Abstract
The myocardium of CD1 mice was examined for the activation of signal transduction pathways leading to cardiac inflammation and subsequent remodeling during Trypanosoma cruzi infection (Brazil strain). The activity of three pathways of the mitogen-activated protein kinases (MAPKs) was determined. Immunoblotting revealed a persistent elevation of phosphorylated (activated) extracellular-signal-regulated kinase (ERK), which regulates cell proliferation. During infection there was a transient activation of p38 MAPK but no activation of Jun N-terminal kinase. Early targets of activated ERK, c-Jun and c-Fos, were elevated during infection, as demonstrated by semiquantitative reverse transcription-PCR. Immunostaining revealed that the endothelium and the interstitial cells were most intensely stained with antibodies to c-Jun and c-Fos. Soon after infection, AP-1 and NF-κB DNA binding activity was increased. Protein levels of cyclin D1, the downstream target of ERK and NF-κB, were induced during acute infection. Immunostaining demonstrated increased expression of cyclin D1 in the vascular and endocardial endothelium, inflammatory cells, and the interstitial areas. Increased expression of the cyclin D1-specific phosphorylated retinoblastoma protein (Ser780) was also evident. Immunoblotting and immunostaining also demonstrated increased expression of proliferating cellular nuclear antigen that was predominantly present in the inflammatory cells, interstitial areas (i.e., fibroblasts), and endothelium. These data demonstrate that T. cruzi infection results in activation of the ERK-AP-1 pathway and NF-κB. Cyclin D1 expression was also increased. These observations provide a molecular basis for the activation of pathways involved in cardiac remodeling in chagasic cardiomyopathy.
Infection with the protozoan hemoflagellate parasite Trypanosoma cruzi causes Chagas' disease. The important manifestations of Chagas' disease include acute myocarditis and chronic cardiomyopathy (37, 57). Chagas' disease continues to be a serious health problem in Mexico and Central and South America and has recently emerged as an opportunistic infection in the setting of AIDS (43).
T. cruzi infection is lifelong, and chagasic heart disease represents a unique interplay of ischemic and inflammatory changes, resulting in cardiac myocyte hypertrophy (2), cardiac remodeling, and the eventual development of chronic cardiomyopathy. Acute chagasic myocarditis plays an important role in the development of chronic cardiomyopathy and is characterized by intense inflammation, myonecrosis, myocytolysis, vasculitis, and numerous parasite pseudocysts. These pathological changes are accompanied by the increased expression of myocardial cytokines, chemokines, nitric oxide synthase, endothelin-1 (ET-1), and kinins (10, 18, 19, 38, 45, 56, 57, 60). Chagasic heart disease is also accompanied by vasculopathy (37, 41), manifested by microvascular spasm and decreased blood flow (37, 57, 58). Similarly, in the other examples of myocardial injury activation of several signaling pathways involving cytokines, chemokines, NF-κB, vascular adhesion molecules, transforming growth factor beta, and ET-1 and the mitogen-activated protein kinases (MAPKs) is observed (4, 9, 23, 24, 30-32, 42, 48, 50, 55, 59).
Eukaryotic cell division has been divided into distinct phases, originating from observations of periods of distinct biological activity. The orderly progression of cells through the phases of the cell cycle is governed by the sequential assembly and activation of holoenzyme complexes, comprised of a regulatory subunit (cyclin) and a catalytic subunit (cyclin-dependent kinase [Cdk]), both of which are distinct for each phase (5, 35, 39). The MAPK pathways, ET-1, and cell cycle-regulatory proteins, including cyclin D1, participate in the regulation of cell proliferation and cardiac remodeling (4, 7, 24, 27, 28, 37, 42, 54). Importantly, cyclin D1, a regulator of cellular proliferation, is itself regulated by extracellular-signal-regulated kinase (ERK), a component of the MAPK pathway and ET-1 (35, 52). The cell cycle-regulatory protein cyclin D1 is an important mediator of G1 phase progression and a downstream target of multiple proliferative signaling pathways, including MAPKs, NF-κB, and activating transcription factor 1 (AP-1) (5, 35, 39, 47).
We found that myocardial injury following T. cruzi infection resulted in increased expression of those proteins known to be associated with cellular proliferation, such as proliferating cell nuclear antigen (PCNA). Since the MAPK pathways have been implicated in cellular proliferation as a result of myocardial injury (48), we undertook an investigation of this pathway in chagasic myocarditis. T. cruzi infection was found to activate ERK and the transcription factors AP-1 and NF-κB as well as the downstream target cyclin D1. These data suggest that activation or induction of these signaling pathways in T. cruzi-induced cardiovascular disease likely contributes to the observed cardiovascular remodeling as assessed by histopathology and analysis of myocardial structure and function (8, 19). Furthermore, these studies into the mechanisms that control the proliferation of cells of the cardiovascular system during T. cruzi infection are important in advancing our understanding of the pathogenesis of chagasic heart disease and in providing possible targets for adjunctive therapy.
MATERIALS AND METHODS
Infection of mice.
Eight- to 10-week-old male CD1 mice (Jackson Laboratories, Bar Harbor, Maine) were infected with 5 × 104 trypomastigotes of the Brazil strain of T. cruzi. Hearts were obtained for histology, immunostaining, and protein and RNA isolation.
Immunoblotting.
Homogenates were obtained from mouse hearts as previously described (20). Approximately 30 to 50 μg of homogenate was dissolved in sample buffer, boiled for 5 min, and separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE). Samples were then transferred onto nitrocellulose membranes (0.2 μM) by electroblotting. Analysis of phosphorylated (activated) ERK, Jun N-terminal kinase (JNK), p38 MAPK, and retinoblastoma protein (pRb) was performed with antibodies obtained from Cell Signaling (Beverly, Mass.). Antibodies to ERK and p38 MAPK were polyclonal, while the antibody to JNK was monoclonal. Analysis of PCNA was performed with a monoclonal antibody obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). The abundance of cyclin D1 protein was determined with a monoclonal cyclin D1 antibody, AB3 (polyclonal) or DCS-6 (monoclonal) (NeoMarkers, Fremont, Calif.). Filters were probed with primary antibodies at a dilution of 1:500, and the appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz) was used at a dilution of 1:5,000. Signals of bound antibodies were visualized with Western blotting protocols (ECL; Amersham Life Science). Coomassie blue staining was used to determine protein integrity, and a GDP-disassociated inhibitor antibody, a gift of P. Scherer, was used to assess equal loading efficiency.
RT-PCR studies.
Total RNA was isolated from mouse hearts with Trizol reagent according to the protocol of the manufacturer (Gibco-BRL, Grand Island, N.Y.). First-strand cDNA was prepared by incubation of 1 μg of total RNA with murine leukemia virus reverse transcriptase and oligo(dT)16 primer at 42°C for 15 min. Then 2 μl of the reaction products was amplified by PCR with 2.5 U of Taq polymerase (Perkin-Elmer, Branchburg, N.J.). PCR amplification consisted of 95°C for 30 s for denaturation, 60°C for 40 s for annealing, and 72°C for 2 min for extension from 25 to 35 cycles in the linear range of the amplification number. The primers used were c-jun forward (5′-GCA TGA GGA ACC GCA TTG CCG CCT CCA AGT-3′), c-jun reverse (5′-CAG TCT GCT GCA TAG AAG GAA CCG-3′), c-fos forward (5′-GAG CTG ACA GAT ACA CTC CAA GCG-3′), and c-fos reverse (5′-CAG TCT GCT GCA TAG AAG GAA CCG-3′). Primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as controls. Aliquots of 10 μl of the PCR products were electrophoresed in a 1.6% agarose gel containing ethidium bromide.
Electrophoretic mobility shift assays for AP-1 and NF-κB.
Cardiac protein extracts were prepared as described by Chandrasekar et al. (9). Briefly, the frozen myocardium was broken into small pieces in the presence of liquid nitrogen, and protein extracts were prepared by homogenizing (Polytron; 4°C; 18,000 rpm) the tissue in a buffer containing 20 mM HEPES (pH 7.8), 300 mM NaCl, 0.4 mM EDTA, 0.5 mM dithiothreitol, 25% glycerol, and 0.5 mM phenylmethylsulfonyl fluoride and centrifuged at 14,000 rpm for 10 min at 4°C. Protein extract concentration was determined with the Bio-Rad assay. The extracts were frozen on dry ice and stored at −80°C. Aliquots of 20 μg of cardiac protein extracts from infected mice and uninfected mice were checked for [32P]AP-1 and [32P]NF-κB consensus sequence oligonucleotide (AP-1: 5′-CGC TTG ATG AGT CAG CCG GAA-3′; NF-κB: 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; from Promega, Madison, Wis.) binding by electrophoretic mobility shift assay as previously described (18). A 50-fold molar excess of unlabeled oligonucleotides (AP-1, NF-κB, and SP-1) was used for competition assays to determine the specificity. In addition, specific antibodies for c-Fos and c-Jun as well as p50 and p65 (Santa Cruz Biotechnology, Santa Cruz, Calif.) were used for a supershift assay to identify the proteins involved in the complexes, as previously described (18).
Immunostaining.
These studies were performed with polyclonal antibodies to c-Fos, c-Jun, and PCNA (Santa Cruz Biotechnology), cyclin D1 (Cell Signaling), and the NF-κB components p50 and p65 (polyclonal antibodies obtained from Santa Cruz Biotechnology). Paraffin-embedded sections were used for these antibodies. For studies on phospho-ERK, hearts were immediately removed, embedded in optimal cutting temperature compound, and flash frozen in liquid nitrogen. Sections (5-μm thick) were then cut with a cryostat kept at −17°C and placed on a Super-Frost Plus slide. Sections were fixed in chilled acetone for 2 min before being immunostained. The subsequent staining protocol was carried out in a moist chamber to avoid dehydration. Each sample was blocked for 60 min with phosphate-buffered saline containing 10% horse serum, 3% bovine serum albumin, and 0.1% Triton X-100 at room temperature. The slides were next incubated for 1 h with primary antibodies, anti-phospho-ERK (polyclonal antibody obtained from Cell Signaling), and an anti-caveolin-3 monoclonal antibody (a generous gift of Robert Campos-Gonzalez, BD Transduction Laboratories) diluted in phosphate-buffered saline-3% bovine serum albumin according to the manufacturer's instructions. The slides were washed thrice for 10 min each in phosphate-buffered saline and incubated with fluorescein-conjugated anti-mouse immunoglobulin antibody and/or a rhodamine-conjugated anti-rabbit immunoglobulin antibody (Jackson ImmunoResearch Inc., West Grove, Pa.) at a 1:150 dilution. The samples were again washed thrice for 10 min each in phosphate-buffered saline and dried briefly. A drop of Slow-Fade antifade reagent (Molecular Probes, Inc., Eugene, Oreg.) was added, the slides were mounted with a standard coverslip, and the edges were sealed with nail polish. The slides were imaged at the Analytical Imaging Facility at the Albert Einstein College of Medicine. Control slides for all reactions were done with the omission of the primary antibodies. In all cases this prevented immunostaining, confirming the specificity of the reactions.
RESULTS
Parasitology and pathology.
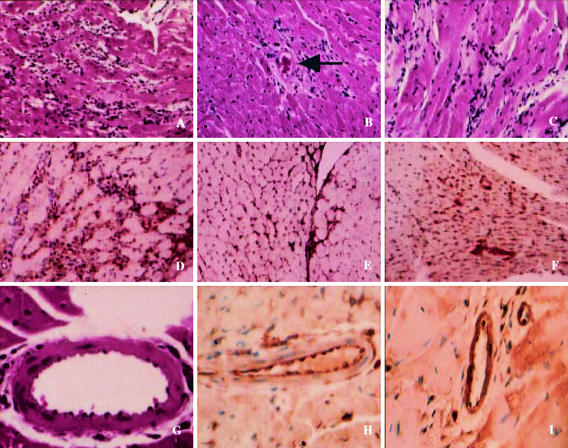
The mortality of Brazil strain-infected CD1 mice was 60% by day 35 postinfection. These data are consistent with our previously published studies on mortality with this strain of T. cruzi in CD1 mice (8). Histopathological examination of the myocardium of Brazil-infected CD1 mice revealed myonecrosis, inflammation, vasculitis, and multiple parasite pseudocysts (Fig. 1A, 1B, 1C, and 1G).
FIG. 1.
Representative histopathology and immunohistochemistry of myocardial sections of CD1 mice infected with the Brazil strain of T. cruzi. (A) Acute myocarditis, demonstrating inflammation, myonecrosis, and pseudocysts. (B) Vasculitis of the coronary artery during acute infection. (C) Chronic myocarditis with inflammation and fibrosis. (D) Acute myocarditis with staining with anti-PCNA antibody. There is intense staining of the interstitial areas containing fibroblasts, endothelium, and inflammatory infiltrates. (E) Myocardial section obtained from a mouse at day 150 postinfection. There is staining of the interstitial areas with anti-PCNA antibody. (F) Myocardial section obtained from a mouse at day 150 postinfection. There is staining of fibroblasts with anti-cyclin D1 antibody within an area of scar formation. (G) Section of the coronary artery of an infected mouse. Note the vasculitis and margination of leukocytes. (H) Coronary artery of an infected mouse 14 days postinfection stained with anti-p65 antibody. (I) Coronary artery of an infected mouse 14 days postinfection stained with anti-c-Fos antibody. Note the positive staining of the endothelium.
Proliferating cell nuclear antigen expression.
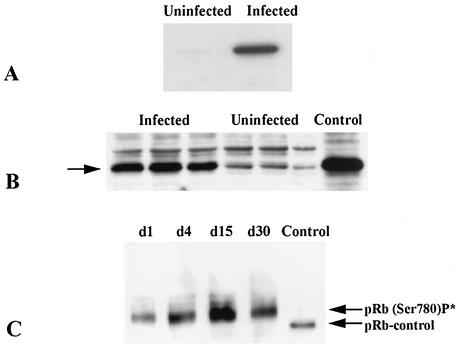
Immunostaining with an anti-PCNA antibody revealed that the inflammatory cells, the interstitial areas (fibroblasts), and the endothelium were the most intensely stained in samples obtained from the myocardium of infected mice (Fig. 1D and 1E). Such staining was evident at 15, 30, 60, and 150 days postinfection. Immunoblotting done at 60 days postinfection detected an increased expression in the myocardium (Fig. 2A).
FIG. 2.
Representative immunoblots of myocardial lysates obtained from infected CD1 mice. (A) PCNA expression was induced 60 days postinfection. Uninfected and infected lysates were probed with anti-PCNA antibody (36 kDa). The experiment was performed on three separate occasions on three different hearts. (B) Cyclin D1 expression 30 days postinfection. This immunoblot demonstrates increased expression (34 kDa). This increased expression was evident as early as day 1 postinfection and persisted until day 60 postinfection. (C) Expression of phosphorylated retinoblastoma protein (pRb) (Ser 780), 110 kDa, was increased. There was no expression prior to infection. In all cases, equal protein loading was determined by Coomassie blue staining or GDP-disassociation inhibitor expression.
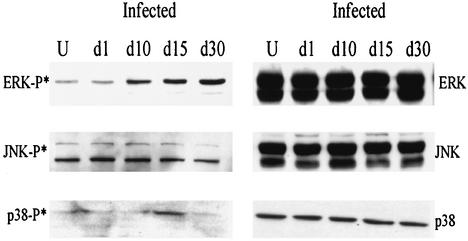
Immunoblotting for phosphorylated ERK, JNK, and p38 MAPK in the myocardium of infected mice.
Immunoblotting with an anti-phospho-ERK antibody revealed a significant increase in phosphorylated ERK abundance from day 1 to day 30 postinfection in the myocardium of infected mice (Fig. 3). Phosphorylated ERK was also detected at day 60 and day 120 postinfection in some samples (data not shown), indicating that Raf-ERK activation persisted in the mouse myocardium in response to T. cruzi infection. There was no change in the abundance of unphosphorylated ERK isoforms following infection. In addition, there was a transient increase in phosphorylated p38 MAPK at day 15 postinfection, while the level of phosphorylated JNK was unchanged (Fig. 3).
FIG. 3.
Representative immunoblots for total and phosphorylated ERK, JNK, and p38 MAPK in the myocardium of infected and uninfected (U) mice days 1 to 30 postinfection.
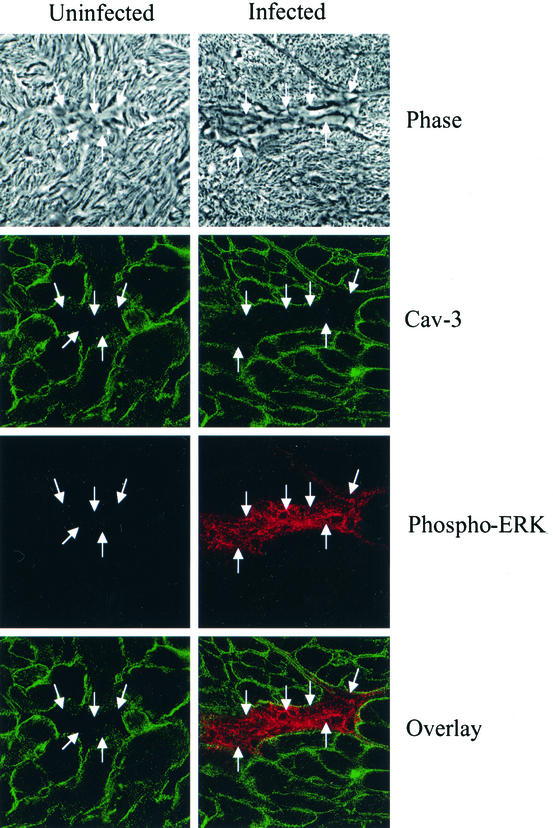
Immunostaining for phosphorylated ERK.
To determine the origin of the activated ERK in the myocardium, we performed immunostaining of frozen myocardial sections obtained from uninfected and infected mice (day 15 postinfection). We used antibodies to phosphorylated ERK and caveolin-3. Anti-caveolin-3 antibody was used as a marker of cardiac myocytes (Fig. 4). There was no phosphorylated ERK staining in the uninfected section. In addition, the double-stained sections did not demonstrate activated ERK in cardiac myocytes but rather in the interstitial areas containing fibroblasts.
FIG. 4.
Phospho-ERK expression. Representative myocardial sections obtained from uninfected and T. cruzi-infected mice 15 days postinfection. The upper panels represent phase contrast microscopy of representative interstitial areas (arrows) composed of fibroblasts and the endothelium. Immunostaining of frozen myocardial sections was performed with antibodies to phosphorylated ERK (red stain). Anticaveolin-3 antibody was used as a marker of cardiac myocytes (green stain). There was no phosphorylated ERK staining in the uninfected section. The double-stained sections did not demonstrate phosphorylated ERK expression in cardiac myocytes.
Semiquantitative RT-PCR for c-fos and c-jun in myocardium of infected mice.
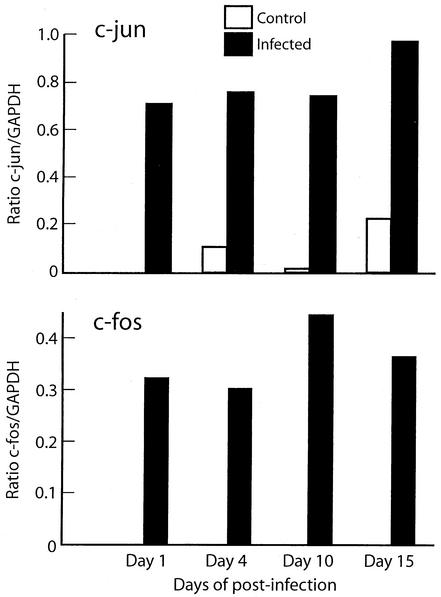
c-fos and c-jun are downstream targets of ERK. Therefore, we performed RT-PCR with specific c-fos and c-jun primers to examine the expression of these early-response genes in response to T. cruzi infection. The results were analyzed by densitometry, and the ratio of the optical density of c-fos to GAPDH and of c-jun to GAPDH was used to demonstrate gene expression. There was no expression in uninfected control mice. There was a significant increase in abundance in infected mice from days 1 to 15 postinfection compared with uninfected mice (Fig. 5). At days 30 and 60 postinfection, the expression of both genes was still detected by RT-PCR (data not shown).
FIG. 5.
Time course of cardiac c-Jun and c-Fos expression. Total cardiac RNA was extracted from infected CD1mice on days 1 to 15 of infection. RT-PCR with specific (A) c-fos and (B) c-jun primers was used and quantitated by densitometry. The ratio of the optical density of c-fos and c-jun to GAPDH was used for this plot.
Immunostaining for c-Jun and c-Fos.
These studies revealed staining of c-Fos and c-Jun in the myocardium, endothelium, endocardium, and capillaries. However, staining was more intense in the vascular endothelium and endocardial endothelium obtained from infected mice (Fig. 1I). c-Fos and c-Jun were detected in the myocardium at early (day 14 postinfection) and late (day 120 postinfection; data not shown) time points.
AP-1 assays.
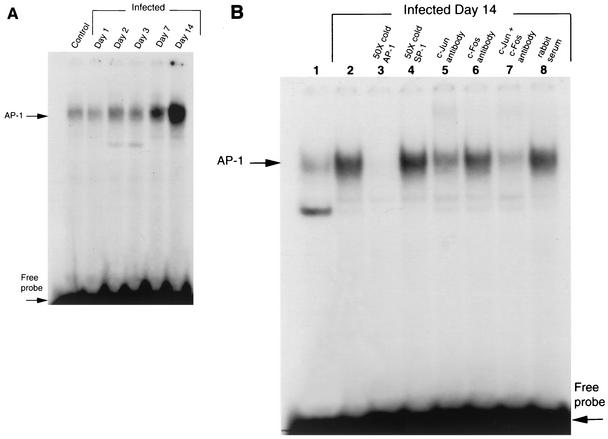
The transcription factor AP-1 is a downstream target of ERK. Therefore, we performed studies to examine consensus sequence oligonucleotide binding specificity and define the proteins involved in the binding complexes. Cardiac protein extracts from infected mice at 15 days postinfection and age-matched uninfected mice were analyzed for [32P]AP-1 consensus sequence oligonucleotide binding by electrophoretic mobility shift assay (Fig. 6B). There was a significant increase in DNA binding activity in infected versus uninfected samples (Fig. 6B, lanes 1 and 2). In lane 3, pretreatment with the 50-fold excess of unlabeled AP-1 but not SP1 (Fig. 6B, lane 4) consensus oligonucleotide completely abolished the signal. Supershift assay with either anti-c-Jun (Fig. 6B, lane 5) or anti-c-Fos (Fig. 6B, lane 6) antibodies decreased the intensity of the signal. Supershift assays with both anti-c-Jun and anti-c-Fos antibodies almost completely abolished the signal (Fig. 6B, lane 7). However, supershift assay with nonimmunized rabbit serum failed to suppress the signal, indicating the effects of c-Jun and c-Fos antibodies interaction with the protein extracts were specific (Fig. 6B, lane 8).
FIG. 6.
(A) Time course of AP-1 DNA binding activity in infected mouse heart. Cardiac protein extracts from infected mice on days 1 to 14 days postinfection were checked for [32P]AP-1 consensus sequence oligonucleotide. (B) AP-1 DNA binding activity in the myocardium of infected mice. Myocardial protein extracts from infected mice at 14 days postinfection and uninfected mice were checked for [32P]AP-1 consensus sequence oligonucleotide binding by electrophoretic mobility shift assay. Lane 1, uninfected control; lanes 2 to 8, protein extract from infected myocardium; lane 3, pretreated with a 50-fold excess of unlabeled AP-1 oligonucleotide prior to incubation with labeled AP-1 oligonucleotide (50X cold); lane 4, pretreated with a 50-fold excess of unlabeled SP-1consensus oligonucleotide prior to incubation with labeled AP-1 oligonucleotide; lane 5, preincubated with anti-c-Jun antibody prior to incubation with labeled AP-1 oligonucleotide; lane 6, preincubated with anti-c-Fos antibody before incubation with labeled AP-1 oligonucleotide, lane 7, preincubated with both anti-c-Jun and anti-c-Fos antibodies prior to incubation with labeled AP-1 oligonucleotide, lane 8, pretreated with nonimmunized rabbit serum prior to incubation with labeled AP-1 oligonucleotide. These data indicate that in the myocardium of infected mice, there was an increase in AP-1 DNA binding activity that was specific. The band contains both c-Jun and c-Fos.
We performed a time course of AP-1 DNA binding activity in the hearts of infected mice. In Fig. 6A, electrophoretic mobility shift assay detected increased AP-1 DNA binding activity from day 2 to day 14 postinfection as a result of infection with T. cruzi. The increased AP-1 DNA binding activity was also found at days 30 and 60 postinfection (data not shown). These studies indicate that AP-1 is activated as a result of infection.
NF-κB assays.
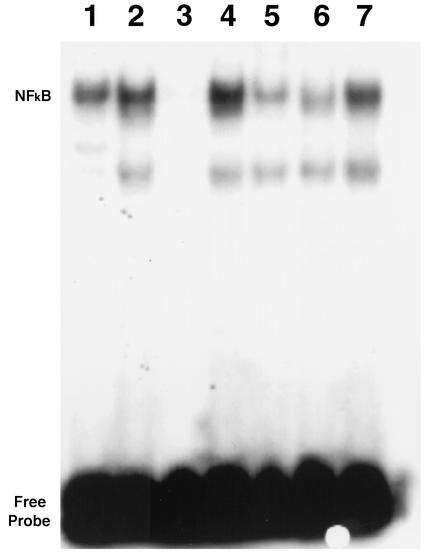
NF-κB is a transcription factor that contributes to the induction of cytokines and cyclin D1. We examined [32P]NF-κB consensus sequence oligonucleotide binding activity (Fig. 7). There was a low background signal in uninfected mice (Fig. 7, lane 1). NF-κB DNA binding was increased in the myocardium of infected mice (Fig. 7, lane 2). Competition assay with unlabeled oligonucleotides (NF-κB and SP-1) indicated that the binding was specific to NF-κB (Fig. 7, lanes 3 and 4). Supershift assays demonstrated that the signal contained p50 and p65 subunits (Fig. 7, lanes 5, 6, and 7). Immunohistochemistry employing antibodies to p50 and p65 showed more intense staining in the vasculature (Fig. 1H).
FIG. 7.
NF-κB DNA binding activity in the myocardium of infected mice. [32P]NF-κB consensus sequence oligonucleotide binding activity was assessed by electrophoretic mobility shift assay with protein extracts from the myocardium of infected and uninfected mice on day 14 of infection. There was a low background signal in uninfected mice (lane 1). Lanes 2 to 7, infected mice; lane 2, infection increases the binding activity; lane 3, pretreatment with a 50-fold excess of unlabeled NF-κB oligonucleotide prior to incubation of labeled NF-κB oligonucleotide; lane 4, protein extract pretreated with a 50-fold excess of unlabeled SP-1consensus sequence oligonucleotide before incubation with labeled NF-κB (this treatment failed to suppress the intensity of the signal, indicating that protein in the signal specifically bound to NF-κB); lane 5, preincubation with antibody specific for p50 prior to incubation with labeled NF-κB oligonucleotide; lane 6, pretreatment with antibody against p65 before incubation with labeled NF-κB oligonucleotide; lane 7, pretreatment with nonimmunized rabbit serum.
Cyclin D1 expression.
Cyclin D1 is an important downstream target of ERK and NF-κB. Western blot revealed that the expression of cyclin D1 was increased by the first day of infection and persisted through day 60 postinfection (Fig. 2B). Immunohistochemistry revealed the most intense staining to be in the vascular and the interstitial areas of infected myocardium (Fig. 1F).
Retinoblastoma protein.
Immunoblotting revealed that the phosphorylation of cyclin D1-specific pRB (Ser780) was increased from day 1 to day 60 postinfection (Fig. 2C). There was no increase in pRb phosphorylation with Ser380 antibody (non-cyclin D1 specific; data not shown). In addition, expression of total pRB was unchanged. These observations underscore the specificity of the cyclin D1 contribution to cardiac remodeling (8, 19).
DISCUSSION
During acute infection, T. cruzi gains access to the cardiac myocytes by first invading endothelial cells, the interstitial areas of the vascular wall, and the myocardium. Thus, damage to these cells and the extracellular matrix occurs as a result of the invasion process, parasite products, and the inflammatory response. Subsequently, remodeling occurs, resulting in structural changes associated with inflammation, necrosis, cardiac myocyte hypertrophy, fibrosis, and ventricular dilation as well as aneurysm formation (57). These phenomena have been demonstrated in human disease and in animal models. However, the mechanisms of cellular proliferation and subsequent cardiac remodeling in Chagas' disease has not been explored in detail.
In the present report as well as previously published studies, we observed evidence of cardiovascular remodeling. This was demonstrated by histopathology, echocardiography, and cardiac magnetic resonance imaging (8, 19). In this paper, immunohistochemistry with anti-PCNA antibody demonstrated evidence of cellular proliferation in the myocardium as early as 15 days postinfection, which did not appear to involve cardiac myocytes. However, Arnaiz et al. (2) recently demonstrated PCNA-positive staining in the inflammatory areas of the myocardium of T. cruzi-infected rats, including cardiac myocytes.
Since the MAPK pathway has been shown to be an important regulator of cellular proliferation and remodeling in animal models of myocardial injury (4, 30, 38, 46, 52), we investigated the activation of this pathway in the myocardium of T. cruzi-infected mice. Of the three major MAPKs in mammalian cells (ERK, JNK, and p38 MAPK), ERK and JNK contributed to the activation of AP-1. By immunoblotting, we demonstrated induction of ERK phosphorylation, which was persistent, in the myocardium of infected mice. Immunostaining suggested that the source of the phosphorylated ERK was the interstitial areas of the infected myocardium. These areas contained fibroblasts and inflammatory cells. Similar areas of uninfected myocardium did not reveal staining. Cardiac myocytes were shown not to be the major source of phosphorylated ERK in infected mice.
The induction of ERK in fibroblasts likely causes induction of factors such as ET-1 and transforming growth factor beta, which have been shown to be important in cardiac myocyte hypertrophy and remodeling (16, 51). Phosphorylated p38 MAPK was only transiently elevated during the 30-day period of observation. An elevation of activated myocardial JNK was not detected by immunoblotting, suggesting that this pathway may not be activated or that the activation may be at a lower level. These data differ from models of myocardial infarction, ischemia, ischemia/reperfusion, and congestive heart failure in which expression of both JNK and p38 MAPK is rapidly increased (48).
Although this is, to our knowledge, the first report of the activation of ERK and the NF-κB-cyclin D1 pathway in the myocardium of T. cruzi-infected mice, there have been similar reports for cultured cells. For example, Villalta et al. (62) demonstrated that trypomastigotes activate MAPKs in macrophages. In addition, Chuenkova and Pereira (13) demonstrated that T. cruzi trans-sialidase induces cell differentiation in PC12 cells via induction of ERK. In addition, viruses and chlamydiae have also been shown to activate ERK in cultured cells (3, 22, 26) as well as in mice (33).
Among the downstream targets of ERK is the family of AP-1 transcription factors composed of c-Jun and c-Fos proteins (29). RT-PCR studies revealed that the expression of mRNAs for c-jun and c-fos was increased as a result of T. cruzi infection. Immunohistochemistry revealed that protein expression of c-Jun and c-Fos was increased in the myocardium of acutely infected mice, especially in the vasculature. In addition, we found that myocardial AP-1 DNA binding activity was increased in the myocardium of infected mice and that the proteins involved were c-Jun and c-Fos. Previous studies from this laboratory have demonstrated that the peptide ET-1 contributes to the pathogenesis of chagasic heart disease (37, 38) and that AP-1 is important in the transcriptional regulation of ET-1 (30). ERK, ET-1, and NF-κB induce the expression of cyclin D1, an important regulator of cell proliferation (35, 52). The present study revealed an increased expression of myocardial cyclin D1 during infection. An increase in the expression of cyclin D1 was observed from days 1 to 60 postinfection by immunoblotting. Immunohistochemical analysis revealed increased staining of fibroblasts in infected myocardium even in chronic infection (e.g., day 120 postinfection). In addition, the phosphorylation of pRB (Ser780), a major substrate of the G1-dependent cyclin-dependent kinases, was increased. The phosphorylation of the cyclin D1-specific pRb was increased, but total pRb expression was unchanged. These observations also underscore the specificity of the cyclin D1 contribution to cardiac remodeling via this protein (pRb).
It is now well established that the transcription factor NF-κB is an important regulator of inflammatory genes. It is in turn activated by a variety of stimuli, including reactive oxygen intermediates, cytokines, hypoxia, and MAPKs. NF-κB plays a role in cardiovascular disease (61). For example, this transcription factor has been shown to contribute to the development of arteriosclerosis and in mediation of apoptosis as well as in the modulation of myocardial ischemia and ischemia/reperfusion injury. To underscore this role, recent studies have demonstrated that inhibition of NF-κB reduced the size of an infarct in an experimental model of myocardial infarction (61). Importantly, recent evidence demonstrates that inflammation is an important component in the development of congestive heart failure of various etiologies. In myocardial samples taken from humans with congestive heart failure, NF-κB has been shown to be activated (61). NF-κB is activated in endothelial cells infected with Borrelia burgdorferi (14) and T. cruzi (18).
Cardiac fibroblasts contribute to cardiac remodeling by influencing the development of fibrosis and cardiac myocyte hypertrophy. Stimulation of cardiac fibroblasts results in the synthesis of ET-1 (30) and transforming growth factor beta (16, 51) and leads to fibrosis, deposition of collagen, and cardiac hypertrophy, as observed in the myocardium of T. cruzi-infected mice (57). Our present study clearly demonstrates that T. cruzi infection activates the ERK-AP-1 pathway, which has been shown to induce the synthesis of ET-1 (30).
Coronary angioplasty and subsequent restenosis are other models of vascular injury that have been investigated extensively. The injury to the vessel results in smooth muscle cell proliferation, migration, and the production of extracellular matrix. Activation of ERK and AP-1 resulting in smooth muscle cell proliferation occurs as a result of balloon injury to the carotid and coronary arteries in animal models and has been reported to be reduced by the administration of PD98059, an inhibitor of the MAPK pathway (6, 17, 34, 36). In addition, shortly following balloon angioplasty, there is a marked induction of cyclins and cyclin-dependent kinases (11, 24, 25, 34, 63). Experimental studies have targeted cyclins and cyclin-dependent kinases in order to reduce neointimal formation in injured vasculature subsequent to balloon angioplasty. For example, antisense oligonucleotides directed against cyclin-dependent kinase 2 and/or cell division cycle 2 and PCNA inhibited smooth muscle cell proliferation (34, 53, 63). Likewise, localized arterial injection of a nonphosphorylatable form of a dominant negative protein gene product (pRb) at the time of angioplasty blocked cellular proliferation (11).
Chagasic heart disease is a result of ischemia and inflammatory injury. Although alterations in myocardial signaling have been explored extensively in other forms of cardiovascular injury, such as that following myocardial infarction and balloon angioplasty, these events have not been fully investigated in chagasic heart disease. Our data are consistent with the idea that activation of ERK, AP-1, and NF-κB and downstream targets such as ET-1 and cyclin D1 contributes to cardiac remodeling following injury due to T. cruzi infection. The possibility of targeting components of the MAPK or the cell cycle pathways is now gaining acceptance as a therapeutic modality for cardiovascular disease, and the data in this paper suggest that this type of adjunctive therapy may be also useful in Chagas' disease (1, 6, 21, 27, 34, 49).
Acknowledgments
This work was supported in part by grants to H.B.T. (NIH AI-12770, American Heart Association GIA), R.G.P. (CA-93596), C.A. (AG-20337), M.P.L. (NIH, American Heart Association GIA), and H.H. (Hirschl/Weil-Caulier Career Scientist Award and New Investigator Award from the American Heart Association) and by the Muscular Dystrophy Association.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alessandri, A., S. Namura, M. A. Moskowitz, and J. V. Bonventre. 1999. MEK1 protein kinase inhibition protects against damage resulting from focal ischemia. Proc. Nat. Acad. Sci. USA 96:12866-12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaiz, M. R., L. E. Fichera, and M. Postan. 2002. Cardiac myocyte hypertrophy and proliferating cell nuclear antigen expression in Wistar rats infected with Trypanosoma cruzi. J. Parasitol. 88:919-925. [DOI] [PubMed] [Google Scholar]
- 3.Barber, S. A., L. Brut, B. R. Douglass, D. S. Herbst, M. C. Zink, and J. E. Clements. 2002. Visna virus-induced activation of MAPK is required for virus replication and correlates with virus-induced neuropathology. J. Virol. 76:817-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogoyevitch, M. A. 2000. Signalling via stress-activated protein kinases in the cardiovascular system. Cardiovasc. Res. 45:826-842. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, G., R. A. Poolman, and J.-M. Li. 1998. Arresting developments in the cardiac myocyte cell cycle: role of cyclin-dependent kinase inhibitors. Cardiovasc. Res. 39:301-331. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald, A. B., A. H. Wagner, C. Webel, and M. Hecker. 2002. Decoy oligodeoxynucleotide against activator protein-1 reduces neointimal proliferation after coronary angioplasty in hypercholesterolemic minipigs. J. Am. Coll. Cardiol. 20:39:732-738. [DOI] [PubMed] [Google Scholar]
- 7.Bueno, O. F., L. J. De Windt, K. M. Tymitz, S. A. Witt, T. R. Kimball, R. Klevitsky, T. E. Hewett, S. P. Jones, D. J. Lefer, C. F. Peng, R. N. Kitsis, and J. D. Molkentin. 2000. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 19:6341-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra, M., J. Shirani, V. Shtutin, et al. 2002. Cardioprotective effects of verapamil on myocardial structure and function in a murine model of chronic Trypanosoma cruzi infection (Brazil strain): an echocardiographic study. Int. J. Parasitol. 32:2007-2015. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekar, B., J. E. Streitman, J. T. Colston, and G. L. Freeman. 1998. Inhibition of nuclear Factor κB attenuates proinflammatory cytokine and inducible nitric-oxide synthase expression in postischemic myocardium. Biochim. Biophys. Acta 1406:91-106. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekar, B., P. C. Melby, D. A. Troyer, and G. L. Freeman. 2000. Differential regulation of nitric oxide synthase isoforms in experimental acute chagasic cardiomyopathy. Clin. Exp. Immunol. 121:112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, M. W., E. Barr, J. Seltzer, Y. Q. Jiang, G. J. Nabel, M. S. Parmacek, and J. M. Leiden. 1995. Cytostatic gene therapy for vascular proliferation with constitutively active form of the retinoblastoma gene. Science 267:518-522. [DOI] [PubMed] [Google Scholar]
- 12.Chen, D., K. Krashinski, D. Chen, A. Sylvester, J. Chen, and P. D. Nisen. 1997. Downregulation of cyclin-dependent-kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27KIP1, an inhibitor of neointima formation in the rat carotid artery. J. Clin. Investig. 99:2334-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuenkova, M. V., and M. A. Pereira. 2001. The T. cruzi trans-sialidase induces PC12 cell differentiation via MAPK/Erk pathway. Neuroreport 12:1-4. [DOI] [PubMed] [Google Scholar]
- 14.Ebnet, K., K. D. Brown, U. K. Siebenlist, M. M. Simon, and S. Shaw. 1997. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J. Immunol. 158:3285-3292. [PubMed] [Google Scholar]
- 15.Factor, S. M., S. Cho, M. Wittner, and H. Tanowitz. 1985. Abnormalities of the coronary microcirculation in acute murine Chagas' disease. Am. J. Trop. Med. Hyg. 34:246-253. [DOI] [PubMed] [Google Scholar]
- 16.Gray, M. O., C. S. Long, J. E. Kalinyak, H.-T. Li, and J. S. Karliner. 1998. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of transforming growth factor β1 and endothelin-1 from fibroblasts. Cardiovasc. Res. 40:352-363. [DOI] [PubMed] [Google Scholar]
- 17.Hu, Y., L., Cheng, B-W., Hochleitner, and Q. Xu. 1997. Activation of mitogen-activated protein kinases (ERK/JNK) and AP-1 Ttranscription factor in rat carotid arteries after balloon injury. Artersclerosis Thromb. Vasc. Biol. 17:2808-2816. [DOI] [PubMed] [Google Scholar]
- 18.Huang, H., T. M. Calderon, J. W. Berman, V. L. Braunstein, L. M. Weiss, M. Wittner, and H. B. Tanowitz. 1999. Infection of endothelial cells with Trypanosoma cruzi activates NF-κB and induces vascular adhesion molecule expression. Infect. Immun. 67:5434-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang. H., J. Chan, M. Wittner, L. A. Jelicks, S. A., Morris, S. M. Factor, L. M. Weiss, V. L. Braunstein, C. J. Bacchi, N. Yarlett, M. Chandra, J. Shirani, and H. B. Tanowitz. 1999. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J. Mol. Cell. Cardiol. 31:75-88. [DOI] [PubMed] [Google Scholar]
- 20.Huang, H., H. B. Tanowitz, J. P. Bilezikian, M. Wittner, L. M. Weiss, and S. A. Morris. 1997. Myocardial G proteins in murine Chagas' disease. J. Parasitol. 83:663-670. [PubMed] [Google Scholar]
- 21.Izumi, Y., S. Kim, M. Namba, H. Yasumoto, H. Miyazaki, M. Hoshiga, Y. Kaneda, R. Morishita, Y. Zhan, and H. Iwao. 2001. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ. Res. 88:1120-1126. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, R. A., S.-M. Huong, and E.-S. Huang. 2000. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: a novel mechanism for activation of p38. J. Virol. 74:1158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan, J. E., Z. Q. Zhao, and J. Vinten-Johansen. 1999. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc. Res. 43:860-878. [DOI] [PubMed] [Google Scholar]
- 24.Kirshenbaum, L. A. 2001. Death-defying pathways linking cell cycle and apoptosis. Circ. Res. 88:978-980. [DOI] [PubMed] [Google Scholar]
- 25.Koyama, H., N. E. Olson, F. F. Dastvan, and M. A. Reidy. 1998. Cell replication in the arterial wall activation of signaling pathway following in vivo injury. Circ. Res. 82:713-721. [DOI] [PubMed] [Google Scholar]
- 26.Krull, M., A. C. Klucken, F. N. Wuppermann, O. Fuhrma, C. Magerl, J. Seybold, S. Hippenstiel, J. H. Hegmann, C. A. Jantos, and N. Suttorp. 1999. Signal transduction pathways activated in endothelial cells following infection with Chlamydia pneumoniae. J. Immunol. 162:4834-4841. [PubMed] [Google Scholar]
- 27.Li, J-M., and G. Brooks. 1999. Cell cycle regulatory molecules (cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors) and the cardiovascular system. Potential targets for therapy? Eur. Heart J. 20:406-420. [DOI] [PubMed] [Google Scholar]
- 28.Li, J-M., R. A. Poolman, and G. Brooks. 1998. Role of G1 phase cyclins and cyclin-dependent kinases during hypertropic growth in rats. Am. J. Physiol. 275:H814-H822. [DOI] [PubMed] [Google Scholar]
- 29.McBride, K., and M. Nemer. 1998. The C-terminal domain of c-Fos is required for activation of an AP-1 site required for activation of an AP-1 site specific for Jun-Fos herterodimers. Mol. Cell. Biol. 18:5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyauchi, T., and T. Masaki. 1999. Pathophysiology of endothelin in the cardiovascular system. Annu. Rev. Physiol. 61:391-415. [DOI] [PubMed] [Google Scholar]
- 31.Nelson, D. P., S. B. Wechsler, T. Miura, A. Stagg, J. W. Newburger, J. E. Mayer, Jr., and E. J. Neufeld. 2002. Myocardial immediate early gene activation after cardiopulmonary bypass with cardiac ischemia-reperfusion. Ann. Thorac. Surg. 73:156-162. [DOI] [PubMed] [Google Scholar]
- 32.Omura, T., M. Yoshiyama, T. Shimada, N. Shimizu, S. Kim, H. Iwao, K. Takeuchi, and J. Yoshikawa. 1999. Activation of mitogen-activated protein kinases in in vivo ischemia/reperfused myocardium in rats. J. Mol. Cell. Cardiol. 31:1269-1279. [DOI] [PubMed] [Google Scholar]
- 33.Opavsky, M. A., T. Martino, M. Rabinovitch, J. Penninger, C. Richardson, M. Petric, C. Trinidad, L. Butcher, J. Chan, and P. P. Liu. 2002. Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J. Clin. Investig. 109:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osuga, H., S. Osuga, F. Wang, R. Fetni, M. J. Hogan, R. S. Slack, A. M. Hakim, J.-E. Ikeda, and D. S. Park. 2000. Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. USA 97:10254-10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pestell. R. G, C. Albanese, A. T. Reutens, J. E. Segall, R. J. Lee, and A. Arnold. 1999. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocrinol. Rev. 20:501-534. [DOI] [PubMed] [Google Scholar]
- 36.Petkova, S. B., A. Ashton, B. Bouzahzah, H. Huang, R. G. Pestell, and H. B. Tanowitz. 2000. Cell cycle molecules and diseases of the cardiovascular system. Frontiers Biosci. 5:d452-460. [DOI] [PubMed] [Google Scholar]
- 37.Petkova, S. B., H. Huang, S. M. Factor, R. G. Pestell, B. Bouzahzah, L. A. Jelicks, L. M. Weiss, S. A. Douglas, M. Wittner, and H. B. Tanowitz. 2001. The role of endothelin in the pathogenesis of Chagas' disease. Int. J. Parasitol. 31:499-511. [DOI] [PubMed] [Google Scholar]
- 38.Petkova, S. B., H. B. Tanowitz, H. I. Magazine, S. M. Factor, J. Chan, R. G. Pestell, B. Bouzahzah, S. A. Douglas, V. Shtutin, S. A. Morris, E. Tsang, L. M. Weiss, G. J. Christ, M. Wittner, and H. Huang. 2000. Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc. Pathol. 9:257-265. [DOI] [PubMed] [Google Scholar]
- 39.Pines, J. 1995. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem. J. 308:697-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiss, K., W. Cheng, A. Giordano, et al. 1996. Myocardial infarction is coupled with the activation of cyclins and cyclin-dependent kinases in myocytes. Exp. Cell Res. 225:44-54. [PubMed] [Google Scholar]
- 41.Rossi, M. 1990. Microvascular changes as a cause of chronic cardiomyopathy in Chagas' disease. Am. Heart J. 120:233-236. [DOI] [PubMed] [Google Scholar]
- 42.Ruwhof, C., and A. van der Laarse. 2000. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc. Res. 47:23-37. [DOI] [PubMed] [Google Scholar]
- 43.Sartori, A. M., M. H. Lopes, B. Caramelli, M. I. Duarte, P. L. Pinto, V. Neto, and S.-Y. Amato. 1995. Simultaneous occurrence of acute myocarditis and reactivated Chagas' disease in a patient with AIDS. Clin. Infect. Dis. 21:1297-1299. [DOI] [PubMed] [Google Scholar]
- 44.Sawada, N., H. Itoh, K. Ueyama, J. Yamashita, K. Doi, T-H. Chun, M. Inoue, K. Masatsugu, T. Saito, Y. Fukunaga, S. Sakaguchi, H. Arai, N. Ohno, M. Komeda, and K. Nakao. 2000. Inhibition of Rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation 101:2030-2033. [DOI] [PubMed] [Google Scholar]
- 45.Scharfstein, J. Activation of bradykinin-receptors by Trypanosoma cruzi: a role for cruzipain in microvascular pathology, p. 17-44. In J. Kelley (ed.), Molecular pathogenesis of Chagas' disease [Online.] Landis Bioscience, Austin, Tex. http://www.eurekah.com.
- 46.Seko, Y., K. Tobe, K. Ueki, T. Kadowaki, and Y. Yazaki. 1996. Hypoxia and hypoxia/reoxygenation activate Raf-1, mitogen-activated protein kinase kinase, mitogen-activated protein kinases, and S6 kinase in cultured rat cardiac myocytes. Circ. Res. 78:82-90. [DOI] [PubMed] [Google Scholar]
- 47.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu, N., M. Yoshiyama, T. Omura, A. Hanatani, S. Kim, K. Takeuchi, H. Iwao, and J. Yoshikawa. 1998. Activation of mitogen-activated protein kinases and activator protein-1 in myocardial infarction in rats. Cardiovasc. Res. 38:116-124. [DOI] [PubMed] [Google Scholar]
- 49.Sriram, V., and C. Patterson. 2001. Cell cycle in vasculoproliferative diseases. Potential interventions and routes. Circulation 103:2414-2419. [DOI] [PubMed] [Google Scholar]
- 50.Sugden, P. H., and A. Clerk. 1998. “Stress-responsive” mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ. Res. 83:345-352. [DOI] [PubMed] [Google Scholar]
- 51.Suwahara, F., H. Kai, K. Tokuda, M. Kai, A. Takeshita, K. Egashira, and T. Imaizumi. 2002. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 106:130-135. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki, E., N. Daisuke, M. Kakoki, H. Hayakawa, A. Goto, M. Omata, and Y. Hirata. 1999. Molecular mechanisms of endothelin-1-induced cell-cycle progression: involvement of extracellular signal-regulated kinase, protein kinase C, and phosphatidylinositol 3-kinase at distinct points. Circ. Res. 84:611-619. [DOI] [PubMed] [Google Scholar]
- 53.Sylvester, A. M., D. Chen, K. Krasinki, and V. Andres. 1998. Role of c-Fos and E2F in the induction of cyclin A transcription and vascular muscle proliferation. J. Clin. Investig. 101:940-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi, E., and B. C. Berk. 1998. MAP kinases and vascular smooth muscle cell function. Acta Physiol. Scand. 164:611-621. [DOI] [PubMed] [Google Scholar]
- 55.Talmor, D., A. Applebaum, A. Rudich, Y. Shapira, and A. Tirosh. 2000. Activation of mitogen-activated protein kinases in human heart during cardiopulmonary bypass. Circ. Res. 12:86:1004-1007. [DOI] [PubMed] [Google Scholar]
- 56.Talvani, A., C. S. Ribeiro, J. C. Aliberti, V. Michailowsky, P. V. Santos, S. M. Murta, A. J. Romanha, I. C. Almeida, J. Farber, J. Lannes-Vieira, J. S. Silva, and R. T. Gazzinelli. 2000. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-γ as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2:851-866. [DOI] [PubMed] [Google Scholar]
- 57.Tanowitz, H. B., S. M. Factor, J. Shirani, A. Ilercil, M. Wittner, J. Scharfstein, and L. V. Kirchhoff. 2002. Recent. developments in the pathology of Chagas disease with emphasis on the cardiovascular system, p. 81-96. In K. M. Tyler et al. (ed.), World class parasites, vol. 7: American trypanosomiasis. Kluwer Academic Publishers, Boston, Mass.
- 58.Tanowitz, H. B., D. K. Kaul, B. Chen, S. A., Morris, S. M. Factor, L. M. Weiss, and M. Wittner. 1996. Compromised microcirculation in acute murine Trypanosoma cruzi infection. J. Parasitol. 82:124-130. [PubMed] [Google Scholar]
- 59.Tarzami, S. T., R. Cheng, W. Miao, R. N. Kitsis, and J. W. Berman. 2002. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. J. Mol. Cell. Cardiol. 34:209-221. [DOI] [PubMed] [Google Scholar]
- 60.Teixeira, M. M., R. T. Gazzinelli, and J. S. Silva. 2002. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 18:262-265. [DOI] [PubMed] [Google Scholar]
- 61.Valen, G., Z.-Q. Yan, and G. K. Hansson. 2001. Nuclear factor kappa-B and the heart. J. Am. Coll. Cardiol. 38:307-314. [DOI] [PubMed] [Google Scholar]
- 62.Villalta, F., Y. Zhang, K. E. Bibb, J. M. Burns, and M. F. Lima. 1998. Signal transduction in human macrophages by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosoma entry through the MAP kinase pathway. Biochem. Biophys. Res. Commun. 249:247-252. [DOI] [PubMed] [Google Scholar]
- 63.Wei, G. L., K. Krasinski, J. M. Isner, K. Walsh, and V. Andres. 1997. Temporally and spatially coordinated expression of cell cycle regulatory factors after angioplasty. Circ. Res. 80:418-426. [PubMed] [Google Scholar]