Abstract
In murine Lyme borreliosis, the absence of γδ T lymphocytes augments the T helper cell 2 type humoral response but does not alter disease susceptibility. Arthropod transmission of Borrelia burgdorferi spirochetes results in similar antibody isotypes when γδ T cells are present, suggesting that vector effects can negate γδ T-cell functions in vivo.
Lyme borreliosis is a multisystem illness caused by infection with the arthropod-borne spirochete Borrelia burgdorferi (7, 27). In the mouse model of Lyme borreliosis, spirochetes introduced intradermally by syringe inoculation or by the tick vector first establish infection in the skin (2, 26), where they may encounter γδ T lymphocytes among the responding immune cells. γδ T cells have been shown to influence the T helper (Th) cell response and alter resistance to disease due to intracellular pathogens (10, 11, 13, 16-18), but their effects on the immune response to a vector-borne extracellular pathogen are unknown. We therefore used mice that had been rendered deficient in γδ T cells either by antibody (Ab) depletion (BALB/c mice) or by targeted disruption of the T-cell receptor (TCR) δ gene (B6.129 TCR δ−/− mice [B6.129P2-Tcrdtm1Mom; Jackson Laboratories, Bar Harbor, Maine] and BALB/c TCR δ−/− mice [29], a gift of Mark Shlomchik, Yale University) to examine the consequences of γδ T-cell deficiency on the course of murine Lyme borreliosis. For Ab depletion, 150 μg of purified hamster anti-mouse γδ TCR monoclonal Ab UC7-13D5 or 150 μl of hamster serum was subcutaneously injected into 4- to 5-week-old BALB/c mice daily, beginning 5 days prior to experimental infection and continuing for a total of 9 days.
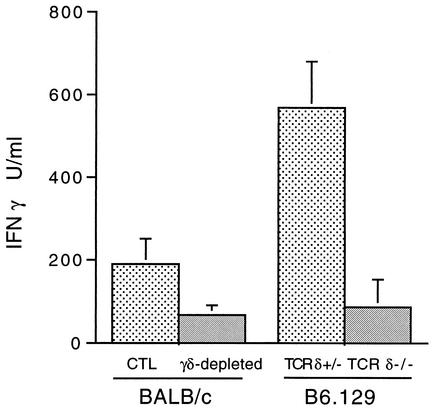
Mice were infected by either intradermal inoculation of 104 cloned N40 spirochetes in 100 μl of Barbour Stoenner Kelly medium (1) or by infestation with five N40-infected Ixodes scapularis nymphs (5). Fourteen days after infection, lymph node cells from γδ T-cell-deficient BALB/c mice produced less gamma interferon (IFN-γ) than controls, as measured by cytokine-specific enzyme-linked immunosorbent assay (ELISA) (Fig. 1). Interleukin 4 was detected in culture supernatants of stimulated lymph node cells, consistent with the known Th2 dominance of BALB/c mice (4, 19), and the level was modestly increased in the absence of γδ T cells (273 versus 408 pg/ml; P < 0.07). A more dramatic reduction in IFN-γ production was noted for B6.129 TCR δ−/− mice (Fig. 1), but no interleukin 4 was detected. Thus, the absence of γδ T cells in B. burgdorferi infection reduces the Th1 cytokine response, consistent with previous studies showing that γδ T cells can direct polarization of αβ Th-cell subsets (11, 17, 18).
FIG. 1.
Lymph node cells from mice deficient in γδ T cells produce less IFN-γ. Production of IFN-γ by popliteal lymph node cells isolated from individual mice was measured by ELISA. Each result is reported as the mean of values obtained for mice within each group ± the standard error of the mean.
ELISA measurement of B. burgdorferi-specific immunoglobulin G (IgG) isotypes (25) reflected the reduction in the Th1 cytokine IFN-γ (Table 1). BALB/c TCR δ−/− mice had a significant rise in endpoint titers of IgG1 (P = 0.0317, Mann- Whitney test) and tended to have lower IgG2a levels. In contrast, B6.129 TCR δ−/− mice exhibited a marked reduction in IgG3 endpoint titers (P = 0.0159, Mann-Whitney test), and titers of IgG2b trended higher. One explanation for the different IgG isotypes induced in the two mouse strains is that γδ T cells serve to decrease the genetically dominant Th2 response in BALB/c mice, whereas they promote a Th1 response in B6.129 mice.
TABLE 1.
Anti-B. burgdorferi IgG and IgG isotype reciprocal endopoint ELISA titersa
| Mouse group or strain | Mean reciprocal endpoint ELISA titer
|
||||
|---|---|---|---|---|---|
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | |
| Syringe inoculated | |||||
| B6.129 (WT) | 43,750 ± 6,250 | 4,800 ± 924 | 1,100 ± 300 | 14,400 ± 4,027 | 9,600 ± 1,848 |
| B6.129 (TCR δ−/−) | 42,560 ± 7,440 | 5,120 ± 784 | 1,840 ± 588 | 18,560 ± 4,571 | 1,280 ± 528b |
| BALB/c (WT) | 17,920 ± 3,135 | 2,880 ± 320 | 12,800 ± 3,505 | 5,440 ± 2,147 | 1,100 ± 392 |
| BALB/c (TCR δ−/−) | 17,920 ± 3,135 | 10,880 ± 1920c | 6,240 ± 4,843 | 5,440 ± 4,468 | 1,120 ± 196 |
| Tick infected | |||||
| BALB/c (WT) | 13,867 ± 2,569 | 10,133 ± 1,736 | 3,267 ± 1,950 | 8,000 ± 1,600 | 4,033 ± 2,673 |
| BALB/c (TCR δ−/−) | 13,867 ± 2,569 | 10,667 ± 1,349 | 5,067 ± 1,855 | 7,200 ± 1,927 | 4,000 ± 1,789 |
Results from a minimum of five mice/group are reported as the mean of reciprocal endpoint ELISA titers ± standard error of the mean. WT, wild type.
Significantly different (P = 0.0159) from value for wild-type mice as calculated by the Mann-Whitney test.
Significantly different (P = 0.0317) from value for wild-type mice as calculated by the Mann-Whitney test.
Saliva from I. scapularis nymphs, the vector for B. burgdorferi, contains factors that inhibit host defenses and retard the development of immunologic resistance to tick infestation (21, 22, 24). Although deposited locally in the skin during tick feeding, tick saliva appears to have systemic effects. The feeding of I. scapularis ticks on mice results in decreased IFN-γ production and downregulation of a Th1-type response by mitogen-stimulated splenocytes (23, 28). Interestingly, in our study, B. burgdorferi-specific IgG isotypes induced in control mice by vector-borne infection paralleled those seen in TCR δ−/− mice infected with cultured spirochetes, supporting a vector-induced bias toward Th2 cytokine patterns. Tick-transmitted spirochetes elicited predominantly IgG1 in wild-type BALB/c mice, at levels comparable to those achieved in BALB/c TCR δ−/− infected with cultured spirochetes (Table 1). The absence of γδ T cells did not lead to a further increase in this Ab subset after vector-borne infection.
Despite the altered cytokine and humoral immune responses in B. burgdorferi-infected γδ T-cell-deficient mice, we observed no difference in the severity or prevalence of disease as assessed by histopathology (3). When examined using TCR δ−/− mice on two backgrounds or after depletion of γδ T cells by monoclonal Ab treatment, no significant differences were noted in arthritis or carditis (Table 2). The relative decrease in B. burgdorferi-specific Th1 responses in TCR δ−/− mice did not lead to protracted episodes of carditis even though CD4+ Th1 cells have been shown to promote resolution of this disease manifestation (6). Two previous studies also failed to show an effect of absence of the Th1 cytokine IFN-γ on murine Lyme arthritis (8, 14). Mean pathogen burden among mouse groups was the same as assessed by quantitative PCR of the spirochete ospA gene in urinary bladders (data not shown).
TABLE 2.
Absence of γδ T cells does not alter disease in B. burgdorferi-infected micea
| Expt or mouse strain + treatment | Day of infection | Results
|
||
|---|---|---|---|---|
| Carditis | Arthritis | Arthritis severity | ||
| Expt 1 | ||||
| BALB/c + UC7-13D5 | 14 | 0/10 | 11/20 | 1.1 ± 0.5 |
| BALB/c + hamster serum | 14 | 0/10 | 9/20 | 1.2 ± 0.5 |
| Expt 2 | ||||
| B6.129 (WT) | 14 | 0/6 | 3/12 | 1.4 ± 0.6 |
| B6.129 (TCR δ−/−) | 14 | 0/6 | 5/12 | 1.1 ± 0.4 |
| B6.129 (WT) | 30 | 0/6 | 4/12 | 1.2 ± 0.3 |
| B6.129 (TCR δ−/−) | 30 | 0/6 | 5/12 | 1.3 ± 0.4 |
| Expt 3 | ||||
| BALB/c (WT) | 14 | 5/5 | 12/13 | 1.0 ± 0.2 |
| BALB/c (TCR δ−/−) | 14 | 5/5 | 15/16 | 1.2 ± 0.3 |
| BALB/c (WT) | 42 | 1/5 | 3/17 | 0.6 ± 0.2 |
| BALB/c (TCR δ−/−) | 42 | 0/3 | 2/12 | 0.3 ± 0.3 |
In experiment 1, BALB/c mice were treated with the indicated agents as described in Materials and Methods and then infected by inoculation of spirochetes into both hind feet. In experiments 2 and 3, mice were infected by inoculation of spirochetes into the skin of the back. Carditis results are reported as the number of mice with acute carditis over the total number of animals examined. Arthritis presence in tibiotarsal joints was assessed and expressed as the number of inflamed joints over the total number of joints examined. Arthritis severity was determined by averaging the individual scores for the most severely affected joint in each mouse ± the standard error of the mean.
B. burgdorferi TCR δ−/− mice were able to develop protective Ab. Both wild-type and TCR δ−/− mice that were passively immunized with 500 μl of 1:5 dilution of immune serum from 45-day-infected TCR δ−/− mice 24 h prior to challenge infection with tick-borne spirochetes were completely protected as assessed by culture and histopathology (data not shown) (3). This was an expected outcome, because for B. burgdorferi infection, protective and arthritis-resolving antibodies arise in the absence of T-cell help (12, 20).
In summary, we used the murine model of Lyme borreliosis to investigate the effect of γδ T cells on the adaptive immune response to a vector-borne extracellular pathogen. While disease expression was not altered, our results show that γδ T cells influence the quality of the humoral immune response to B. burgdorferi introduced through the skin and suggest that tick transmission of spirochetes negates the γδ T-cell effect. γδ T-cell effector functions have been implicated in a variety of inflammatory and infectious processes (9, 15), yet the degree to which these cells play a part in the host immune response remains uncertain. In mammals, the location of these cells at sites of anatomic barriers to the environment suggests a primary role in the early immune response against agents, including arthropod vectors, which can penetrate the barrier. Given the similarity between IgG isotypes induced by vector-borne infection in wild-type mice to those of TCR δ−/− mice, it is possible that the Th cell bias attributed to tick saliva may be due to its actions on dendritic epidermal γδ T cells. These cells may contribute to the local cytokine milieu for maturing dendritic cells in the skin and influence the priming of α/β T-cell responses in the lymph nodes. Although γδ T-cell effects do not alter the outcome from B. burgdorferi infection, our results have important implications for other vector-borne pathogens transmitted through the skin.
Acknowledgments
We thank Debbie Beck for excellent technical assistance and Ruth Montgomery for editorial review of the manuscript.
This work was supported by NIH AR42637, AI50604, and AR47058 and the Arthritis Foundation (L.K.B.) and NIH AI26815 (S.W.B.).
Editor: F. C. Fang
REFERENCES
- 1.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:419-420. [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease following intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47:605-613. [DOI] [PubMed] [Google Scholar]
- 4.Bix, M., Z.-E. Wang, B. Thiel, N. J. Schork, and R. M. Locksley. 1998. Genetic regulation of commitment to interleukin 4 production by a CD4+ T cell-intrinsic mechanism. J. Exp. Med. 188:2289-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bockenstedt, L. K., E. Hodzic, S. Feng, K. W. Bourrel, A. deSilva, R. R. Montgomery, E. Fikrig, J. D. Radolf, and S. W. Barthold. 1997. Borrelia burgdorferi strain-specific Osp C-mediated immunity in mice. Infect. Immun. 65:4661-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bockenstedt, L. K., I. Kang, C. Chang, D. Persing, A. Hayday, and S. W. Barthold. 2001. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect. Immun. 69:5264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bockenstedt, L. K., and S. E. Malawista. 1995. Lyme disease, p. 1234-1249. In R. R. Rich (ed.), Clinical immunology. Mosby-Year Book, St. Louis, Mo.
- 8.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carding, S. R., and P. J. Egan. 2002. γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2:336-345. [DOI] [PubMed] [Google Scholar]
- 10.Cardona, A. E., and J. M. Teale. 2002. γ/δ T cell-deficient mice exhibit reduced disease severity and decreased inflammatory response in the brain in murine neurocysticercosis. J. Immunol. 169:3163-3171. [DOI] [PubMed] [Google Scholar]
- 11.Ferrick, D. A., M. D. Schrenzel, T. Mulvania, B. Hsieh, W. G. Ferlin, and H. Lepper. 1995. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature 373:255-257. [DOI] [PubMed] [Google Scholar]
- 12.Fikrig, E., S. W. Barthold, M. Chen, I. S. Grewal, J. Craft, and R. A. Flavell. 1996. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J. Immunol. 157:1-3. [PubMed] [Google Scholar]
- 13.Fu, Y.-X., C. E. Roark, K. Kelly, D. Drevets, P. Campbell, R. O'Brien, and W. Born. 1994. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J. Immunol. 153:3101-3115. [PubMed] [Google Scholar]
- 14.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayday, A. C. 2000. γδ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18:975-1026. [DOI] [PubMed] [Google Scholar]
- 16.Hiromatsu, K., Y. Yoshikai, G. Matsuzaki, S. Ohga, K. Muramori, K. Matsumoto, J. A. Bluestone, and K. Nomoto. 1992. A protective role of γ/δ T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 175:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber, S., C. Shi, and R. C. Budd. 2002. Gamma delta T cells promote a Th1 response during coxsackievirus B3 infection in vivo: role of Fas and Fas ligand. J. Virol. 76:6487-6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber, S. A., D. Graveline, W. K. Born, and R. L. O'Brien. 2001. Cytokine production by Vgamma(+)-T-cell subsets is an important factor determining CD4(+)-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J. Virol. 75:5860-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, I., S. W. Barthold, D. H. Persing, and L. K. Bockenstedt. 1997. T helper cell cytokines in the early evolution of murine Lyme arthritis. Infect. Immun. 65:3107-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKisic, M. D., and S. W. Barthold. 2000. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect. Immun. 68:5190-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuttall, P. A., G. C. Paesen, C. H. Lawrie, and H. Wang. 2000. Vector-host interactions in disease transmission. J. Mol. Microbiol. Biotechnol. 2:381-386. [PubMed] [Google Scholar]
- 22.Ribeiro, J. M. C., G. T. Makoul, J. Levine, D. R. Robinson, and A. R. Spielman. 1985. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick. Ixodes dammini. J. Exp. Med. 161:332-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoeler, G. B., S. A. Manweiler, and S. K. Wikel. 1999. Ixodes scapularis: effects of repeated infestations with pathogen-free nymphs on macrophage and T lymphocyte cytokine responses of BALB/c and C3H/HeN mice. Exp. Pathol. 92:239-248. [DOI] [PubMed] [Google Scholar]
- 24.Schoeler, G. B., and S. K. Wikel. 2001. Modulation of host immunity by haematophagous arthropods. Ann. Trop. Med. Parasitol. 95:755-771. [DOI] [PubMed] [Google Scholar]
- 25.Shanafelt, M.-C., I. Kang, S. W. Barthold, and L. K. Bockenstedt. 1998. Modulation of murine Lyme borreliosis by interruption of the B7/CD28 T cell costimulatory pathway. Infect. Immun. 66:266-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shih, C.-M., R. J. Pollack, S. R. Telford, III, and A. Spielman. 1992. Delayed dissemination of Lyme disease spirochetes from the site of deposition in the skin of mice. J. Infect. Dis. 166:827-831. [DOI] [PubMed] [Google Scholar]
- 27.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-124. [DOI] [PubMed] [Google Scholar]
- 28.Zeidner, N., M. L. Mbow, M. Dolan, R. Massung, E. Baca, and J. Piesman. 1997. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a Th2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect. Immun. 65:3100-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuany-Amorim, C., C. Ruffie, S. Haile, B. B. Vargaftig, P. Pereira, and M. Pretolani. 1998. Requirement for γδ T cells in allergic airway inflammation. Science 280:1265-1267. [DOI] [PubMed] [Google Scholar]