold defect in expression of the quorum-regulated luciferase operon (luxCDABE) transferred from V. harveyi (see below).
Since HapR is a critical activator of hapA (14) and controls other quorum-regulated genes of V. cholerae (30), we attempted to restore protease production in E7946 mpc with a hapR expression plasmid (pBBR-hapR). The pBBR-hapR plasmid only weakly restored protease production in E7946 mpc as assessed by zymogram analysis (Fig. 2A) and a quantitative azocasein protease assay (Fig. 2B). As a control, we found that pBBR-hapR significantly increased protease production by N16961, a V. cholerae strain that is a natural hapR mutant (30) (Fig. 2), and also fully restored protease production to hapR mutants of V. cholerae E7946 and C6706 (data not shown). These results, in combination with sequencing of the hapR gene from E7946 mpc, suggested that mpc is not a hapR mutant. A plasmid expressing hapA under the control of the lac promoter (pBBR-hapA) fully restored protease production by E7946 mpc, implying that E7946 mpc did not have a defect in protease translation or secretion (data not shown). The hapA gene of E7946 mpc was sequenced (including 170 bp of promoter sequence) and was found to be identical to that of wild-type E7946. Taken together, the above results suggested that the defect in E7946 mpc might be upstream of hapR and might result from a constitutively active allele of luxO, a known negative regulator of hapR (30).
We sequenced the luxO and luxU genes from E7946 and E7946 mpc and discovered a single nucleotide change at nucleotide 311 of luxO (T to an A) that results in a leucine-to-glutamine change at amino acid 104 of the LuxO protein. We identified the LuxO protein from E7946 mpc as LuxO(Con). Work with V. harveyi has established that constitutively active alleles of luxO can be generated by mutation of the site at which LuxO is phosphorylated (e.g., D47E) or at a second site (e.g., F94W) which is thought to switch LuxO to an “open” and constitutively active conformation (7). The mutation observed in E7946 mpc maps near the second site and may therefore result in a constitutively active LuxO protein.
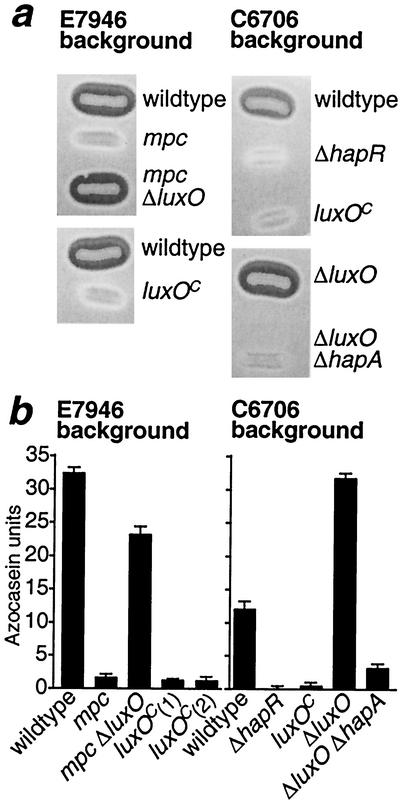
To prove that E7946 mpc expresses a constitutively active form of luxO, we conducted two experiments. First, we deleted luxO(Con) from the E7946 mpc background and found that the resultant E7946 mpc ΔluxO strain produces normal or even elevated levels of protease (Fig. 3). This finding confirms that the Tn5 insertions in toxS and dcuA that are still harbored by E7946 mpc ΔluxO are probably not responsible for the protease-deficient phenotype. Second, we transferred the luxO(Con) allele into clean wild-type E7946 and C6706 backgrounds. The single point mutation in luxO(Con) was sufficient to eliminate protease production by E7946 and C6706 (Fig. 3). Taken together, our results demonstrate that the L104Q substitution in LuxO(Con) is a necessary and sufficient cause of the protease-deficient phenotype of E7946 mpc.
FIG. 3.
(A) Milk plate assay for protease activity. Single colonies of the isolated strains were patched to agar plates containing 1% nonfat milk, incubated overnight at 37°C, and photographed against a black background. Protease activity is detected by clearing of the opaque milk proteins. luxOC indicates the luxO allele from E7946 mpc (encoding the L104Q substitution). (B) Cell-free supernatants from cultures of indicated strains were assayed for digestion of azocasein in triplicate. Error bars indicate standard deviation. Two independent isolates of E7946 luxO(Con), designated luxOC(1) and luxOC(2), were tested.
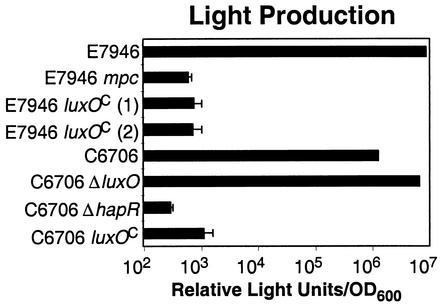
This conclusion was supported by an analysis of light production by various strains carrying the pBB1 cosmid (Fig. 4). pBB1 contains the luciferase (luxCDABE) operon from V. harveyi, and previous work has demonstrated that this operon is quorum regulated in V. cholerae (21). At high cell density (12 h of growth), wild-type E7946 and C6706 strains carrying pBB1 produce significant amounts of light whereas luxO(Con) mutants of E7946 or C6706 exhibit almost a 10,000-fold defect in light production (Fig. 4). Strains harboring luxO(Con) thus recapitulate the constitutively “dark” phenotype of hapR null mutants and exhibit a phenotype that is the converse of the constitutively “bright” luxO null mutants.
FIG. 4.
Luminescence of V. cholerae strains harboring a cosmid containing the luxCDABE operon from V. harveyi. The indicated strains were diluted in triplicate and allowed to grow for 12 h at 30°C. The results are representative of three experiments. luxOC, LuxO(Con).
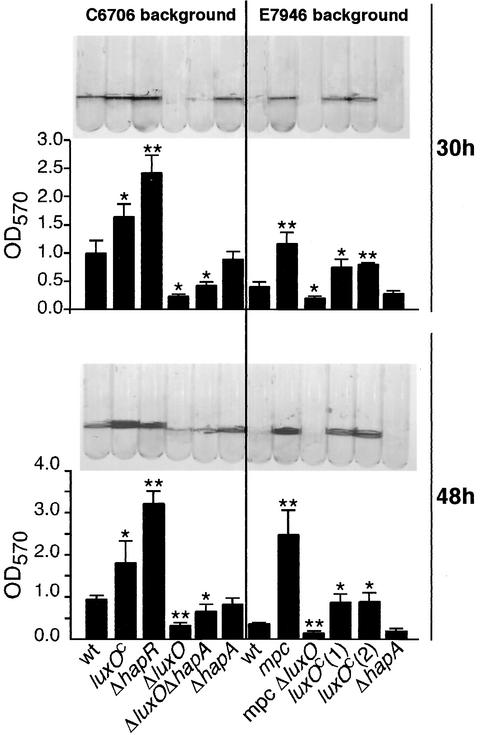
During the course of these analyses, we noted that overnight cultures of E7946 mpc often contained aggregated bacterial biomass, in contrast to E7946, which formed relatively uniform cell suspensions. We therefore suspected that the E7946 mpc strain might have an altered propensity for biofilm formation. We tested the ability of E7946 mpc to form a biofilm on the wall of a glass culture tube and found that E7946 mpc consistently formed thicker biofilms than did the parental E7946 strain (Fig. 5). To confirm that the biofilm phenotype was due to the mutation in luxO and not some other alteration in E7946 mpc, we tested derivatives of E7946 and C6706 harboring the constitutive luxO(Con) allele and found that these strains also formed thicker biofilms than did the wild-type strains (Fig. 5). luxO(Con) was necessary for the enhanced biofilm formation by E7946 mpc, since deletion of luxO from E7946 mpc eliminated biofilm formation. The phenotype of luxO(Con) strains resembled that of hapR mutant strains (Fig. 5) (30), as expected, since a constitutively active LuxO should always repress hapR.
FIG. 5.
Biofilm production on glass tubes in static cultures incubated at room temperature. After the indicated time, planktonic cells were rinsed away with distilled water, and the remaining adherent biofilm-associated cells were stained with crystal violet and photographed. The amount of biofilm formation for each strain was quantified by solubilizing the stained biofilm with DMSO and measuring the OD570. Each strain was tested in quadruplicate at each time point. Error bars indicate standard deviation. A single asterisk (*) indicates that biofilm formation was significantly different (P < 0.05) from the corresponding parental wild-type (wt) strain, whereas a double asterisk (**) indicates a P of <0.005. luxOC indicates the luxO allele from E7946 mpc (encoding the L104Q substitution). Two independent isolates of E7946 luxO(Con), designated luxOC(1) and luxOC(2), were tested. The results are representative of three experiments.
The reason why luxO null mutants fail to produce significant biofilms remains under investigation. We tested the possibility that increased protease production in luxO mutants interfered with biofilm formation by examining biofilm formation in a ΔluxO ΔhapA double mutant (Fig. 5). This strain resembled the single ΔluxO mutant and failed to produce normal biofilms, suggesting that overproduction of proteases is not solely responsible for the biofilm defect of luxO mutants; in addition, ΔhapA mutants of C6706 or E7946 did not produce significantly thicker biofilms than did their wild-type parental strains (Fig. 5). Therefore, it seems unlikely that enhanced biofilm formation by strains harboring the constitutive luxO(Con) allele is due solely to repression of protease production in these strains. Interestingly, ΔluxO ΔhapA mutants consistently produced slightly but significantly (P < 0.05) thicker biofilms than did ΔluxO mutants (Fig. 5). Thus, it remains possible that HA protease plays a minor role in biofilm formation, at least in the luxO mutant background. We are investigating whether exopolysaccharide genes or other factors are the primary target of LuxO with respect to biofilm formation.
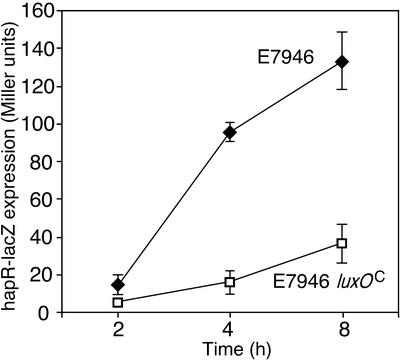
We wished to dissect further the mechanism by which LuxO interferes with the function of hapR. Results discussed above raised the possibility that LuxO might act posttranscriptionally on HapR, since expression of hapR from the pBBR-hapR plasmid failed to complement fully the E7946 mpc protease deficiency (although pBBR-hapR did correct a hapR deletion) (Fig. 2). In contrast, previous work (30) demonstrated that luxO deficiency resulted in increased hapR transcription at low cell densities. To test whether constitutive luxO acts transcriptionally on hapR, we transferred a hapR-lacZ transcriptional fusion into E7946 and E7946 luxO(Con) derivatives from which the chromosomal lacZ gene had been deleted. We found that the hapR gene was transcriptionally repressed in the strain harboring the LuxO(Con) protein (Fig. 6). The reason why the pBBR-hapR plasmid fails to restore protease production to luxO(Con) strains may be that this construct contains 200 bp of sequence upstream of the hapR start codon and that this 200 bp sequence may be the target of the transcriptional repression mediated by LuxO. This hypothesis is not yet conclusively demonstrated; however, it is worth noting that another hapR expression plasmid (pJZ146) (30), which lacks this 200-bp upstream sequence and instead drives hapR expression from a tac promoter, fully restored protease production to luxO(Con) strains (data not shown).
FIG. 6.
hapR transcription is repressed by constitutive LuxO activity. The chromosomal lacZ gene was deleted from the indicated strains, and a transcriptional hapR-lacZ fusion was introduced at the chromosomal hapR locus. Overnight cultures were diluted into fresh LB medium and allowed to regrow, and samples were taken at the indicated time points for β-galactosidase assays (20). luxOC, LuxO(Con).
It should also be noted that although HA protease accumulates at high cell densities and is regulated by LuxO (30; also see above), we have not presented direct evidence demonstrating that HA protease expression is regulated by quorum sensing. In fact, a recent paper (2) demonstrated that supplementation of cultures with spent culture media (presumably containing autoinducers) had no effect on HA protease production by strain C7258. In contrast, hapA expression was induced by nutrient limitation and repressed by glucose (2), suggesting that expression of HA protease at high cell densities may be due to nutrient limitation rather than to the accumulation of quorum signals. Indeed, a thorough genetic analysis of the quorum-sensing systems in V. cholerae (21) suggests that nonquorum signals may feed into the LuxO/HapR signaling pathway.
Our results add to our understanding of the quorum-sensing signaling cascade in V. cholerae. This cascade appears to be central to a number of important virulence-related phenotypes, including CT production, protease secretion, and biofilm formation. Previous work (30) demonstrated that a luxO mutant formed poor biofilms, overproduced protease, and was avirulent in vivo. The present work complements and extends these findings by demonstrating that a constitutively active luxO allele enhances biofilm formation and inhibits protease production. We demonstrate that constitutively active luxO represses the transcription of hapR. The identification of a novel single amino acid substitution that leads to a constitutively active form of LuxO will be invaluable in understanding the structural basis of LuxO activation. Taken together, our results place LuxO as a central regulator that controls a switch between two distinct phenotypic phases of V. cholerae: in the “LuxO-ON” phase, V. cholerae is permissive for CT production, forms biofilms, and ceases protease production, whereas in the “LuxO-OFF” phase, biofilm formation is repressed, hapR is expressed and represses the ToxR regulon, and proteases are produced. It will be of interest to determine whether (and how) these phenotypic phases allow V. cholerae to survive in the environment and to colonize the human intestinal tract.
Acknowledgments
Russell Vance is supported by a postdoctoral fellowship from the Damon Runyon Cancer Research Fund. Jun Zhu is supported by an NRSA fellowship from the NIH. This work was also supported by NIH grant AI-18045.
We thank D. Provenzano for helpful discussions and for assistance with the zymogram analysis, K. Fullner and C. Walchle for initially identifying the Tn5 insertion in toxS, M. Miller and B. Bassler for strains and advice, and K. Klose for pKEK229.
Editor: D. L. Burns
REFERENCES
- 1.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Sequence and function of LuxO, a negative regulator of luminescence in Vibrio harveyi. Mol. Microbiol. 12:403-412. [DOI] [PubMed] [Google Scholar]
- 2.Benitez, J. A., A. J. Silva, and R. A. Finkelstein. 2001. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect. Immun. 69:6549-6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bortner, S. R. 1988. Biochemical and genetic studies of a Vibrio cholerae extracellular protease. Ph.D. thesis. Harvard University, Cambridge, Mass.
- 4.Correa, N. E., C. M. Lauriano, R. McGee, and K. E. Klose. 2000. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol. Microbiol. 35:743-755. [DOI] [PubMed] [Google Scholar]
- 5.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman, J. A., and B. L. Bassler. 1999. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 31:665-677. [DOI] [PubMed] [Google Scholar]
- 8.Fullner, K. J., W. I. Lencer, and J. J. Mekalanos. 2001. Vibrio cholerae-induced cellular responses of polarized T84 intestinal epithelial cells are dependent on production of cholera toxin and the RTX toxin. Infect. Immun. 69:6310-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fullner, K. J., and J. J. Mekalanos. 1999. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and E1 Tor biotypes of Vibrio cholerae. Infect. Immun. 67:1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fullner, K. J., and J. J. Mekalanos. 2000. In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin. EMBO J. 19:5315-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hase, C. C., and R. A. Finkelstein. 1991. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J. Bacteriol. 173:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, G., L. Zhang, and R. G. Birch. 2000. Rapid amplification and cloning of Tn5 flanking fragments by inverse PCR. Lett. Appl. Microbiol. 31:149-153. [DOI] [PubMed] [Google Scholar]
- 14.Jobling, M. G., and R. K. Holmes. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26:1023-1034. [DOI] [PubMed] [Google Scholar]
- 15.Klose, K. E., V. Novik, and J. J. Mekalanos. 1998. Identification of multiple sigma54-dependent transcriptional activators in Vibrio cholerae. J. Bacteriol. 180:5256-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, Jr., and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 17.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135-1147. [DOI] [PubMed] [Google Scholar]
- 18.Lin, W., K. J. Fullner, R. Clayton, J. A. Sexton, M. B. Rogers, K. E. Calia, S. B. Calderwood, C. Fraser, and J. J. Mekalanos. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 96:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mel, S. F., K. J. Fullner, S. Wimer-Mackin, W. I. Lencer, and J. J. Mekalanos. 2000. Association of protease activity in Vibrio cholerae vaccine strains with decreases in transcellular epithelial resistance of polarized T84 intestinal epithelial cells. Infect. Immun. 68:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 22.Miller, V. L., V. J. DiRita, and J. J. Mekalanos. 1989. Identification of toxS, a regulatory gene whose product enhances toxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 171:1288-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitra, R., P. Figueroa, A. K. Mukhopadhyay, T. Shimada, Y. Takeda, D. E. Berg, and G. B. Nair. 2000. Cell vacuolation, a manifestation of the El Tor hemolysin of Vibrio cholerae. Infect. Immun. 68:1928-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skorupski, K., and R. K. Taylor. 1999. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol. Microbiol. 31:763-771. [DOI] [PubMed] [Google Scholar]
- 25.Tacket, C. O., G. Losonsky, J. P. Nataro, L. Comstock, J. Michalski, R. Edelman, J. B. Kaper, and M. M. Levine. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J. Infect. Dis. 172:883-886. [DOI] [PubMed] [Google Scholar]
- 26.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 27.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 28.World Health Organization. 2000. WHO report on global surveillance of epidemic-prone infectious diseases, p. 39-54. World Health Organization, Geneva, Switzerland.
- 29.Wu, Z., D. Milton, P. Nybom, A. Sjo, and K. E. Magnusson. 1996. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb. Pathog. 21:111-123. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]