Abstract
The luxS gene product is an integral component of LuxS/autoinducer-2 (AI-2) quorum-sensing systems in bacteria. A putative luxS gene was expressed at comparable levels by Borrelia burgdorferi strain 297 cultivated either in vitro or in dialysis membrane chambers implanted in rat peritoneal cavities. Although the borrelial luxS gene functionally complemented a LuxS deficiency in Escherichia coli DH5α, AI-2-like activity could not be detected within B. burgdorferi culture supernatants or concentrated cell lysates. Finally, a luxS-deficient mutant of B. burgdorferi was infectious at wild-type levels when it was intradermally needle inoculated into mice, indicating that expression of luxS probably is not required for infectivity but, at the very least, is not essential for mammalian host adaptation. Our findings also challenge the notion that a LuxS/AI-2 quorum-sensing system is operative in B. burgdorferi.
Quorum sensing is a cell-to-cell communication system that modulates a wide variety of bacterial phenotypes, including virulence expression (3, 11, 19). One of the quorum-sensing systems is the autoinducer-2 (AI-2)-dependent system, which utilizes a small cell-permeable signaling molecule (AI-2) whose synthesis is dependent on the product of a luxS gene. This AI-2-dependent quorum-sensing system is common among diverse bacteria, inasmuch as conserved LuxS orthologs exist in over 30 bacterial species (19). Genome analysis (20) previously revealed the presence of a luxS homolog (BB0377) in Borrelia burgdorferi strain B31. In addition, it has been shown that spirochete cell density influences the expression of a number of B. burgdorferi membrane lipoproteins, such as OspC, P7.5, Mlps, and P35 (9, 14, 25). These combined observations have led to the notion that B. burgdorferi may utilize quorum sensing as a means of adaptation during one or more phases of its complex life cycle. Stevenson and Babb (21) previously showed that B. burgdorferi open reading frame BB0377 was able to complement a luxS deficiency in Escherichia coli DH5α, thereby supporting a luxS designation for open reading frame BB0377. Furthermore, the addition of cell-free supernatants of E. coli DH5α harboring the B. burgdorferi luxS gene to B. burgdorferi cultures (at 23 or 34°C) induced the differential regulation of 25 to 30 borrelial proteins, ostensibly from the presence of AI-2 in the E. coli culture medium (21). However, AI-2-like activity was not detectable in high-density culture supernatants of B. burgdorferi strain B31, N40, or 297, nor was the expression of the luxS gene in B. burgdorferi evaluated (21). Nevertheless, it was concluded that the LuxS/AI-2 quorum-sensing system is operative in B. burgdorferi (21). The objectives of the present study were to examine directly the expression of luxS in B. burgdorferi, evaluate AI-2-like activity emanating from luxS expression, and determine whether LuxS was required for mammalian infectivity by the Lyme disease spirochete.
Generation of luxS mutants.
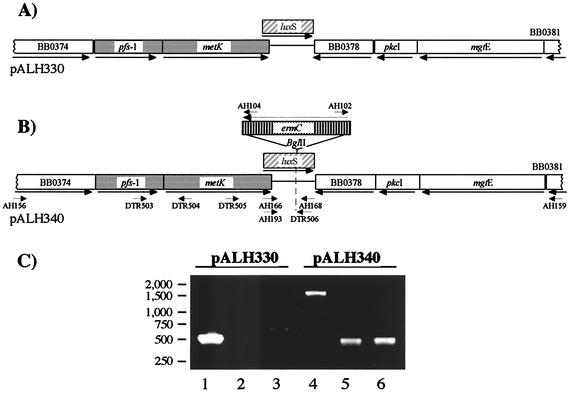
Infectious B. burgdorferi strain 297 (12) and all other bacterial strains and plasmid constructs used in this study are listed in Table 1. The overall strategy for targeted gene disruption in B. burgdorferi strain 297 was described previously (8). All PCR primers were designed on the basis of strain B31 sequence information (5) (http://www.tigr.org), and their approximate positions are depicted in Fig. 1B. PCR was routinely performed with the Expand High Fidelity PCR System (Roche Diagnostics). First, a 5,014-bp region of the luxS gene with flanking sequences from strain 297 was PCR amplified by using primers priAH156 (5′-CTGCTAGTGCCTATTCTGCTATC) and priAH159 (5′-GGTTCCTGCAAATGTTGTTCAGAG) and ligated into pGEMT-easy (Promega, Madison, Wis.) to yield pALH330 (Fig. 1A). The luxS gene was then disrupted by inserting an erythromycin resistance marker, ermC (8, 18), into the unique BglII site of pALH330 to yield the suicide plasmid pALH340 (Fig. 1B). To minimize polar effects, ermC was inserted in an orientation opposite to that of luxS; the orientation was confirmed by PCR (Fig. 1C) with primers internal to ermC (priAH102 and priAH104 [8]) in combination with priAH166 and priAH168 (see below).
TABLE 1.
Strains and recombinant plasmids
| Strain or plasmid | Description | Source and/or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17(rK− mK+) deoR thi-1 supE44 λ−gyrA96 relA1; does not express AI-2 | GIBCO/Life Technologies |
| V. harveyi | ||
| BB170 | luxN::Tn5; AI-2 sensor strain | V. Sperandio (22) |
| BB152 | Positive control, synthesizes AI-2 | V. Sperandio (22) |
| B. burgdorferi | ||
| 297 | Nonclonal, infectious human isolate | 12 |
| BbAH308 | Clonal, infectious luxS mutant | This study |
| BbAH309 | Clonal, infectious luxS mutant | This study |
| Plasmids | ||
| pGEM-T | TA cloning vector, high-copy-number; Ampr | Promega |
| pHAT20 | Expression vector with His tag; Ampr | Clontech |
| pALH133 | pGEM-T, ermC from pJRS233; Ampr Ermr | 8 |
| pALH330 | pGEM-T, 5,014-bp fragment (analogous B31 chromosome 385038-390052); Ampr | This study |
| pALH340 | pALH330, except luxS::ermC (BglII [1 kb]); Ampr Ermr | This study |
| pALH470 | pHAT20Ω(luxS, AgeI/KpnI [560 bp]); Ampr | This study |
FIG. 1.
Construction of the suicide plasmid (pALH340) for disruption of B. burgdorferi luxS. (A) pGEMT-easy-based pALH330 contains the cloned B. burgdorferi luxS (hatched box) and flanking sequences (only the relevant portion of the plasmid is shown). Two genes, pfs-1 and metK (gray boxes), along with luxS, comprise a three-gene operon. (B) luxS was insertionally disrupted with ermC (vertical stripes), yielding pALH340. Short arrows indicate approximate primer locations. (C) Agarose gel electrophoresis patterns of amplicons for pALH330 (lanes 1 to 3) and pALH340 (lanes 4 to 6). The primers used to generate products were as follows: lanes 1 and 4, priAH166 and priAH168; lanes 2 and 5, priAH104 and priAH166; lanes 3 and 6, priAH102 and priAH168. PCR using combinations of ermC- and luxS-specific primers confirmed the reverse-transcriptional orientation of ermC relative to luxS in pALH340 (lanes 5 and 6).
Fifteen micrograms of purified pALH340 was used for electroporation of strain 297 (8), after which spirochetes were added to 2 ml of fresh BSK-H medium supplemented with antibiotic mixture for Borrelia (Sigma Chemical Co., St. Louis, Mo.). Following 36 h of recovery in the absence of erythromycin, the entire transformation mixture was added to 50 ml of prewarmed BSK-H medium (Sigma) containing erythromycin (0.06 μg/ml). Erythromycin-resistant spirochetes could easily be distinguished (via dark-field microscopy) from nonviable (nonmotile) cells after 10 to 14 days of incubation at 37°C. No viable spirochetes were detected in a control culture of BSK-H plus erythromycin inoculated with mock-electroporated wild-type bacteria, indicating that spirochetes were efficiently killed and that spontaneous erythromycin resistance did not occur among the population.
It was postulated that further selection and enrichment of transformed spirochetes under conditions that more closely mimic the mammalian host environment (as opposed to in vitro growth on solid medium) might assist with the recovery of luxS mutants that retained the ability to infect and replicate within mammalian tissues. To approach this, 10 ml of the erythromycin-resistant mutant pool or a mock-electroporated control pool (both diluted to 1,000 spirochetes per ml in fresh BSK-H plus erythromycin) were placed into sterile dialysis membrane chambers (DMCs), which were then implanted into rat peritoneal cavities (1). After 15 days, motile spirochetes were not detectable in DMCs inoculated with mock-electroporated wild-type bacteria, again indicating that initial erythromycin selection efficiently eliminated all wild-type B. burgdorferi. In contrast, luxS-disrupted, erythromycin-resistant spirochetes within DMCs replicated to normal levels (3.0 × 106 to 4.5 × 106 per ml), providing the first indication that luxS may not be required for the growth of B. burgdorferi during mammalian host-adapted conditions. Furthermore, PCR analysis of genomic contents from the pool of erythromycin-resistant spirochetes within DMCs did not reveal any amplicons representative of wild-type luxS (not shown), suggesting that erythromycin resistance correlated with the presence of ermC within luxS. As a preliminary assessment of whether the luxS-deficient mutants might be capable of infecting mice, approximately 105 spirochetes recovered from the DMCs were intradermally needle inoculated into each of five C3H/HeJ mice (6). Ear punch biopsies (6) were cultured in BSK-H plus erythromycin; 2 weeks postinfection, cultured biopsies from all five mice yielded motile spirochetes after 1 week in culture.
One of the resultant ear punch biopsy-positive cultures was then diluted in BSK-H and plated on solid selective medium (pBSK plus erythromycin) (17) to isolate single colonies. Two clonal isolates were randomly selected, designated BbAH308 and BbAH309, and subjected to PCR analysis to confirm luxS gene disruption (8). BbAH309 was chosen for further characterization because plasmid profiling (4) of this mutant (via PCR) indicated that it contained all plasmids except cp32-6 (not shown). Moreover, when the in vitro growth of BbAH309 in BSK-H medium was compared with that of wild-type 297 under various temperature (37°C) and pH (7.5 or 8.0) conditions over 5 days, starting inocula of 103 spirochetes grew at comparable rates and to comparable levels (ca. 2.5 × 107 cells per ml) (not shown).
Expression of luxS and its genetic organization in B. burgdorferi.
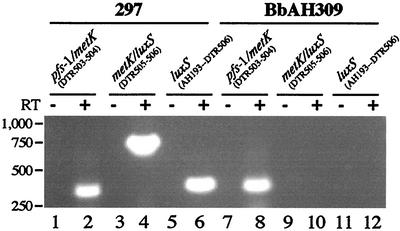
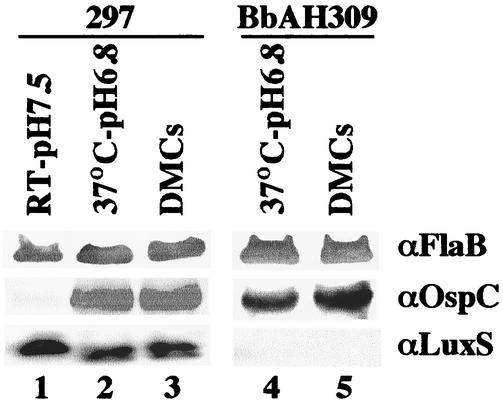
Heretofore, expression of the putative luxS gene in B. burgdorferi had not been evaluated. Expression of luxS by wild-type 297 and mutant BbAH309 initially was assessed by reverse transcriptase PCR (RT-PCR) with the Titan one-step RT-PCR system (Roche Molecular Biochemicals, Indianapolis, Ind.) according to manufacturer's instructions. Spirochetes propagated at 37°C (pH 6.8) were harvested in the late-logarithmic phase of growth (ca. 2 × 107 cells per ml). Total RNA was extracted (15) and DNase treated, and 500-ng quantities of RNA were subjected to RT-PCR (26) with the primers priDTR506 (5′-GATTTCGGAAAAAAGCCATGAGAC) and priAH193 (5′-ATGGGAAAAATTAGATTTTGTAAAAAAAATAC [the start codon is in boldface]). For all RT-PCRs, a negative control lacking RT was included (Fig. 2, lanes 5 and 11). As shown in Fig. 2, wild-type 297 expressed luxS RNA when cultivated at 37°C (pH 6.8) (parameters analogous to the feeding-tick condition [15, 25]) (lane 6), whereas BbAH309 did not (lane 12). Next, we sought to determine whether mRNA detected by RT-PCR accounted for detectable levels of LuxS. For this purpose, we first constructed and expressed in E. coli a 6-histidine fusion protein of LuxS by standard protocols (6) and used the purified protein to generate polyclonal antisera in rats (10). LuxS antibodies in antisera were further affinity purified (HiTrap affinity columns; Amersham Biosciences, Piscataway, N.J.) to yield a monospecific antibody preparation. Wild-type 297 and luxS-deficient BbAH309 were cultivated in vitro under various conditions or in DMCs implanted in rat peritoneal cavities. As shown in Fig. 3, LuxS was detectable by immunoblotting at comparable levels within wild-type B. burgdorferi cultivated in vitro (lanes 1 and 2) or in DMCs (lane 3). These results were consistent with those of previous gene array analyses (15), which also showed that the luxS gene of infectious B. burgdorferi strain B31 was expressed at similar levels when spirochetes were cultivated in vitro or in DMCs. As expected, LuxS was not detectable in BbAH309 grown either in vitro or in DMCs (Fig. 3, lanes 4 and 5). Of note, it was previously postulated that OspC expression by B. burgdorferi might be affected by LuxS-produced AI-2 when spirochetes replicate within a mammalian host (21). However, in the present study, OspC expression was unaffected in the luxS-deficient mutant cultivated within DMCs (Fig. 3, lane 5), suggesting that OspC expression is not influenced by a LuxS/AI-2 quorum-sensing system when replicating in a mammalian host-adapted state.
FIG. 2.
RT-PCR analysis of luxS expression in wild-type B. burgdorferi 297 (lanes 1 to 6) and a luxS mutant (BbAH309) (lanes 7 to 12) cultured at 37°C (pH 6.8). + and −, PCRs with or without RT, respectively. Primer locations are depicted in Fig. 1B, and the primer pairs used are indicated above the pairs of the corresponding gel lanes. Lanes 2 and 4 show the operonic organization of pfs-1, metK, and luxS in wild-type 297. Lane 6 shows luxS expression in wild-type 297, whereas no similar RT-PCR product is detectable in the luxS mutant (lane 12). The absence of product in lane 10 is due to disruption of luxS by ermC.
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting of wild-type (strain 297) and luxS-deficient (strain BbAH309) B. burgdorferi. For organisms cultivated in vitro at 37°C or at room temperature (RT) (lanes 1, 2, and 4), each strain was cultivated in BSK-H medium to the late-logarithmic stage (ca. 2 × 107 spirochetes per ml). To obtain spirochetes in a mammalian host-adapted state, B. burgdorferi strains were cultivated in DMCs (lanes 3 and 5) (1). A total of 5 × 107 spirochetes were loaded in each gel lane. To detect FlaB, conventional immunoblotting was performed with murine monoclonal antibody 8H3-33 (1). Detection of FlaB was used as a loading control for each gel lane. To detect OspC, rat polyclonal antiserum was used as a primary antibody (1). OspC expression was used, in part, as an indicator that in vitro-cultivated borreliae were suitably adapted to the various growth conditions; OspC expression was low in spirochetes cultivated in BSK-H medium (pH 7.5) at room temperature, as has been reported previously (25). To detect low-abundance LuxS, chemiluminescence (Western Lightening; Perkin-Elmer Life Sciences, Boston, Mass.) was employed with affinity-purified rat antibodies (see text).
Stevenson and Babb (21) reported that the B. burgdorferi luxS gene cloned into E. coli functionally complemented the natural LuxS deficiency of E. coli DH5α (22). In the present study, we obtained similar results (not shown), implying that the product of the enzymatic reaction catalyzed by B. burgdorferi LuxS, when synthesized in E. coli, is functional in heterologous AI-2-dependent quorum-sensing systems. However, thus far we have been unable to detect the presence of any AI-2-like activity (via Vibrio harveyi bioluminescence) either in culture supernatants of B. burgdorferi 297 grown to a high density at 37°C (pH 6.8) or in cell lysates concentrated 100-fold (not shown). The simplest explanation of these findings is that even if an AI-2-like molecule is synthesized by the action of LuxS in B. burgdorferi, AI-2 may not achieve levels sufficient to function in a LuxS/AI-2-type quorum-sensing system.
Schauder et al. (20) first proposed that B. burgdorferi luxS might be in a three-gene operon with two other accessory genes, pfs-1 and metK, involved in S-adenosylmethionine (SAM) utilization pathways. This hypothesized operonic organization of pfs-1-metK-luxS was assessed by RT-PCR with the primers priDTR503 (5′-GCTGCAATAGCTCAAGTAGCACAC) and priDTR504 (5′-CTATTTATTTCTCTCCTGCTATTAC) (353-bp product for pfs-1-metK), as well as priDTR505 (5′-CTACCGGAGACACTGGGCTTACAG) and priDTR506 (see above; 770-bp product for metK-luxS). As shown in Fig. 2, wild-type 297 cultured at 37°C (pH 6.8) yielded RT-PCR products linking pfs-1 to metK (lane 2) and metK to luxS (lane 4), indicating that pfs-1, metK, and luxS are transcriptionally linked. In mutant BbAH309, the pfs-1 and metK genes are still transcribed together (lane 8), whereas primers priDTR505 and priDTR506 did not yield an RT-PCR product for metK-luxS due to disruption of the luxS gene (lane 10).
A luxS-deficient mutant of B. burgdorferi retains infectivity for mice.
The luxS-deficient mutant BbAH309 was used to verify retention of the mouse infectivity phenotype following its clonal selection. In these experiments, groups of five mice were inoculated intradermally with 100 spirochetes of either wild-type 297 or BbAH309. After 2 weeks, ear punch biopsies were obtained from all mice and were cultured in BSK-H medium under standard conditions (6). One week later, motile spirochetes were observed by dark-field microscopy in all cultures from both groups of mice. Given that five of five mice became infected by low levels of inocula, BbAH309 thus appeared to be fully infectious at wild-type levels. These findings suggest that even if a LuxS/AI-2 quorum-sensing system is operative at some stage(s) in the life cycle of B. burgdorferi, expression of the luxS gene is not required for mammalian host adaptation and probably is not required for infection. Finally, to our knowledge, this is the first report of a genetically manipulated B. burgdorferi mutant that has retained its ability to infect laboratory mice, although recently we also obtained evidence that a csrA (carbon storage regulator A) (16) mutant of B. burgdorferi 297 also is infectious for mice.
Summary and implications.
Given recent attention to the importance of quorum sensing in bacterial virulence expression (3, 11, 19), it has been tempting to speculate that quorum sensing may be operative as a regulatory mechanism governing differential antigen expression in B. burgdorferi. However, based on results presented here, it appears that a LuxS/AI-2 system is not involved in the overall mammalian infectious process or, at the very least, in mammalian host adaptation by B. burgdorferi. This is consistent with the notion that the mammalian phase of Lyme borreliosis tends to be paucibacillary, and thus it is counterintuitive as to how quorum sensing would be beneficial under such conditions. Nonetheless, our results do not preclude that such a system might be operative when B. burgdorferi is harbored within tick midguts, where replicative bursts that accompany tick feeding culminate in higher spirochete densities (13). Also, our mouse infection experiments were performed with intradermal needle inoculation, a route which is not equivalent to natural tick infection (7). Therefore, further experiments examining the influence of LuxS on the tick transmission of B. burgdorferi are warranted.
Despite the initial report by Stevenson and Babb (21), there still is not direct evidence that B. burgdorferi exploits quorum sensing as a mechanism of gene regulation. In fact, Winzer et al. (24) have cautioned that the presence of a luxS gene product and the potential ability to synthesize AI-2 molecules should not be accepted as sole evidence for quorum sensing. Given the present paucity of additional supporting data, one therefore must continue to entertain the possibility that B. burgdorferi may not utilize a LuxS/AI-2 quorum-sensing system in any stage of its life cycle. This hypothesis also is consistent with our findings that AI-2-like activity was not detectable in either culture supernatants or 100-fold-concentrated cell lysates of B. burgdorferi 297. Therefore, AI-2 either may not be synthesized by B. burgdorferi or, alternatively, is a metabolic intermediate that is rapidly catabolized (23, 24). Regarding AI-2 synthesis in other bacteria, MetK is a SAM synthase. SAM subsequently is converted to S-adenosylhomocysteine, which is then acted upon by Pfs for conversion to S-ribosylhomocysteine (2, 23). LuxS cleaves S-ribosylhomocysteine to homocysteine and 4,5-dihydroxy-2,3-pentanedione, which then most likely cyclizes in the presence of borate to form AI-2 (a furanosyl borate diester) (2). Inasmuch as this pathway is common in bacteria, it has been proposed that AI-2 merely may be a dispensable by-product of methionine metabolism that leaves the cell via simple diffusion (23) and thus serves as an autoinducer for quorum sensing in only some species of bacteria (24). In this regard, although we showed that luxS was expressed in B. burgdorferi and was capable of complementing the LuxS deficiency of E. coli DH5α, these findings may not be relevant to borrelial LuxS functioning in an AI-2-dependent quorum-sensing system, akin to what has been noted by Winzer et al. (24). It thus may not be coincidental that two other essential components of an AI-2-dependent quorum-sensing system, LuxP (periplasmic binding protein) and LuxQ (sensor kinase), thus far have not been identified in B. burgdorferi. Taken together, these findings indicate that B. burgdorferi may not have the molecular machinery requisite for sensing and responding to AI-2-like molecules, even if AI-2 is stably produced and excreted.
Acknowledgments
We thank Justin Radolf, Melissa Caimano, and Christian Eggers for sharing plasmid primer sequence information prior to publication, Martin Goldberg and Patrick Conley for antibody production and purification, Vanessa Sperandio for assistance with V. harveyi AI-2 assays and preparation of the manuscript, and Xiaofeng Yang for manuscript review.
This work was supported by grant AI-45538 from the Lyme disease program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by a grant (Advanced Research Program) from the Texas Higher Education Coordinating Board. A.H. was supported by Training Grant T32-AI-07520 from the National Institute of Allergy and Infectious Diseases.
Editor: J. T. Barbieri
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 3.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 5.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 6.Hagman, K. E., P. Lahdenne, T. G. Popova, S. F. Porcella, D. R. Akins, J. D. Radolf, and M. V. Norgard. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Indest, K. J., R. Ramamoorthy, M. Sole, R. D. Gilmore, B. J. B. Johnson, and M. T. Philipp. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect. Immun. 65:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahdenne, P., S. F. Porcella, K. E. Hagman, D. R. Akins, T. G. Popova, D. L. Cox, L. I. Katona, J. D. Radolf, and M. V. Norgard. 1997. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect. Immun. 65:412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 12.Norton Hughes, C. A., C. B. Kodner, and R. C. Johnson. 1992. DNA analysis of Borrelia burgdorferi NCH-1, the first north-central U.S. human Lyme disease isolate. J. Clin. Microbiol. 30:698-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piesman, J., J. R. Oliver, and R. J. Sinsky. 1990. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am. J. Trop. Med. Hyg. 42:352-357. [DOI] [PubMed] [Google Scholar]
- 14.Ramamoorthy, R., and M. T. Philipp. 1998. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect. Immun. 66:5119-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321-1330. [DOI] [PubMed] [Google Scholar]
- 17.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi, p. 253-259. In J. A. Nickoloff (ed.), Methods in molecular biology. Humana Press, Totowa, N.J. [DOI] [PMC free article] [PubMed]
- 18.Sartakova, M., E. Dobrikova, and F. C. Cabello. 2000. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schauder, S., and B. L. Bassler. 2001. The language of bacteria. Genes Dev. 15:1468-1480. [DOI] [PubMed] [Google Scholar]
- 20.Schauder, S., K. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson, B., and K. Babb. 2002. LuxS-mediated quorum sensing in Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun. 70:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 96:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winzer, K., K. R. Hardie, N. Burgess, N. Doherty, D. Kirke, M. T. G. Holden, R. Linforth, K. A. Cornell, A. J. Taylor, P. J. Hill, and P. Williams. 2002. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 148:909-922. [DOI] [PubMed] [Google Scholar]
- 24.Winzer, K., K. R. Hardie, and P. Williams. 2002. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch. Curr. Opin. Microbiol. 5:216-222. [DOI] [PubMed] [Google Scholar]
- 25.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 26.Yang, X., T. G. Popova, K. E. Hagman, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 1999. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect. Immun. 67:6008-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]