Abstract
Staphylococcus aureus causes a wide variety of diseases. Major virulence factors of this organism include enterotoxins (SEs) that cause both food poisoning and toxic shock syndrome. Recently, a novel SE, tentatively designated SEL, was identified in a pathogenicity island from a bovine mastitis isolate. The toxin had a molecular weight of 26,000 and an isoelectric point of 8.5. Recombinant SEL shared many biological activities with SEs, including superantigenicity, pyrogenicity, enhancement of endotoxin shock, and lethality in rabbits when administered in subcutaneous miniosmotic pumps, but the protein lacked emetic activity. T cells bearing the T-cell receptor β chain variable regions 5.1, 5.2, 6.7, 16, and 22 were significantly stimulated by recombinant SEL.
Staphylococcus aureus is an important human and animal pathogen, in part due to production of superantigen exotoxins (SAgs). The spectrum of SAg-mediated illnesses ranges from relatively benign food poisoning to life-threatening toxic shock syndrome (TSS) (2, 7). The major secreted SAgs of S. aureus include TSS toxin 1 (TSST-1) and enterotoxin (SE) serotypes A to Q, excluding F.
Crystallographic studies of SAgs have shown that these molecules have similar three-dimensional structures (2, 7). The toxins have a short N-terminal α helix that leads into a β barrel structure known as the B domain or oligosaccharide-binding (O/B) fold. The O/B fold is connected to a C-terminal wall of β strands by a central diagonal α helix, forming domain A (β grasp fold). All SAgs have these features, but some differ slightly in that they have small additional loops. The most notable of these is a cysteine loop structure present in many of the SEs and streptococcal pyrogenic exotoxin A (SPE A). This loop is thought to be important for emetic activity in SEs (4). Recently, SEs I, K, and Q, which lack the cysteine loop structure, have been identified; these toxins were shown to be superantigenic, but the emetic activity was significantly reduced in magnitude (SEI) or lacking (SEK and SEQ) (8-10). In addition, TSST-1, which lacks cysteine residues, was shown to be nonemetic (14).
This study was undertaken to purify and characterize a new toxin, designated SEL, whose coding sequence had been detected as an open reading frame in a pathogenicity island (SaPIbov) from a bovine mastitis S. aureus isolate.
The gene sel was cloned from S. aureus TSS isolate MN Don. PCR primers were chosen based on the sequences of SaPIbov and SaPI4 (3). PCR primers including several hundred nucleotides at either end of sel were included in the original clone. The PCR product was electrophoresed in 1% agarose, purified, and cloned into the TA vector pGEM T-easy (Promega, Madison, Wis.), resulting in the plasmid pPMO031. This plasmid was transformed into Escherichia coli XL-1 Blue. The entire sequence of sel was determined by automated sequencing (Biomedical Genomics and Advanced Genetic Analysis Centers, University of Minnesota). To examine the biochemical and biological properties of SEL, a sel signal sequence deletion mutation was cloned into pET28b by PCR amplification of the pGEM T-easy insert. NcoI and BamHI restriction sites were encoded in the primers for in-frame insertion of the signal sequence deletion mutation into pET28b (the resultant plasmid is referred to as pPMO032). The deleted signal peptide of SEL was replaced with N-terminal methionine and glycine residues. The N-terminal sequence of recombinant SEL (rSEL) was determined experimentally to be MGNGDVGPGP (single-letter designations) (Ben Madden, Mayo Clinic, Rochester, Minn.). Transformation of pPMO032 into E. coli XL-1 Blue was followed by purification of the plasmid and verification of the construct by restriction digestion with NcoI and BamHI. Subsequent to verification, the plasmid was introduced into E. coli BL21(DE3) for expression by using the ptac system.
The pET28b clone containing sel (pPMO032) in BL21(DE3) was grown to early logarithmic phase in beef heart dialysate (15) plus kanamycin (50 μg/ml) at 37°C with shaking and then induced with 200 μM isopropyl thiogalactoside. After growth overnight under the same conditions, the cultures were treated with 4 volumes of absolute ethanol for 48 h to precipitate toxins, and then preparative thin-layer isoelectric focusing (15) was performed. Final purification was accomplished by using a gel filtration column (Bio-Rad Laboratories, Hercules, Calif.) containing Sephadex G-75 (Sigma Chemical Company, St. Louis, Mo.). Purity was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in which 10 μg of rSEL gave a homogeneous band of the appropriate molecular weight (5). The protein concentration was assessed by using the Bradford assay (Bio-Rad), and protein was stored in the lyophilized state until used in biological and biochemical assays. The isoelectric point (pI) was determined by measuring the pH of the rSEL fraction eluted from the pH 7 to 9 isoelectric focusing plate.
Rabbit splenocyte proliferation was used to assess the superantigenicity of rSEL (1).
American Dutch belted rabbits were given injections of toxin in phosphate-buffered saline (PBS) at doses of 10, 1, and 0.1 μg/kg of body weight intravenously, followed by a sublethal dose of endotoxin (10 μg/kg) to assess both pyrogenicity and the ability to enhance susceptibility to endotoxin shock (13). Six American Dutch belted rabbits were implanted with miniosmotic pumps containing 200 μl (200 or 500 μg) of either TSST-1 or rSEL (11). Lethality of the toxins was assessed over a period of 15 days. The stimulation profile of rSEL for human T cells, with regard to the T-cell receptor β chain variable region (TRBV), was determined by flow cytometry (9, 10). The emetic capacity of rSEL compared to that of SEC1 was analyzed by a standard monkey feeding assay (14). Each toxin was dissolved in flavored fruit punch (Lyons-Magnus, Clovis, Calif.) and, using a sterile syringe, was fed to young adult pigtail monkeys (Macaca nemestrina) weighing between 5 and 10 kg. Prior to use in experiments, the animals were trained to accept fluids willingly from a syringe so that physical restraint or anesthesia was not needed. The minimal emetic dose for SEC1 in this assay was 0.1 to 1.0 μg/kg.
The gene sel was cloned from S. aureus clinical isolate MN Don. Concurrently sel was identified and sequenced from a strain of S. aureus isolated from a bovine mastitis case (3). The gene was shown to be part of an S. aureus pathogenicity island in both strains (SaPI4 and SaPIbov, respectively). The amino acid sequences predicted from the sel genes identified were virtually identical, with a single amino acid change from methionine to isoleucine (M195I) in the SEL from strain MN Don.
Primary sequence analysis showed that SEL belongs in a new subfamily of SEs (group V), along with the other recently described novel SEs, SEI, -K, and -Q (7). Primary sequence comparison with members of other previously described subfamilies also revealed several potentially important structural elements. Like other recently described SAgs, SEL was more similar to other SEs at the carboxyl terminus than at the amino terminus, and the toxin contained residues known to be important in Zn2+ coordination (6, 12). SEL contained no cysteine residues, and the region corresponding to the characteristic cysteine loop in classical SEs was absent in this toxin.
The biochemical properties of rSEL are somewhat different from those of previously described SEs. The putative mature protein was about 20 amino acids shorter than the average SE, though it was similar to SEK and -Q. The mature SEL sequence was 218 amino acids in length, with a predicted molecular weight of 25,219. The mature protein as purified from the pET cloning and expression system had an observed pI of 8.5. This was similar to the predicted pI of 8.1 based on the mature sequence including an N-terminal methionine and glycine added to the expressed protein. The N-terminal 10 amino acids of the purified recombinant toxin were MGNGDVGPGP, as determined by protein sequencing.
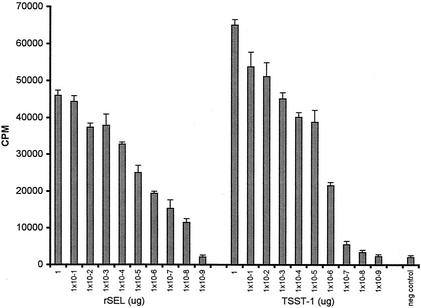
The purified rSEL was active in in vitro and in vivo tests of superantigenicity and TSS, respectively. rSEL was tested for superantigenicity in a standard assay of rabbit splenocyte stimulation (Fig. 1). Maximal stimulation of splenocyte proliferation was observed at a toxin concentration of 1.0 μg/well, but the protein was significantly mitogenic at doses as low as 10−8 μg/well. An rSEL dose approximately 100-fold-lower than the TSST-1 dose was required to induce significant lymphocyte proliferation. Significant fever (>0.5°C) was induced in three rabbits per group 4 h after injection with rSEL at all three concentrations tested (10, 1, and 0.1 μg/kg). The toxin was lethal at two of the three doses (10 and 1 μg/kg), as tested in the endotoxin enhancement model of TSS, with two of three and three of three animals succumbing compared to none of three at the 0.1-μg/kg exotoxin dose (P = 0.05 by Fischer's exact test when deaths of animals receiving 1 μg of rSEL/kg followed by endotoxin were compared to deaths of animals receiving 0.1 μg of exotoxin/kg followed by endotoxin). rSEL was tested for lethality in a rabbit miniosmotic pump model of TSS at doses of 200 and 500 μg/rabbit over a period of 15 days. The toxin was nonlethal at 200 μg and lethal in one of three rabbits at 500 μg. This contrasts with TSST-1, which was lethal for all three rabbits exposed to 200 μg over the 15-day period.
FIG. 1.
Rabbit splenocyte mitogenicity of rSEL compared to that of TSST-1. Splenocytes (2 × 105/well) in quadruplicate wells of a microtiter plate were stimulated for 3 days with the indicated amounts of rSEL or TSST-1, and then 1 μCi of [3H]thymidine was added to each well. Cells were then reincubated for another day, and DNA was harvested onto glass fiber filters. Counts per minute were determined by scintillation counting. Negative-control wells (neg control) contained splenocytes only.
rSEL was administered orally to 5 M. nemestrina monkeys at doses of 6.5 (n = 1), 10 (n = 2), and 100 (n = 2) μg/kg. None of the animals exhibited an emetic response or any other enteric symptoms characteristic of classical SEs. In contrast, 50 μg of SEC1/kg induced emesis in two of two animals.
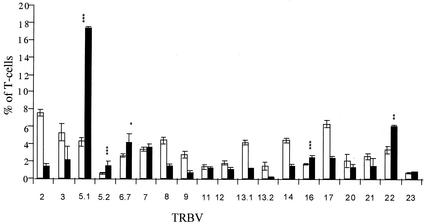
The TRBV profile of human T cells stimulated with rSEL was examined by flow cytometry with monoclonal antibodies directed against the repertoire of human TRBV subsets. It was observed that T cells bearing TRBV 5.1, 5.2, 6.7, 16, and 22 were preferentially activated by the toxin (Fig. 2). T cells with TRBV 5.1, 5.2, and 16 were statistically highly significantly stimulated (P < 0.005), and T cells with TRBV 5.1 had the highest level of proliferation (18% of T cells, averaged over five study subjects tested), implying a likely biological significance for this TRBV subset. We observed that primarily CD4+ T cells but also CD8+ T-cell populations were expanded in some cases. TRBV 16- and 23-bearing T cells were also enhanced in all five study subjects, but not in a statistically significant manner. In these cases, the percent reduction of CD8+ T cells bearing these TRBV chains was responsible for the lack of statistical significance. The rise in TRBV 16 and 23 CD4+ T cells in response to rSEL was significant (P < 0.05). The proliferation of cells bearing TRBV 7 in samples stimulated with rSEL was variable; it was enhanced twofold in one study subject's lymphocytes compared to that following stimulation with anti-CD3 antibodies. Samples from only three of five study subjects contained increased levels of this T-cell type, however. The populations of T cells bearing certain TRBV subsets were reduced, consistent with the effect of SAgs. Examples of this effect include TRBV 2, 8, 13.1, and 14. These apparent reductions in certain subsets of T cells do not result from apoptosis or other loss of those subsets but rather reflect the significant overrepresentation of the stimulated T-cell subsets relative to those that are not stimulated.
FIG. 2.
TRBV profile of rSEL. Peripheral blood mononuclear cells from five study subjects were stimulated with either anti-CD3 (white bars), which stimulates all T cells, or rSEL (black bars), which selectively stimulates T cells dependent on the TRBVs. Cells were stained with monoclonal antibodies against listed TRBVs, and the results were evaluated by flow cytometry. The percentages of T cells expressing the listed TRBVs are shown. Results from all five study subjects are reflected in each case, except for those for TRBV 6.7, which are the averages of four of five study subjects (results for the fifth study subject were not obtained). P values were determined by using the paired Student t test (∗, P = 0.046; ∗∗, P = 0.009; ∗∗∗, P < 0.003). Error bars represent the standard errors for each data set.
The data presented in this work indicate that a novel enterotoxin gene, sel, was present, together with two different sec molecular variants, in pathogenicity islands SaPIbov and SaPI4 (3). rSEL had biological activities, excluding emesis, similar to those of previously described SEs and other SAgs in general. Analysis of the sequence of sel showed that the gene and its encoded protein fit into a new subfamily of SEs, along with the recently described SEI, SEK, and SEQ (7).
rSEL was superantigenic and pyrogenic and enhanced the toxic effects of endotoxin in a rabbit model. It was only weakly lethal in a miniosmotic pump model of TSS in rabbits. This reduced effectiveness in the miniosmotic pump model may be caused by the instability of the purified protein in vivo or intrinsic lack of activity over the 15-day time period. However, the toxin was fully capable of enhancing susceptibility to endotoxin in rabbits in a 24-h assay. rSEL was a potent T-cell SAg, capable of stimulating high levels of both CD4+ and CD8+ T-cell proliferation. The activity was comparable to that of other SAgs in this regard. SEL was also a potent inducer of fever in rabbits, with a minimum pyrogenic dose consistent with those for other SAgs.
Interestingly, at a dose of 100 μg/kg, rSEL did not cause emetic responses in monkeys. This dose of toxin is 100- to 1,000-fold in excess of the amount of SEC1 required to cause emesis in this model (14). It is possible that this lack of emetic activity resulted in part from an inability to form a disulfide bond characteristic of the classical SEs such as SEA to SEE. Those SAgs that lack the loop previously have been shown to be only weakly emetic or nonemetic (8-10). In addition, Hovde and colleagues (4) showed that by changing the cysteine residues in SEC3 to alanines the emetic activity of the molecule was lost. It is possible that the ability to form disulfide bonds constrains other aspects of the SE structure such that the molecules retain emetic activity. From our studies it was also clearly shown that emetic activity was separable from superantigenicity, in that rSEL was not emetic yet the toxin was superantigenic. This is consistent with previous data obtained in studies comparing the biological activities of SPE A, TSST-1, and SEC1 (14).
rSEL stimulated T cells in a manner similar to that of previously described SAgs, but it had a TRBV profile distinct from those previously observed. As indicated above we have shown that T cells bearing TRBV 5.1, 5.2, 6.7, 16, and 22 are significantly stimulated by rSEL in vitro.
Finally, it is noteworthy that rSEL has the strongest homology with other SEs in the C-terminal region, where major histocompatibility complex class II molecules may be bound by certain SAgs in a high-affinity site and which contains a Zn2+ molecule (6, 7). These residues, important for Zn2+ binding in SEA, -H, and -K and SPE C, are present in the same positions relative to one another in rSEL (His17, His209, and Asp211). We do not know if Zn2+ is present in rSEL, but clearly the positions of these coordination residues suggest that rSEL has this high-affinity site.
Acknowledgments
This work was supported by research grants from the NIH (AI22159, AI2840, RR15587, HL37260, AR41256, and HL36577), the USDA NRI (99-35201-8581), and the Idaho Agricultural and Experiment Station. Primate experiments were conducted at the University of Washington Regional Primate Facility (NIH grant RR00166) with technical assistance from Debra Glanister and Ed Novak. P.M.O. was supported by a National Science Foundation fellowship and a USPHS/NIAID training grant (5 T32 AI07421).
Tim Leonard is gratefully acknowledged for help in generating figures.
Editor: J. D. Clements
REFERENCES
- 1.Barsumian, E. L., P. M. Schlievert, and D. W. Watson. 1978. Nonspecific and specific immunological mitogenicity by group A streptococcal pyrogenic exotoxins. Infect. Immun. 22:681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald, J. R., S. R. Monday, T. J. Foster, G. A. Bohach, P. J. Hartigan, W. J. Meaney, and C. J. Smyth. 2001. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J. Bacteriol. 183:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovde, C. J., J. C. Marr, M. L. Hoffmann, S. P. Hackett, Y. I. Chi, K. K. Crum, D. L. Stevens, C. V. Stauffacher, and G. A. Bohach. 1994. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol. Microbiol. 13:897-909. [DOI] [PubMed] [Google Scholar]
- 5.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 6.Li, Y., H. Li, N. Dimasi, J. K. McCormick, R. Martin, P. Schuck, P. M. Schlievert, and R. A. Mariuzza. 2001. Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHC class II. Immunity 14:93-104. [DOI] [PubMed] [Google Scholar]
- 7.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 8.Munson, S. H., M. T. Tremaine, M. J. Betley, and R. A. Welch. 1998. Identification and characterization of staphylococcal enterotoxin types G and I from Staphylococcus aureus. Infect. Immun. 66:3337-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orwin, P. M., D. Y. Leung, H. L. Donahue, R. P. Novick, and P. M. Schlievert. 2001. Biochemical and biological properties of staphylococcal enterotoxin K. Infect. Immun. 69:360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orwin, P. M., D. Y. Leung, T. J. Tripp, G. A. Bohach, C. A. Earhart, D. H. Ohlendorf, and P. M. Schlievert. 2002. Characterization of a novel staphylococcal enterotoxin-like superantigen, a member of the group V subfamily of pyrogenic toxins. Biochemistry 41:14033-14040. [DOI] [PubMed] [Google Scholar]
- 11.Parsonnet, J., Z. A. Gillis, A. G. Richter, and G. B. Pier. 1987. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect. Immun. 55:1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schad, E. M., I. Zaitseva, V. N. Zaitsev, M. Dohlsten, T. Kalland, P. M. Schlievert, D. H. Ohlendorf, and L. A. Svensson. 1995. Crystal structure of the superantigen staphylococcal enterotoxin type A. EMBO J. 14:3292-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlievert, P. M. 1982. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect. Immun. 36:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlievert, P. M., L. M. Jablonski, M. Roggiani, I. Sadler, S. Callantine, D. T. Mitchell, D. H. Ohlendorf, and G. A. Bohach. 2000. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect. Immun. 68:3630-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlievert, P. M., K. N. Shands, B. B. Dan, G. P. Schmid, and R. D. Nishimura. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J. Infect. Dis. 143:509-516. [DOI] [PubMed] [Google Scholar]