Abstract
Toxoplasma gondii is an opportunistic intracellular parasite. Infection with the high-virulence T. gondii strain RH induces inflammatory cytokine overproduction and uncontrolled apoptosis in lymphoid organs. Here, we show by fluorescent terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay and binding of fluorescein isothiocyanate-conjugated VAD-FMK, an irreversible pan-caspase inhibitor, that parasite-triggered apoptosis occurs among CD4+, CD8+, B220+, Gr-1+, and NK1.1+ splenic populations. Caspases 8 and 9 were activated during infection, implicating cell surface death receptors and mitochondria in apoptosis. Induction of apoptosis was absent among all cell populations in both interleukin-12 (IL-12) p40- and Fas ligand (FasL)-negative mice. STAT-1 phosphorylation correlated with onset of apoptosis during infection, but in the absence of IL-12 p40 and functional FasL, activation of this transcription factor failed to occur. The results demonstrate T. gondii-induced activation of multiple apoptotic pathways, dependent upon both IL-12 p40 and FasL, that may play a role in the lethal pathology of infection.
Toxoplasma gondii is a widely distributed intracellular parasite of the class Apicomplexa. Acute-stage disease, characterized by dissemination of rapidly multiplying tachyzoites, is followed by chronic infection, during which the parasite forms quiescent cysts in tissues of the central nervous system and skeletal muscle (8). The host mounts a strong type 1 cytokine response to T. gondii, with high levels of the proinflammatory mediators interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) (11, 47, 55). These cytokines are required for control of infection through mechanisms such as macrophage activation and concurrent nitric oxide production, as well as through the activity of IFN-γ-inducible GTP-binding protein (IGTP) and related genes (57). Nevertheless, the immune response is itself tightly regulated, since excess production of proinflammatory cytokines can result in lethal immunopathology (10, 12, 32, 33, 39).
T. gondii has been classified into three lineages, based on restriction fragment length polymorphism analysis of different genes (21, 52). Tachyzoites of the prototypic high-virulence (type I) strain RH are uniformly lethal in mice during acute infection (22, 51). In contrast, low-virulence (type II or III) strains, such as ME49, do not normally kill the host but establish a long-term chronic infection associated with formation of bradyzoite-containing cysts in tissues of the brain and skeletal muscle (2, 20). Type I strains have been associated with cases of human ocular toxoplasmosis and severe congenital disease, suggesting that parasites belonging to this category are also more virulent in humans than type II and III strains (9, 15).
We, and others, recently reported that infection with high-virulence T. gondii strains resulted in IFN-γ overproduction and high levels of apoptosis, in contrast to low-virulence infection (10, 39). Thus, mice infected with type I strain RH tachyzoites displayed greatly increased levels of apoptotic cell death in the spleen immediately prior to death. Importantly, this did not appear to be directly attributable to parasite-induced tissue destruction but instead may have been driven by high levels of proinflammatory mediators induced by the RH parasite strain. Therefore, pathology during toxoplasmosis may, in part, be the result of uncontrolled apoptotic death induced by high-virulence strains such as RH.
Execution of programmed cell death (PCD) involves a cascade of proteolytic events characterized by cleavage of a series of procaspase zymogens into active aspartate proteases, or caspases (41, 62). Three signals induce caspase (Casp) activation. One involves death receptor engagement, resulting from an interaction between TNF-α and TNF receptor I (TNFRI), or Fas ligand (FasL) and Fas, and leading to Casp-8 activation (1, 4, 14, 27). A second pathway is initiated by changes in mitochondrial membrane potential. The latter may be induced by the presence of toxic agents, stress, or growth factor deprivation. Cytochrome c, translocated from the inner mitochondrial membrane to the cytoplasm, activates APAF-1, which then cleaves procaspase 9 to the active Casp-9 form (29, 31). Finally, PCD may be triggered by cytolytic T cells. As a result of T-cell receptor engagement, effector cells release the pore-forming molecule perforin and insert caspase-activating granzyme molecules into the target cell cytosol (18, 45, 56). Casp-8 and Casp-9, activated by the above stimuli, continue to cleave a large array of effector caspases (e.g., Casp-3, -6, and -7), which in turn attack a variety of target molecules essential for cellular structural integrity, thus mediating cell death (53).
The importance of mitochondria in PCD is underscored by molecular interactions between antiapoptotic (e.g., Bcl-2, Bcl-XL, and Bcl-w) and proapoptotic (e.g., Bax, Bak, and Bcl-XS) members of the Bcl-2 family (13, 19). These factors become functional through dimerization in the outer mitochondrial membrane (40, 46). The signaling outcome depends on ratios of pro- and antiapoptotic complexes. Additional complexity is added by the presence of the proapoptotic factor Bid, a Bcl-2 family member which exists as an inactive protein in the cytoplasm but can be cleaved by Casp-8 into an active fragment (35, 59). Active Bid translocates to the mitochondria and induces cytochrome c release and activation of Casp-9 (61).
In the present study we examined apoptotic pathways triggered during acute infection with high-virulence strain RH tachyzoites. In agreement with others (39), we found that CD4+ and CD8+ T cells, B lymphocytes, NK cells, and granulocytes were induced to undergo PCD. Biochemical analysis of caspase activation suggested that multiple apoptotic pathways were activated by parasite infection. PCD was further examined in a panel of gene knockout mouse strains. Parasite-induced apoptosis continued to occur in p47phox−/− (Phox−/−) infected mice but was substantially decreased in TNFRI−/−, inducible nitric oxide synthase (iNOS)−/−, IFN-γ−/−, and especially IL-12 p40−/− and FasL−/− strains. Interestingly, we could correlate PCD with the presence of activated signal transducer and activator of transcription STAT-1. We conclude that high-virulence Toxoplasma infection provides a profoundly strong stimulus for PCD, resulting in activation of multiple proapoptotic pathways among different cell types. We hypothesize that global leukocyte apoptosis contributes to early death in mice infected with RH and other high-virulence type I T. gondii strains.
MATERIALS AND METHODS
Mice.
B6.129S7-Ifngtm1Ts (IFN-γ−/−), B6.129-Il12btm1Jm (IL-12 p40−/−), B6.129P2-Nos2tm1Lau (iNOS−/−), B6Smn.C3H-Tnfsf6gld (FasL−/−), and B6.129-Tnfrsftm1Mak (TNFRI−/−) mice, and C57BL/6J controls, were obtained from The Jackson Laboratory (Bar Harbor, Maine). p47phox (Phox−/−) breeder pairs were kindly provided by S. M. Holland (National Institute of Allergy and Infectious Diseases, Bethesda, Md.) and bred at Cornell University. Female mice 8 to 10 weeks of age were used throughout this study. Mice were housed under specific-pathogen-free conditions in the animal facility of the College of Veterinary Medicine at Cornell University, which is accredited by the American Association for Accreditation of Laboratory Animal Care.
Parasites and infection.
Tachyzoites of strain RH were maintained by biweekly passage on human foreskin fibroblast monolayers in fibroblast medium, composed of Dulbecco's modified Eagle medium (Life Technologies, Gaithersburg, Md.) supplemented with 1% fetal calf serum (HyClone, Logan, Utah), 100 U of penicillin/ml, and 0.1 mg of streptomycin (Life Technologies)/ml. One thousand tachyzoites were inoculated intraperitoneally (i.p.) into mice. Groups of three mice were sacrificed at 12, 24, and 48 h postinfection and then at 5 and 8 days postinfection, and spleens were harvested for analysis. Unless otherwise indicated, splenocytes from three mice per group were pooled, red blood cells were lysed by using Red Blood Cell Lysis Buffer (Sigma Chemical Co., St. Louis, Mo.), and samples were examined by flow cytometric or Western blot.
Analysis of apoptotic cell populations.
For measurement of early apoptosis, freshly isolated splenocytes were resuspended at 107 cells/ml in complete medium consisting of Dulbecco's modified Eagle medium (Life Technologies) supplemented with 10% fetal calf serum (HyClone), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 30 mM HEPES, 100 U of penicillin/ml, 0.1 mg of streptomycin/ml, and 50 mM 2-mercaptoethanol (supplements from Life Technologies). The irreversible pan-caspase inhibitor peptide VAD-FMK, conjugated to fluorescein isothiocyanate (FITC; Promega, Madison, Wis.), was added at a final concentration of 10 μM. After a 20-min incubation at 37°C, splenocytes were washed, Fc receptors were blocked with 10% normal mouse serum (Jackson ImmunoResearch Laboratories, West Grove, Pa.), and cells were stained for surface markers with monoclonal phycoerythrin (PE)-conjugated antibodies (Ab) directed against mouse CD4, CD8, NK1.1, Gr-1 (Ly-6G) (all from BD PharMingen, San Diego, Calif.), and B220 (Caltag Laboratories, Burlingame, Calif.). Alternatively, for late-phase apoptosis, freshly isolated splenocytes were fixed in 3.7% formalin, permeabilized, and subjected to terminal deoxynucleotide transferase (TdT)-mediated labeling of nick-end DNA strands with FITC-conjugated dUTP (TUNEL) by using a commercially available kit and following the manufacturer's protocol (Boehringer Mannheim, Indianapolis, Ind.). Cells were then surface stained as above. All flow cytometric data were acquired on a FACScalibur flow cytometer and analyzed with CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Western blotting.
Cells (108 per sample) were lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, composed of 62.5 mM Tris-HCl (pH 6.8), 2% (wt/vol) SDS, 10% glycerol, 50 mM dithiothreitol, and 0.01% (wt/vol) bromphenol blue. To facilitate gel loading, DNA in the lysate was fragmented by sequential passage through 18-, 23-, and 27-gauge needles. Samples (20 μl) were subjected to electrophoresis on an SDS-PAGE gel (10 or 12% polyacrylamide) under reducing conditions and were transferred to nitrocellulose membranes. After transfer, membranes were blocked for 2 h at room temperature with 1× Tris-buffered saline plus 5% nonfat dry milk and were incubated overnight at 4°C with the recommended dilution of primary Ab to the full-length and cleaved forms of poly(ADP-ribose) polymerase (PARP), Casp-9 (Cell Signaling, Beverly, Mass.), Casp-8 (BD PharMingen), and the total and phosphorylated forms of STAT-1 (Cell Signaling). Bound Ab were detected with a horseradish peroxidase-conjugated secondary Ab and an enhanced chemiluminescence system (LumiGLO; Cell Signaling).
RESULTS
T. gondii triggers apoptosis in multiple cell types.
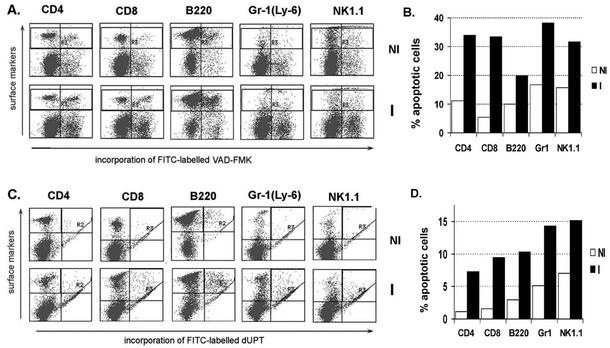
We previously reported that after initial cell proliferation, all splenocyte populations decreased to levels at or below those of noninfected animals. This suggested that cell death may be a widespread phenomenon during RH infection. We therefore sought to identify which spleen cell populations were undergoing apoptosis. Freshly isolated splenocytes from noninfected or 5-day-RH-infected animals were loaded with the fluorescently-labeled irreversible pan-caspase inhibitor VAD-FMK-FITC, surface stained for membrane markers (CD4, CD8, B220, Gr-1, and NK1.1), and analyzed by flow cytometry. We determined that apoptotic cell death, detected by the presence of activated caspases to which the inhibitor bound, was not limited to one splenic population but was triggered in CD4+ and CD8+ T cells, B cells, NK cells, and granulocytes (Fig. 1A and B). We obtained similar results at 7 days postinfection using fluorescent TUNEL reagents (Fig. 1C and D). No TUNEL-positive cells were detected at 5 days postinfection (data not shown), a time when caspase activation was maximal. This difference likely relates to the fact that caspase activation precedes DNA degradation during progression of PCD.
FIG. 1.
Apoptosis occurs in multiple splenic populations during infection. (A) Five days after infection with 103 RH tachyzoites, spleens were removed and splenocytes were first incubated with VAD-FMK-FITC and then surface stained for cell-specific markers. (B) Bar graph summarizing the data in panel A, showing that CD4+ and CD8+ T cells, B lymphocytes, NK cells, and granulocytes undergo PCD as a result of infection. (C) At 7 days postinfection, splenocytes were subjected to TUNEL and surface marker staining and were studied by flow cytometry. (D) Bar graph summarizing the data in panel C. Open bars, samples from noninfected mice (NI); solid bars, samples from 5-day-RH-infected animals (I). Data are representative of three independent experiments.
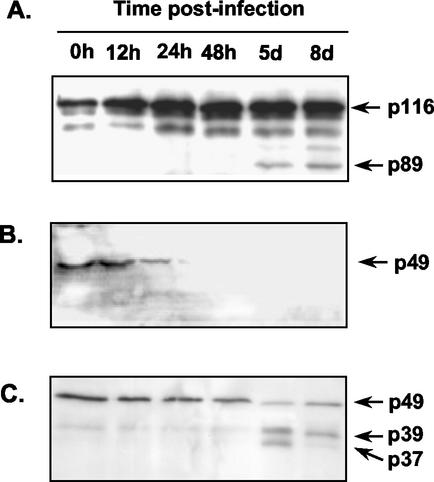
We previously showed the presence of a major population of TUNEL-positive cells in the spleens of mice infected for 8 days with the high-virulence type I T. gondii strain RH (10). In order to further confirm that PCD was activated, we assayed for the presence of cleaved PARP. Intact PARP is a crucial 116-kDa enzyme involved in multiple cell survival-related functions, including DNA repair (49). PARP cleavage by activated effector caspases, an event specific to apoptosis but not necrosis, renders the enzyme no longer functional (7, 30). To assess PARP cleavage after T. gondii infection, we examined cell lysates obtained at given time points from the spleens of mice inoculated i.p. with 103 tachyzoites of the high-virulence strain RH. Western blot analysis of lysates, using an Ab recognizing both full-length and cleaved PARP peptides, showed the appearance of the diagnostic p89 fragment, indicative of apoptosis at 5 and 8 days postinfection (Fig. 2A).
FIG. 2.
T. gondii induces degradation of PARP and cleavage of Casp-8 and Casp-9. At the indicated times following i.p. injection of 103 strain RH tachyzoites into C57BL/6J mice (WT), spleens were collected, cells were lysed, and lysates were subjected to SDS-PAGE and Western blot analysis. (A) The presence of caspase-cleaved PARP was detected with an Ab recognizing both the full-length enzyme and its cleaved fragment. The full-length (p116) and cleaved (p89) PARP molecules are indicated by arrows. (B) An Ab directed against the pro-Casp-8 peptide detects a band that decreases in intensity by 24 h postinfection and falls to undetectable levels thereafter, indicative of Casp-8 activation. (C) An Ab recognizing both pro-Casp-9 (p49) and its active cleavage products shows that the pro-Casp-9 band decreases in intensity by 48 h postinfection. Two active forms of Casp-9 are found at 5 days postinfection. The upper band, p39, results from pro-Casp-9 cleavage by activated Casp-3. The lower band, p37, results from Casp-9 self-cleavage. These experiments were repeated four times with similar results.
Infection results in activation of multiple apoptotic pathways.
Apoptosis induced through death receptors, such as Fas or TNFRI, leads to activation of Casp-8. However, Casp-9 is activated by the mitochondrially induced pathway of PCD, after release of cytochrome c and APAF-1 activation. This is linked to loss of mitochondrial membrane potential resulting from stress and inflammation. Therefore, in order to assess if either the receptor-mediated or the mitochondrially induced pathway was activated by the parasite, we examined levels of the proforms (inactive) and cleaved forms (active) of Casp-8 and Casp-9.
Remarkably, we found a rapid degradation of the pro-Casp-8 protein to undetectable levels, as early as 48 h postinfection (Fig. 2B). This result suggested a receptor-mediated induction of caspase activation. We also found that the levels of pro-Casp-9 (p49) diminished during the course of infection. Thus, cleaved Casp-9 was clearly present by 5 days postinfection (Fig. 2C). The two shorter protein bands represent the p37 and p39 peptides, a consequence of pro-Casp-9 proteolysis. Cleavage at Asp368, resulting in the p39 peptide, is due to Casp-3 activity, and the p37 Casp-9 fragment results from self-cleavage at Asp353 (31). For reasons presently unclear, the latter band appeared only at 5 days postinfection, fading by 8 days. Activation of Casp-9 suggests a role for the mitochondrial pathway of PCD during T. gondii infection.
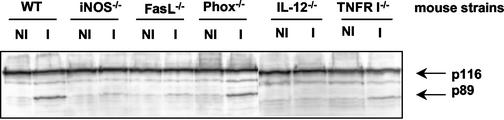
We next assessed apoptosis in a panel of gene knockout mouse strains undergoing acute RH infection. Western blot analysis for the presence of degraded PARP showed a strong p89 band in samples from infected wild-type (WT) and Phox−/− mice. However, PARP degradation was visibly less in samples from infected TNFRI−/− mice, greatly decreased in samples from infected iNOS−/− and FasL−/− mice, and absent in samples from IL-12 p40−/− mice (Fig. 3).
FIG. 3.
Reduced levels of PARP cleavage in gene knockout mice infected with T. gondii. The indicated WT and gene knockout animals were infected with 103 strain RH parasites, and PARP cleavage was assessed in splenocyte lysates from noninfected (NI) and 6-day-postinfection (I) samples. Full-length, active PARP appears at 116 kDa, and the caspase-cleaved inactive fragment is detected at 89 kDa. This experiment was repeated twice with similar results.
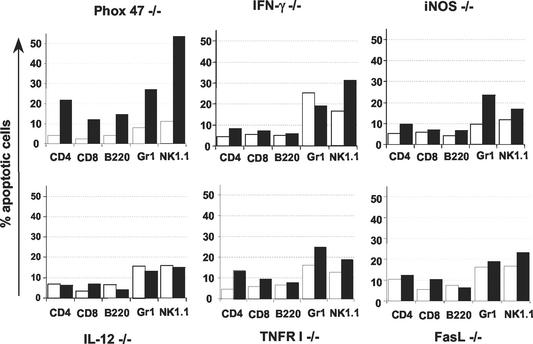
Because several of the infected knockout mouse strains displayed lower levels of PARP degradation, we sought to determine if, in a given mouse strain, a specific splenic population would lack apoptosis. We therefore incubated splenocytes from infected mice with VAD-FMK-FITC, surface stained for membrane markers, and compared data to those obtained from noninfected samples (Fig. 4) and WT controls (see Fig. 1B). All cells from Phox−/− mice that were examined displayed apoptosis, similarly to cells from WT mice. Splenocytes corresponding to samples with reduced PARP degradation showed lower proportions of caspase-positive cells after infection than were observed in samples from Phox−/− and WT mice. Cells from IL-12 p40−/− mice exhibited the lowest levels of inhibitory peptide binding, consistent with the total lack of PARP degradation shown in Fig. 3. Notably, for any given gene knockout mouse strain, levels of apoptosis were equivalently affected among all cell types.
FIG. 4.
Reduced apoptosis in infected gene knockout mice occurs uniformly among all splenic populations. Mice were infected i.p. with 103 RH tachyzoites, and splenocytes were analyzed 6 days later in comparison with cells from noninfected animals. Freshly isolated cells were incubated with VAD-FMK-FITC and were PE surface stained for cell-specific markers. Graphs represent summaries of the fluorescence-activated cell sorter data. Open bars, samples from noninfected mice; solid bars, samples from infected animals. This experiment was repeated twice with similar results.
PCD is correlated with STAT-1 activation.
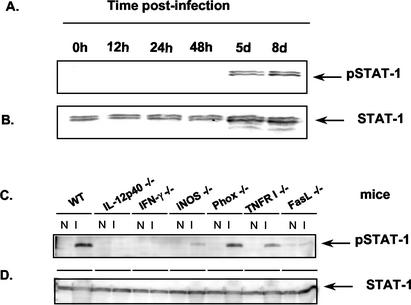
In previous studies, we and others found that T cells from mice infected with high-virulence strains of T. gondii secrete excessively high levels of proinflamatory cytokines such as IFN-γ (10, 39). We hypothesized that these proinflamatory signals may trigger apoptosis in spleens of infected mice. Because IFN-γ signals through STAT-1 (37), we examined its activation during the course of acute T. gondii infection. We found high levels of activated STAT-1 (detected as phospho-STAT-1) at 5 and 8 days postinfection (Fig. 5A), time points corresponding to the presence of degraded PARP (Fig. 2A). We then determined if STAT-1 activation in the infected gene knockout mice predicted the onset of apoptosis as determined by PARP degradation. Indeed, activated STAT-1 was detected at high levels in WT and Phox−/− samples, was present at substantially lower levels in cells from infected TNFRI−/−, iNOS−/−, and FasL−/− mice, and was undetectable in IL-12 p40−/− and IFN-γ−/− spleen cell lysates (Fig. 5C).
FIG. 5.
STAT-1 activation correlates with the induction of apoptosis during Toxoplasma infection. Spleen cell lysates were prepared after i.p. infection with 103 RH tachyzoites and were subjected to Western blot analysis using Ab directed against activated STAT-1 (A and C) and total STAT-1 (B and D). (A and B) Samples from WT mice obtained at the indicated time points. (C and D) Cell lysates from the indicated noninfected (N) and 6-day-postinfection (I) knockout mice were analyzed. The Ab used recognizes an epitope common to both the STAT-1α (p91) and STAT-1β (p84) splice variants; thus, STAT-1 appears as a dimer in these blots. Data are representative of four (panels A and B) and two (panels C and D) experiments independently performed.
DISCUSSION
Immune stimulation with a single antigen, or during infection with a pathogen, induces antigen-specific lymphocyte proliferation, as well as production of cytokines and antibodies. The initial response is later down-regulated by diverse mechanisms. Activation-induced cell death eliminates antigen-responsive cells by apoptosis and has been shown to occur during protozoan infection (1, 28, 42). Another mechanism regulating the immune response is production of modulatory cytokines (e.g., IL-10 and transforming growth factor β), which counteract the pathological effect of excessive proinflammatory factors (12, 16, 25, 26, 50). In previous work comparing mice infected with low- and high-virulence T. gondii strains, we and others determined that in the latter case lethality is associated with excessive levels of proinflammatory cytokines such as IFN-γ and extensive splenic lymphocyte death (10, 39). Therefore, we hypothesized that splenic apoptosis during acute toxoplasmosis is induced by the high levels of proinflamatory cytokines present, and we sought to define the pathways involved.
Here, we first show that apoptosis is a generalized event in the spleen after RH infection, in that all lymphocyte populations studied have increased levels of apoptosis (Fig. 1). Similar phenomena have previously been described for secondary lymphoid organs after either T. gondii or Plasmodium chabaudi chabaudi infection (17, 34) and also for nonlymphoid organs such as the liver after Entamoeba histolytica infection (48). Nevertheless, the present results demonstrate a strikingly widespread induction of PCD during T. gondii infection. Thus, cells of both lymphoid and myeloid lineages were triggered by the parasite to undergo apoptosis during acute infection. While RH infection induces massive splenic apoptosis, the proportion of infected cells does not rise above 2% (10). This strongly argues against a direct effect of Toxoplasma in inducing apoptosis, suggesting instead an immunopathological basis underlying parasite-induced PCD.
Both the Fas-FasL and TNF-α-TNFRI pathways may be activated in the end phases of the immune response, in order to modulate excessive responses with possible pathological outcomes and to remove antigen-specific lymphocytes after microbial clearance (6). Indeed, these pathways have been implicated in down-regulating the inflammatory cell infiltrates at the site of lesion during Leishmania major and Trypanosoma cruzi infections (3, 5, 24, 36, 58). We show here that infected FasL−/− and TNFRI−/− mice display low levels of PARP degradation (Fig. 3) and that splenocytes bind low levels of caspase inhibitor, relative to those in infected WT animals (Fig. 4). This indicates that both pathways are involved in Toxoplasma-induced apoptosis. Furthermore, Casp-8 undergoes remarkably rapid degradation in splenocytes from infected WT mice (Fig. 2B). We conclude that T. gondii triggers extracellular pathways of apoptosis through both TNF-α-TNFRI and Fas-FasL interactions.
A role for the mitochondrially induced apoptotic pathway is suggested by our finding that Casp-9 is activated during acute T. gondii infection. This pathway may be activated as a response to stress, and a candidate mediator would be nitric oxide, since iNOS−/− mice display greatly reduced levels of apoptosis (44). Nevertheless, because the mitochondrial pathway may be triggered by Casp-8 through the activity of Bid (Fig. 1) (35), it is possible that Casp-9 activation is mediated by Fas-FasL and TNF-α-TNFRI engagement. Arguing against the latter, we did not detect Bid activation at any point during the acute phase of T. gondii infection (data not shown).
We report here for the first time in an in vivo parasite infection model the tight correlation between apoptosis and STAT-1 activation (Fig. 5). Thus, STAT-1 phosphorylation occurred in WT animals concurrently with the timing of apoptosis, and STAT-1 activation was reduced or absent in those knockout mouse strains in which levels of apoptosis were reduced. Activation of STAT-1, the only known signal transducer and activator of transcription responding to IFN-γ, has been linked to apoptosis in the cases of ischemia-induced cell death in cardiomyocytes and keratinocyte apoptosis after epithelial injury in vivo, while STAT-1-null mice showed resistance to apoptotic stimuli (38, 54). In vitro studies on the mechanisms by which STAT-1 activation may trigger apoptosis have linked the molecule to induction of Casp-1 and Fas-FasL, but the precise mechanisms involved remain obscure (54, 60). The studies reported here suggest that protozoan infection triggers apoptosis in multiple cell types through multiple pathways and that STAT-1 activation is correlated with the presence of PCD. While IFN-γ serves as the major activator of STAT-1, other molecules such as IL-6 and IL-10, as well as platelet-derived growth factor and epidermal growth factor, may activate this transducing molecule (37, 43). Thus, it is possible that the lack of apoptosis in infected FasL−/− and iNOS−/− animals points to a direct role for STAT-1 in PCD triggered through Fas and nitric oxide, although the mechanism linking STAT-1 with FasL and iNOS is not presently clear. This may be an event independent of IFN-γ, as cultures of splenocytes from infected FasL−/− and iNOS−/− animals harbored high levels of IFN-γ (data not shown). Our laboratory is currently further investigating the role of STAT-1 during T. gondii infection.
Apoptosis has previously been linked to acute T. gondii infection (28). Recent studies have demonstrated induction of PCD during high-virulence infection (10, 39). Induction of apoptosis has also been reported during ocular toxoplasmosis, and in Peyer's patch CD4+ T cells in orally infected mice (23, 34). The latter effect was dependent on IFN-γ and was linked to FasL up-regulation. Therefore, it is possible that IFN-γ exerts its proapoptotic effect at least partly through up-regulation of this death receptor.
Why T. gondii would induce such a generalized cell death is not clear. In the context of the mouse, infection with RH and other type I parasite strains is uniformly lethal, and dysregulated apoptosis driven by overproduction of inflammatory mediators may contribute to early death in this model. Inasmuch as human infection with type I strains is not lethal, it is possible that induction of apoptosis would serve as a parasite defense strategy temporarily disabling the immune system and allowing the establishment of long-term infection.
Acknowledgments
This work was supported by NIH grant AI47888.
Editor: J. M. Mansfield
REFERENCES
- 1.Alderson, M. R., T. W. Tough, T. Davis-Smith, S. Braddy, B. Falk, K. A. Schooley, R. G. Goodwin, C. A. Smith, F. Ramsdell, and D. H. Lynch. 1995. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 181:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boothroyd, J. 1993. Population biology of Toxoplasma: clonality, virulence, and speciation (or not). Infect. Agents Dis. 2:100-102. [PubMed] [Google Scholar]
- 3.Castanos-Velez, E., S. Maerlan, L. M. Osorio, F. Aberg, P. Biberfeld, A. Orn, and M. E. Rottenberg. 1998. Trypanosoma cruzi infection in tumor necrosis factor receptor p55-deficient mice. Infect. Immun. 66:2960-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinnaiyan, A. M., K. O'Rourke, M. Tewari, and V. M. Dixit. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505-512. [DOI] [PubMed] [Google Scholar]
- 5.Conceicao-Silva, F., M. Hahne, M. Schronter, J. Louis, and J. Tschopp. 1998. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur. J. Immunol. 28:237-245. [DOI] [PubMed] [Google Scholar]
- 6.Crispe, I. N. 1999. Death and destruction of activated T lymphocytes. Immunol. Res. 19:143-157. [DOI] [PubMed] [Google Scholar]
- 7.Duriez, P. J., and G. M. Shah. 1997. Cleavage of poly(ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem. Cell Biol. 75:337-349. [PubMed] [Google Scholar]
- 8.Frenkel, J. K. 1988. Pathophysiology of toxoplasmosis. Parasitol. Today 4:273-278. [DOI] [PubMed] [Google Scholar]
- 9.Fuentes, I., J. M. Rubio, C. Ramirez, and J. Alvar. 2001. Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J. Clin. Microbiol. 39:1566-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavrilescu, L. C., and E. Y. Denkers. 2001. IFN-gamma overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J. Immunol. 167:902-909. [DOI] [PubMed] [Google Scholar]
- 11.Gazzinelli, R. T., A. Brezin, Q. Li, R. B. Nussenblatt, and C. C. Chan. 1994. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-α and IFN-γ. Exp. Parasitol. 78:217-229. [DOI] [PubMed] [Google Scholar]
- 12.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent upon CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 13.Gonzalez-Garcia, M., R. Perez-Ballestero, L. Ding, L. Duan, L. H. Boise, C. B. Thompson, and G. Nunez. 1994. bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development 120:3033-3042. [DOI] [PubMed] [Google Scholar]
- 14.Grell, M., G. Zimmermann, D. Hulser, K. Pfizenmaier, and P. Scheurich. 1994. TNF receptors TR60 and TR80 can mediate apoptosis via induction of distinct signal pathways. J. Immunol. 153:1963-1972. [PubMed] [Google Scholar]
- 15.Grigg, M. E., J. Ganatra, J. C. Boothroyd, and T. P. Margolis. 2001. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 184:633-639. [DOI] [PubMed] [Google Scholar]
- 16.Heinzel, F. P., R. M. Rerko, F. Ahmed, and E. Pearlman. 1995. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J. Immunol. 155:730-739. [PubMed] [Google Scholar]
- 17.Helmby, H., G. Jonsson, and M. Troye-Blomberg. 2000. Cellular changes and apoptosis in the spleens and peripheral blood of mice infected with blood-stage Plasmodium chabaudi chabaudi AS. Infect. Immun. 68:1485-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heusel, J. W., R. L. Wesselschmidt, S. Shresta, J. H. Russell, and T. J. Ley. 1994. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 76:977-987. [DOI] [PubMed] [Google Scholar]
- 19.Hockenbery, D. M., Z. N. Oltvai, X. M. Yin, C. L. Milliman, and S. J. Korsmeyer. 1993. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75:241-251. [DOI] [PubMed] [Google Scholar]
- 20.Howe, D. K., S. Honore, F. Derouin, and L. D. Sibley. 1997. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J. Clin. Microbiol. 35:1411-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howe, D. K., and L. D. Sibley. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human diseases. J. Infect. Dis. 172:1561-1566. [DOI] [PubMed] [Google Scholar]
- 22.Howe, D. K., B. C. Summers, and L. D. Sibley. 1996. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii. Infect. Immun. 64:5193-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, M. S., J. D. Schwartzman, G. R. Yeaman, J. Collins, R. Seguin, I. A. Khan, and L. H. Kasper. 1999. Fas-FasL interaction involved in pathogenesis of ocular toxoplasmosis in mice. Infect. Immun. 67:928-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, F. P., D. Xu, E. O. Esfandiari, W. Sands, X. Q. Wei, and F. Y. Liew. 1998. Mice defective in Fas are highly susceptible to Leishmania major infection despite elevated IL-12 synthesis, strong Th1 responses, and enhanced nitric oxide production. J. Immunol. 160:4143-4147. [PubMed] [Google Scholar]
- 25.Hunter, C. A., L. A. Ellis-Neyes, T. Slifer, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F. G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 158:3311-3316. [PubMed] [Google Scholar]
- 26.Hunter, C. A., and J. S. Remington. 1994. Regulation of IL-12 and TNF-α induced NK cell production of IFN-γ by IL-1β and TGF-β. Eur. Cytokine Netw. 5:145. [Google Scholar]
- 27.Juo, P., C. J. Kuo, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8:1001-1008. [DOI] [PubMed] [Google Scholar]
- 28.Khan, I. A., T. Matsuura, and L. H. Kasper. 1996. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int. Immunol. 8:887-896. [DOI] [PubMed] [Google Scholar]
- 29.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 30.Lazebnik, Y. A., S. H. Kaufmann, S. Desnoyers, G. G. Poirier, and W. C. Earnshaw. 1994. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371:346-347. [DOI] [PubMed] [Google Scholar]
- 31.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 32.Liesenfeld, O., H. Kang, D. Park, T. A. Nguyen, C. V. Parkhe, H. Watanabe, T. Abo, A. Sher, J. S. Remington, and Y. Suzuki. 1999. TNF-α, nitric oxide and IFN-γ are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21:365-376. [DOI] [PubMed] [Google Scholar]
- 33.Liesenfeld, O., J. Kosek, J. S. Remington, and Y. Suzuki. 1996. Association of CD4+ T cell-dependent, IFN-γ-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liesenfeld, O., J. C. Kosek, and Y. Suzuki. 1997. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect. Immun. 65:4682-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 36.Martins, G. A., L. Q. Vieira, F. Q. Cunha, and J. S. Silva. 1999. Gamma interferon modulates CD95 (Fas) and CD95 ligand (Fas-L) expression and nitric oxide-induced apoptosis during the acute phase of Trypansoma cruzi infection: a possible role in immune response control. Infect. Immun. 67:3864-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meraz, M. A., J. M. White, K. C. Sheehan, E. A. Bach, S. J. Rodig, A. S. Dighe, D. H. Kaplan, J. K. Riley, A. C. Greenlund, D. Campbell, K. Carver-Moore, R. N. DuBois, R. Clark, M. Aguet, and R. D. Schreiber. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84:431-442. [DOI] [PubMed] [Google Scholar]
- 38.Mohan, R. R., R. R. Mohan, W. J. Kim, G. R. Stark, and S. E. Wilson. 2000. Defective keratocyte apoptosis in response to epithelial injury in stat 1 null mice. Exp. Eye Res. 70:485-491. [DOI] [PubMed] [Google Scholar]
- 39.Mordue, D. G., F. Monroy, M. La Regina, C. A. Dinarello, and L. D. Sibley. 2001. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 167:4574-4584. [DOI] [PubMed] [Google Scholar]
- 40.Newmeyer, D. D., D. M. Farschon, and J. C. Reed. 1994. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell 79:353-364. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson, D. W., and N. A. Thornberry. 1997. Caspases: killer proteases. Trends Biochem. Sci. 22:299-306. [DOI] [PubMed] [Google Scholar]
- 42.Nunes, M. P., R. M. Andrade, M. F. Lopes, and G. A. DosReis. 1998. Activation-induced T cell death exacerbates Trypanosoma cruzi replication in macrophages cocultured with CD4+ T lymphocytes from infected hosts. J. Immunol. 160:1313-1319. [PubMed] [Google Scholar]
- 43.Rane, S. G., and E. P. Reddy. 2002. JAKs, STATs and Src kinases in hematopoiesis. Oncogene 21:3334-3358. [DOI] [PubMed] [Google Scholar]
- 44.Richter, C., M. Schweizer, A. Cossarizza, and C. Franceschi. 1996. Control of apoptosis by the cellular ATP level. FEBS Lett. 378:107-110. [DOI] [PubMed] [Google Scholar]
- 45.Russell, J. H., and T. J. Ley. 2002. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20:323-370. [DOI] [PubMed] [Google Scholar]
- 46.Sato, T., M. Hanada, S. Bodrug, S. Irie, N. Iwama, L. Boise, C. B. Thompson, E. Golemis, L. Fong, H. G. Wang, and J. C. Reed. 1994. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc. Natl. Acad. Sci. USA 91:9238-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, L. Showe, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-γ mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 48.Seydel, K. B., and S. L. Stanley, Jr. 1998. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent pathway of apoptosis. Infect. Immun. 66:2980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shall, S., and G. de Murcia. 2000. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat. Res. 460:1-15. [DOI] [PubMed] [Google Scholar]
- 50.Sher, A., D. F. Fiorentino, P. Caspar, E. Pearce, and T. Mosmann. 1991. Production of IL-10 by CD4+ lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J. Immunol. 144:2713-2716. [PubMed] [Google Scholar]
- 51.Sibley, L. D., and J. C. Boothroyd. 1992. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359:82-85. [DOI] [PubMed] [Google Scholar]
- 52.Sibley, L. D., D. G. Mordue, C. Su, P. M. Robben, and D. K. Howe. 2002. Genetic approaches to studying virulence and pathogenesis in Toxoplasma gondii. Philos. Trans. R. Soc. Lond. B 357:81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stephanou, A., T. M. Scarabelli, B. K. Brar, Y. Nakanishi, M. Matsumura, R. A. Knight, and D. S. Latchman. 2001. Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J. Biol. Chem. 276:28340-28347. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 56.Talanian, R. V., X. Yang, J. Turbov, P. Seth, T. Ghayur, C. A. Asiano, K. Orth, and C. J. Froelich. 1997. Granule-mediated killing: pathways for granzyme B-initiated apoptosis. J. Exp. Med. 186:1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor, G. A., C. M. Collazo, G. S. Yap, K. Nguyen, T. A. Gregorio, L. S. Taylor, B. Eagleson, L. Secret, E. A. Southon, S. W. Reid, L. Tessarollo, M. Bray, D. W. McVicar, K. L. Komschlies, H. A. Young, C. A. Biron, A. Sher, and G. F. Vande Woude. 2000. Pathogen-specific loss of host resistance in mice lacking the IFN-γ-inducible gene IGTP. Proc. Natl. Acad. Sci. USA 97:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vieira, L. Q., M. Goldschmidt, M. Nashleanas, K. Pfeffer, T. Mak, and P. Scott. 1996. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J. Immunol. 157:827-835. [PubMed] [Google Scholar]
- 59.Wang, K., X. M. Yin, D. T. Chao, C. L. Milliman, and S. J. Korsmeyer. 1996. BID: a novel BH3 domain-only death agonist. Genes Dev. 10:2859-2869. [DOI] [PubMed] [Google Scholar]
- 60.Xu, X., X. Y. Fu, J. Plate, and A. S. Chong. 1998. IFN-γ induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res. 58:2832-2837. [PubMed] [Google Scholar]
- 61.Yin, X. 2000. Bid, a critical mediator for apoptosis induced by the activation of Fas/TNF-R1 death receptors in hepatocytes. J. Mol. Med. 78:203-211. [DOI] [PubMed] [Google Scholar]
- 62.Zhivotovsky, B., A. Gahm, M. Ankarcrona, P. Nicotera, and S. Orrenius. 1995. Multiple proteases are involved in thymocyte apoptosis. Exp. Cell Res. 221:404-412. [DOI] [PubMed] [Google Scholar]