Abstract
Immunoreactive proteins of Ehrlichia canis and Ehrlichia chaffeensis that have been characterized include a family of 28-kDa major outer membrane proteins (p28) and two large antigenically divergent surface glycoprotein orthologs. We previously demonstrated that recombinant E. canis p28 and the 140- and 200-kDa glycoproteins gp140 and gp200, respectively, react strongly with serum antibodies from suspect canine ehrlichiosis cases that were positive for E. canis by immunofluorescent antibody test and in various phases of acute or chronic infection (J. Clin. Microbiol. 39:315-322, 2001). The kinetics of the antibody response to these potentially important vaccine and immunodiagnostic candidates is not known. Acute-phase serum antibody responses to whole-cell E. canis lysates and recombinant p28, gp140, and gp200 were monitored for 6 weeks in dogs experimentally infected with E. canis. Irrespective of the inoculation route, a T-helper 1-type response was elicited to E. canis antigens consisting of immunoglobulin G2 antibodies exclusively in both acute and convalescent phases in most dogs. Analysis of immuoreactive antigens for peak intensity and relative quantity identified major immunoreactive E. canis antigens recognized early in the infection as the 19-, 37-, 75-, and 140-kDa proteins. Later in infection, additional major immunoreactive E. canis proteins were identified, including the 28-, 47-, and 95-kDa proteins and the recently identified 200-kDa glycoprotein. All dogs had developed antibody against the recombinant gp140, gp200, and p28 in the convalescent phase. Immunoreactivity and antibody response kinetics suggest that major immunoreactive proteins identified are immunodominant, but early recognition suggests increased dominance by some antigens.
Canine monocytic ehrlichiosis is a globally distributed tick-borne rickettsial disease of dogs caused primarily by the obligate intracellular bacterium, Ehrlichia canis (41). E. canis causes a serious acute disease in dogs, which exhibit clinical signs and hematologic abnormalities, including depression, anorexia, weight loss, fever, bleeding, thrombocytopenia, and anemia (15). Following the acute phase, dogs may eliminate the infection (12) or develop a mild asymptomatic chronic infection, lasting from months to years that may progress into a severe chronic infection (9, 12): some dogs can lapse directly into a severe chronic phase 6 to 12 weeks postinfection (7). In the severe chronic phase, impaired production of blood cells associated with bone marrow hypoplasia is irreversible, resulting in a less-favorable prognosis and an outcome that is more likely to be fatal (7).
Immunoreactive E. canis proteins with masses ranging from 19 to 110 kDa have been reported to react with convalescent-phase antisera from E. canis-infected dogs, and proteins consistently identified as major antigens are 25 to 30 kDa and 42 to 47 kDa (3, 30, 37). Progress towards an effective E. canis subunit vaccine has been advanced by the identification and molecular characterization of several major immunoreactive proteins and corresponding orthologs in Ehrlichia chaffeensis. Molecularly characterized antigens include a family of major outer membrane proteins (p28) (23, 33, 46, 48), two large antigenically distinct glycoproteins (45, 46), and orthologs of the Ehrlichia ruminantium major antigenic protein 2 (MAP2) (1, 2).
Genetic conservation has been reported for major immunoreactive protein genes (p28 and gp140) in North American E. canis isolates, suggesting that identification of immunoprotective antigen(s) could be regionally efficacious (23, 24, 46). Similarly, the antigenic profiles of both North and South American E. canis isolates were nearly identical genetically and antigenically, suggesting that E. canis vaccines developed for the North American isolates may be useful in South America (42). Antigenic conservation is also important to note with regard to the development of subunit immunodiagnostics. The antigenic conservation of immunoreactive proteins has been demonstrated in several studies showing strong and consistent immunoreactivity of multiple immunoreactive proteins in dogs with E. canis infections diagnosed by indirect immunofluorescence antibody (IFA) testing (22, 34). In contrast, genetic diversity of E. chaffeensis has been described, leading to the identification of three genogroups based the diversity of p28 genes (20, 38, 47), and may be partially responsible for the inconsistent reactivity of antibodies from human monocytic ehrlichiosis patients with p28 proteins by Western immunoblotting.
Protective immune mechanisms against E. canis are likely to involve both cellular and humoral immune components. Partial immunity to challenge has been documented in dogs that recovered from acute and severe chronic canine ehrlichosis (7), but a more recent study suggests that E. canis infection does not provide immunity against reinfection and a reduction in clinical signs (3). Antibody has been shown to suppress the growth of E. canis in vitro (16, 17), and evidence supporting a major role for antibody in immunity to Ehrlichia infections has recently been provided by experiments in which passive transfer of antibodies provided protection against lethal E. chaffeensis infection in SCID mice (44). Additional studies have concluded that antibodies against specific p28 linear epitopes located in a hypervariable region mediate this protection (18, 19). Although the kinetics of antibody responses to individual E. canis immunoreactive proteins have not been adequately addressed, serum antibody responses to whole antigen in dogs with experimental and natural E. canis infections consist primarily of immunoglobulin G2 (IgG2) (13).
Development of effective subunit vaccines and useful immunodiagnostics for E. canis is dependent on accurate and comprehensive identification of immunoprotective antigens and characterization of protective host immune responses. In this study, we describe the quantitative and qualitative characteristics of antibody response kinetics to E. canis antigens in dogs during acute infection with E. canis and comprehensively define the major immunoreactive proteins of E. canis, including a newly recognized 200-kDa glycoprotein (gp200).
MATERIALS AND METHODS
Experimental animals.
Twenty-four healthy 11-month-old male and female Walker hounds with body weights of 20 to 30 kg were obtained from the Louisiana State University School of Medicine breeding colony maintained by the Department of Laboratory Animal Medicine. Males were castrated 2 weeks before use, but females remained intact. All animals were housed in enclosed runs within an indoor climate-controlled kennel at Louisiana State University. Food and water were supplied ad libitum throughout the study. The animal care facility operates under the guidelines of the American Association for the Accreditation of Laboratory Animal Care. The experimental protocol for this study was approved by the Institutional Animal Care and Use Committee at Louisiana State University (protocol no. 00-048). Prior to inclusion in this study, all dogs were given physical and hematologic examinations to confirm a normal status and screened for a negative antibody status to E. canis by an IFA test. Dogs were randomly assigned to six groups of four dogs each.
Experimental design.
Groups of dogs were inoculated either subcutaneously (s.c.) or intradermally (i.d.) on the dorsal midline between scapulae with cell culture-passaged E. canis (Louisiana isolate) as previously described (11) with the following modifications. One 2-ml vial of E. canis-infected dog bone marrow (DBM) cells was mixed by inversion, and three dilutions (1:100, 1:500, and 1:1,000) of stock inoculum were made. The inoculum (0.5 ml) was loaded into 1-ml syringes for injection. An extra syringe filled with the 1:1,000 dilution was taken to the kennel on ice and returned to the laboratory to verify infectivity by titration in cell culture. Groups of dogs (one group s.c., one group i.d. for each dilution) received the 1:100, 1:500, and 1:1,000 dilutions of inoculum on day 0. Dogs were monitored for 6 weeks postinoculation. At the completion of study, dogs that developed E. canis infections were administered a doxycycline regimen (10 mg/kg of body weight twice a day [b.i.d.] for 10 days, per os). The following parameters were measured at 0, 7, 14, 21, 28, 35, 42 days postinoculation and 2 weeks posttreatment (day 56) for this study: platelet concentration and anti-E. canis serum antibody (IgG) titer. Dogs with E. canis infections were identified by concurrence of increased anti-E. canis antibodies and decreased platelet concentrations (<200 × 103/μl).
Hematology.
Venous blood was collected from the jugular vein in tripotassium EDTA-containing tubes (Becton-Dickinson, Rutherford, N.J.). Platelet concentrations were determined with a multichannel electronic cell counter (System 9000; Serono-Baker, Allentown, Pa.) validated for canine blood cells. Wright-stained blood smears were also performed to exclude platelet clumping as a cause for decreased platelet concentrations.
Inoculum.
E. canis was propogated in a DBM cell line created at Louisiana State University (R. E. Corstvet) as described previously (11). The cells have not been completely characterized, but they are phenotypically and functionally similar to macrophages and were originated from a primary culture of canine bone marrow cells. Cells were maintained in Fisher's medium (GIBCO, Grand Island, N.Y.) containing 20% horse serum (HyClone, Logan, Utah), 2 mM l-glutamine, and 0.2% hydrocortisone (Sigma, St. Louis, Mo.) in a humidified 37°C incubator with 5% CO2 in tightly capped flasks. E. canis was cultured in DBM cells until the percentage of infected cells reached 70% as determined by IFA. The infected cells were collected and frozen in liquid nitrogen in 2-ml aliquots with 10% dimethyl sulfoxide. Frozen stocks were maintained in liquid nitrogen for 11 weeks prior to inoculation as previously described (11). Titrations of inoculum were performed prior to freezing, 7 days postfreezing, and on the day of inoculation. Cultured monolayers of DBM cells were inoculated with 1 ml of serial 10-fold dilutions of the inoculum. The cultures were incubated for 21 days, and infectivity was determined by an IFA test as described previously (11).
Purification of E. canis antigen.
E. canis was cultured in DH82 and purified with a 32% Percoll gradient as previously described (43).
Gel electrophoresis and protein blotting.
Purified E. canis (50 μg) (Jake, a North Carolina isolate) was subjected to sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 3-N-morpholinopropanesulfonic acid (MOPS) running buffer (NuPAGE; Invitrogen, Carlsbad, Calif.) under reducing conditions with 4 to 12% gradient Bis-Tris acrylamide gels with a two-dimensional well (NuPAGE; Invitrogen). The same E. canis antigen preparation was used for all experiments. An antioxidant (Invitrogen) was added to the upper chamber buffer during electrophoresis and to the transfer buffer during protein blotting to prevent partial protein renaturation as a result of disulfide bond reoxidation. Antigen was heated at 70°C for 10 min in lauryl dodecyl sulfate sample buffer (NuPAGE; Invitrogen) containing the reducing agent dithiothreitol (Invitrogen) prior to loading. A protein standard was included for molecular mass determination (Precision protein standards, broad range, prestained; Bio-Rad, Hercules, Calif.). Proteins were transferred to supported nitrocellulose (BA85; Schleicher & Schuell, Keene, N.H.) with a semidry protein blotting unit (Bio-Rad) with 2× transfer buffer (NuPAGE; Invitrogen) at 15 V for 30 min. Electrophoresis and protein blotting were also performed similarly with individual E. canis recombinant proteins (p28, gp200, and gp140).
Western blotting.
Membranes were incubated with blocking buffer consisting of Tris-buffered saline (TBS [pH 8.0])-3% nonfat milk for 1 h, and damp blots were placed in a slotted blotting apparatus (Multiscreen; Bio-Rad). Primary antibodies were diluted (1:100) in the blocking buffer, and 450 μl was placed in each channel for 1 h at room temperature and slowly rocked. Membranes were removed from the apparatus, washed with buffer (TBS-0.05% Tween 20), and incubated for 1 h with affinity-purified alkaline phosphatase-labeled goat anti-dog IgG (H & L) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) diluted 1:5,000 in blocking buffer. Membranes were washed and incubated for 30 min at room temperature in a 5-bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium (BCIP-NBT) substrate (Kirkegaard & Perry Laboratories) to visualize bound antibody. A one-dimensional gel and blot analysis package (Quantity One; Bio-Rad) was used to determine molecular mass, peak intensity, and relative quantity of immunoreactive E. canis antigens in each lane. Proteins were identified as major immunoreactive antigens based on peak intensity and the average relative quantity (measured intensity expressed as a percentage of the total intensity of all bands in a lane). Major immunoreactive proteins on days 21, 28, and 35 were determined by identification of antigens with an average relative quantity of >3.0 that were identified by at least 75% of the reactive dog sera. Comprehensive identification of all major immunreactive proteins was determined on day 42 as described above, except antigens reactive with sera from all dogs were included in the analysis.
ELISA.
Antibody responses (IgM, IgG1, and IgG2) to E. canis whole-cell lysates were evaluated with an enzyme-linked immunosorbant assay (ELISA). The optimal antigen concentration for antibody detection was determined by serial antigen titration. Assay plates (Immulon I) were coated with E. canis whole-cell lysate diluted in coating buffer (Kirkegaard & Perry Laboratories) and incubated at room temperature for 2 h. Plates were washed four times with buffer (TBS-Tween 20) and then incubated in blocking buffer (TBS-3% nonfat milk) for 1 h and washed. The primary antibody (100 μl) diluted 1:100 in blocking buffer was added to the plates, which were then incubated for 1 h at 37°C. The plates were washed, and affinity-purified goat anti-dog IgG1 or IgM or sheep anti-dog IgG2 (Bethyl Laboratories, Montgomery, Tex.) diluted 1:1,000 in blocking buffer was added, and the mixture was incubated for 1 h at 37°C. Bound antibody was visualized with BluePhos substrate (BCIP and a proprietary tetrazolium) (Kirkegaard & Perry Laboratories) for color development. Plates were read on a tuneable plate reader (Molecular Devices, Sunnyvale, Calif.) at A620 after incubation at room temperature for 30 min. The sample absorbance was plotted as the optical density at 620 nm (OD620).
E. canis recombinant protein expression and purification.
E. canis recombinant proteins used in this study were expressed and purified as described previously (22, 24, 46).
IFA test.
Antigen slides were prepared from DBM cells infected with E. canis (Louisiana isolate) as described previously (22). Sera were diluted twofold in saline from 1:40 to the endpoint titer. Fifteen microliters of diluted serum was added to each well, and then the wells were incubated for 30 min. Slides were washed, and an affinity-purified fluorescein isothiocyanate (FITC)-labeled rabbit anti-dog IgG (H & L) secondary antibody (Kirkegaard & Perry Laboratories) diluted 1:80 was added, and the mixture was incubated for 30 min. Slides were viewed with a UV microscope with filters for fluorescein.
RESULTS
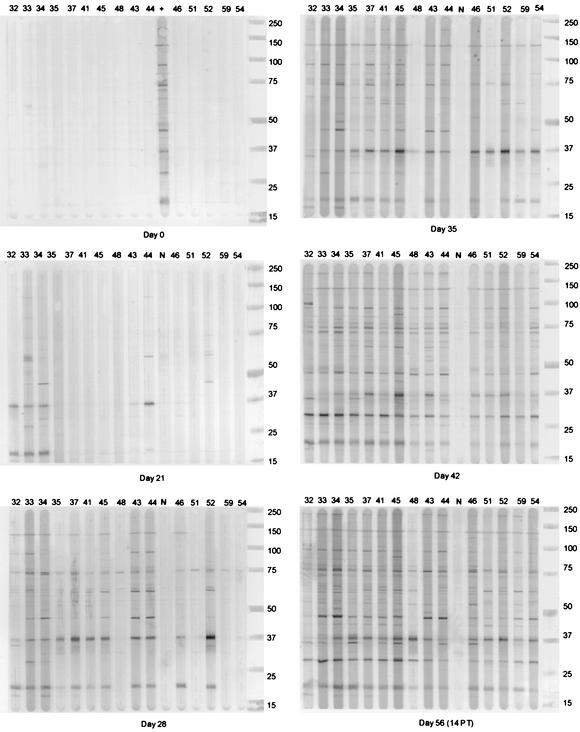
Fifteen of the 24 dogs experimentally inoculated (i.d. or s.c.) with different dilutions (1:100, 1:500, and 1:1,000) of tissue culture-grown E. canis developed canine ehrlichiosis. All infected dogs developed anti-E. canis antibody as determined by an IFA test and thrombocytopenia (<200 × 103/μl) by 5 weeks postinoculation (Table 1). All dogs (eight of eight) that received the 1:100 dilution of inoculum and five of eight that received the 1:500 dilution became infected, but only two of eight that received the 1:1,000 dilution were infected. The route of inoculation did not affect the efficiency of infection; however, the i.d. inoculation appeared to induce an earlier antibody response in the dogs than the s.c. inoculation (Table 1 and Fig. 1). This was particularly evident in the dogs that received the 1:100 inoculum dilution. The two dogs that received the 1:1,000 inoculum dilution and became infected developed antibody later (day 35) than the majority of dogs (day 28) inoculated with 1:100 or 1:500 dilutions (Table 1 and see Fig. 1 and 3).
TABLE 1.
IFA antibody titers and platelet counts in dogs experimentally infected with E. canis
| Dog (inoculum and route) | IFA antibody titer/no. of platelets (103)/per μl on:
|
||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42a | |
| 32 (1:100 i.d.) | <40/259 | <40/216 | <40/268 | 160/183 | 640/<10 | 1,280/31 | 1,280/38 |
| 33 (1:100 i.d.) | <40/253 | <40/240 | <40/179 | 640/<10 | 2,560/11 | 2,560/31 | 5,120/12 |
| 34 (1:100 i.d.) | <40/352 | <40/362 | <40/155 | 640/20 | 2,560/11 | 5,120/26 | 10,240/15 |
| 35 (1:100 i.d.) | <40/274 | <40/282 | <40/295 | <40/280 | 40/197 | 1,280/24 | 2,560/10 |
| 37 (1:100 s.c.) | <40/257 | <40/264 | <40/242 | <40/245 | 320/47 | 5,120/<10 | 10,240/<10 |
| 41 (1:100 s.c.) | <40/308 | <40/313 | <40/290 | <40/278 | 160/135 | 2,560/<10 | 5,120/<10 |
| 45 (1:100 s.c.) | <40/328 | <40/319 | <40/326 | <40/230 | 5,120/70 | 5,120/35 | 10,240/<10 |
| 48 (1:100 s.c.) | <40/278 | <40/300 | <40/243 | <40/272 | <40/301 | <40/268 | 640/98 |
| 43 (1:500 i.d.) | <40/280 | <40/272 | <40/286 | <40/207 | 2,560/20 | 5,120/22 | 2,560/17 |
| 44 (1:500 i.d.) | <40/281 | <40/267 | <40/260 | 80/206 | 1,280/10 | 2,560/36 | 5,120/11 |
| 46 (1:500 s.c.) | <40/292 | <40/295 | <40/287 | <40/210 | 640/23 | 1,280/19 | 2,560/20 |
| 51 (1:500 s.c.) | <40/229 | <40/297 | <40/299 | <40/282 | <40/258 | 80/302 | 640/<10 |
| 52 (1:500 s.c.) | <40/318 | <40/319 | <40/274 | <40/262 | 1,280/64 | 2,560/<10 | 5,120/<10 |
| 59 (1:1,000 i.d.) | <40/239 | <40/299 | <40/279 | <40/260 | <40/254 | 640/135 | 2,560/<10 |
| 54 (1:1,000 s.c.) | <40/307 | <40/317 | <40/307 | <40/316 | 40/260 | 5,120/10 | 1,280/<10 |
Treated with doxycycline.
FIG. 1.
Kinetic antibody responses (IgG) to E. canis whole-cell lysates in 15 dogs experimentally infected s.c. or i.d. with E. canis. The dog number is shown above each corresponding lane. A positive control (+) was included on days 0, 7, and 14, and a negative control (N) was included on days 21, 28, 35, 42, and 56 postinoculation. The day 0 immunoblot is representative of results obtained for days 7 and 14. Day 56 was 2 weeks postinitiation of doxycycline treatment (PT). Dogs 32, 33, 34, and 35 received a 1:100 inoculum dose i.d., and dogs 37, 41, 45, and 48 received the same dose s.c. Dogs 43 and 44 received a 1:500 inoculum dose i.d., and dogs 46, 51, and 52 received the same dose s.c. Dog 59 received a 1:1,000 inoculum dose i.d., and dog 54 received the same dose s.c. Protein standards representing 15 to 250 kDa (Precision protein standards; Bio-Rad) are shown to the right of each blot.
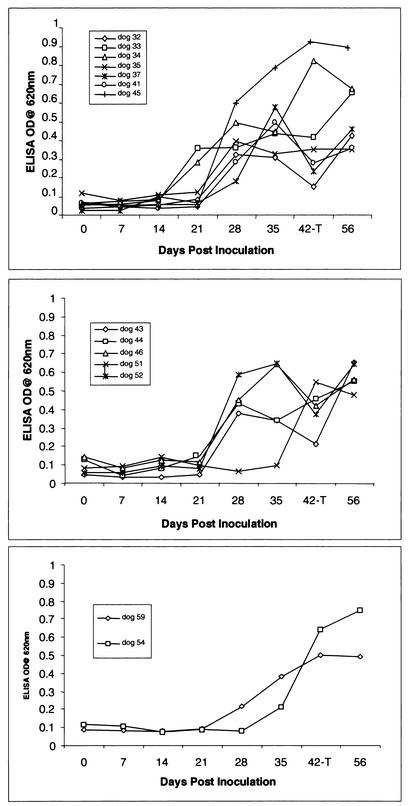
FIG. 3.
Detection of anti-E. canis IgG2 responses to E. canis whole-cell antigen in dogs experimentally infected with E. canis on days 0, 7, 14, 21, 28, 35, 42, and 56 by ELISA. Sera were diluted 1:100, and values were plotted as the OD620. Dogs were inoculated with a 1:100 (top), 1:500 (middle), or 1:1,000 (bottom) dilution of E. canis.
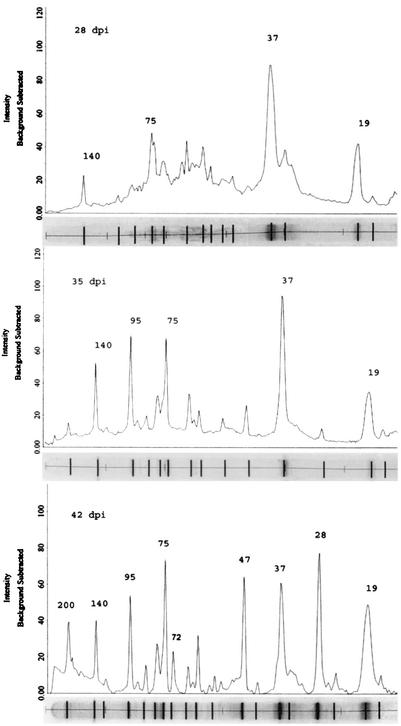
Major and minor immunoreactive antigens were identified by Western blots based on the peak intensity and average relative quantity of all reactive antigens on day 42 (Table 2 and Fig. 1 and 2). A slightly greater sensitivity in detecting antibody was observed by immunblotting with E. canis whole-cell lysates as compared to the ELISA (Fig. 1 and 3). Antigens were recognized by Western blotting in at least six dogs on day 21 compared to two dogs positive by ELISA and four dogs positive by IFA (Table 1 and Fig. 1 and 3). The two antigens in E. canis lysates that exhibited the earliest antibody reactivity in the acute-phase response were a 37-kDa protein and a 19-kDa protein—perhaps the ortholog of E. ruminantium MAP2. Some dogs that received the highest inoculum concentrations (1:100 and 1:500 dilutions) by the i.d. route consistently exhibited the earliest antibody responses (day 21), which were primarily directed at the 37- and 19-kDa proteins (Table 1 and Fig. 1). Similar responses on day 21 were not observed in any dogs from companion groups that received the inoculum s.c. All dogs had detectable E. canis-specific antibody by Western immunoblotting on day 28, and all dogs exhibited an antibody response to three or more antigens by day 35 postinoculation (Fig. 1). Major immunoreactive antigens, recognized earliest in the immune response (days 21 and 28), were the 19-, 37-, 75- and 140-kDa proteins (Table 3 and Fig. 1 and 2). The minor antigens were 72- and 79-kDa proteins. The major immunoreactive antigens recognized later (days 28, 35, and 42) in the infection were the 28-, 47-, 72-, 95-, and 200-kDa proteins (Table 3 and Fig. 1 and 2).
TABLE 2.
Major and minor E. canis immunoreactive antigens
| Antigen | E. canis immunoreactive antigens by immunoblot (mass in kDa) |
|---|---|
| Majora | 19, 28, 37, 47, 72, 75, 95, 140, 200 |
| Minor | 25, 34, 61, 65, 79 |
Relative average quantity of >3.0 of on day 42.
FIG. 2.
Peak intensity of immunoblot from a representative dog (dog 37) on days 28, 35, and 42 postinoculation. Molecular mass calculations, peak intensity, and average relative quantity of immunoreactive antigens were determined by the Quantity One gel analysis software package (Bio-Rad). The corresponding immunoblot of reactive antigens identified by computer analysis is shown below the peak intensity graph. Peak intensity and the average relative quantity were used to identify major immunoreactive proteins. Major immunoreactive proteins identified for each time interval are labeled above the corresponding peak.
TABLE 3.
Kinetic antibody response to E. canis major immunoreactive antigens
| No. of dayspostinoculation | E. canis major immunoreactive proteins (mass in kDa)a |
|---|---|
| 21 | 19, 37 |
| 28 | 19, 37, 75, 140 |
| 35 | 19, 37, 47, 75, 95, 140, 200 |
| 42 | 19, 28, 37, 47, 72, 75, 95, 140, 200 |
Relative average quantity of >3.0.
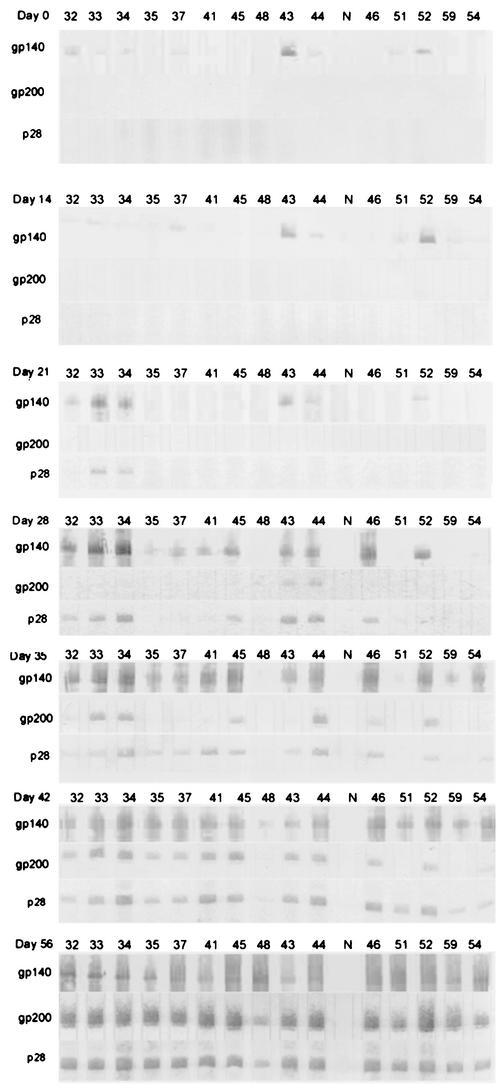
The kinetics of the antibody responses to recombinant proteins p28, gp140, and gp200 were similar to those demonstrated with whole-cell lysates (Fig. 4). The recombinant protein gp140 reacted nonspecifically with at least half of the dog sera prior to inoculation. This reactivity was not detectable in Western immunoblots with E. canis whole-cell lysates as an antigen (Fig. 1). In the 15 dogs that became infected and developed anti-E. canis antibody, an increase in antibody to gp140 over background levels was detectable on day 21 in 3 dogs, and 2 dogs exhibited antibody specific for the p28. By day 28, 11 dogs had developed antibodies to gp140, 7 dogs had developed antibody to the p28 protein, and 2 dogs had developed antibody to the gp200 protein. Antibody to gp200 developed later in the infection than antibodies to gp140 and p28, but by day 42, 12 of the 15 dogs recognized all three proteins, and in convalescent-phase sera obtained 2 weeks posttreatment, antibodies were detectable to the three recombinant proteins in all dogs.
FIG. 4.
Anti-recombinant E. canis gp140, gp200, and p28 antibody detection by Western immunoblotting on days 0, 7, 14, 21, 28, 35, 42, and 56. Lanes are labeled with the corresponding dog number.
By ELISA, the IgG2 isotype exclusively was found to be produced against E. canis whole-cell lysates by most dogs that developed canine ehrlichiosis (Fig. 3). A minimal increase in anti-E. canis IgG1 and IgM antibodies was detected by ELISA on day 28 only, in 4 of the 15 dogs (dogs 30, 35, 41, and 45) but was undetectable by day 35 postinoculation (data not shown). Antibody was detected earlier in the dogs that received the highest inoculum dose (1:100), but the levels of antibody detected 2 weeks posttreatment (day 56) or 8 weeks postinoculation were similar among all dogs. Of the dogs receiving the 1:100 inoculum dose, two dogs had detectable antibody by ELISA at 21 days postinoculation, while four of five dogs receiving the 1:500 dose and one of two dogs receiving the 1:1,000 dose developed a detectable antibody response on day 28.
Major immunoreactive antigens gp200, gp140, and p28 were confirmed to correspond to previously molecularly characterized and reported immunoreactive E. canis antigens (21, 24, 34, 46). These antigens were identified with polyclonal antisera produced against the gp200, gp140, and p28 recombinant proteins (data not shown).
DISCUSSION
The identification of all of the major immunoreactive proteins of E. canis recognized by the host immune response is an important step towards the identification of candidates for subunit vaccines and immunodiagnostics. Previous studies aimed at identification of major immunoreactive E. canis proteins have been deficient in the identification of large immunoreactive proteins (4, 32, 39), and none has substantially addressed the kinetics of antibody response to determine antigen recognition in different phases of the immune response. The aim of this study was to identify all major immunoreactive antigens, determine the kinetics of antibody responses to these antigens, and characterize the antibody response to E. canis in dogs experimentally infected by routes that best represent a tick bite inoculation.
Some major antigens recognized early, including the 37- and 75-kDa proteins, have not been well characterized. However, others, including the 200- and 140-kDa proteins and the corresponding orthologs in E. chaffeensis, have been described previously (21, 22, 45, 46) and are among the first glycoproteins described in pathogenic bacteria (25). The gp200 and gp140 proteins are genetically and antigenically distinct from the E. chaffeensis orthologs, and thus they may be important for species-specific immunity and will be useful as species-specific immunodiagnostic antigens (21, 22). gp200 has been shown to be an especially sensitive immunodiagnositic antigen for E. canis infections compared to IFA and provides species specificity (21). While gp140 was previously shown to be an immunoreactive protein (22), the nonspecific reactions we have observed suggest that it may not be useful as an immunodiagnostic antigen in its current form. The 19-kDa protein, the target of an antibody response earliest in the acute phase, may be the ortholog of E. ruminantium MAP2 that has been previously characterized and shown to be a useful serodiagnostic antigen (2). The kinetics of antibody response to the major immunoreactive proteins of E. canis suggest that some antigens such as the 37- and 19-kDa proteins detect antibody earliest in the acute phase and therefore may be advantageous for diagnostics aimed at detecting early-acute-phase infections. However, the use of these proteins as immunodiagnostic antigens may not be of any added benefit, because most dogs do not present with clinical signs until 21 to 40 days postinfection.
Many of the major immunoreactive proteins identified in this study are similar in mass to those described previous reports, but differences in the assignment of molecular masses to these proteins make direct comparison difficult. However, several major immunoreactive proteins consistently identified in those reports appear to be homologous with the 19-, 28-, 37-, 47-, and 75-kDa antigens described in this study. Major immunoreactive antigens described in this study and not consistently identified in previous reports are the 95-, 140-, and 200-kDa proteins, although the 110-kDa protein described in several reports may be homologous to the 95-kDa protein identified in this study. Differences in molecular masses among these previously published reports and ours may be related to electrophoresis methodology, protein standards, or available methods for molecular mass determination.
The molecularly characterized multigene family of 28-kDa major outer membrane proteins was expected to be recognized early in the acute infection, but kinetics suggested that antibodies against these proteins developed later in the infection. This finding is in contrast to previously reported early reactivity of a 28-kDa immunoreactive antigen in dogs intravenously inoculated with E. canis-infected cells (14). Notably, another study of experimental infection by intravenous E. canis inoculation reported antibody reactivity by one of four dogs that was consistent with our findings (39). This dog had early recognition of antigens of ∼47 and 37-kDa, but reactivity of the 30-kDa (p28) antigen was observed 2 weeks later; unfortunately, the three remaining dogs showed very little reactivity to proteins other than the 30-kDa antigen. The reasons for this substantially increased E. canis antigen reactivity in our study compared to that in the previous report are not clear, but a likely explanation is immunoblot sensitivity.
In our experiments, we were able to resolve proteins with masses of between ∼15 and 250 kDa, which enabled the consistent identification of larger immunoreactive proteins, including gp140 and gp200. A significant difference was noted with respect to a single major protein band identified in our study compared with numerous immunoreactive proteins in the 23- to 30-kDa range identified in previous studies. It has been established that the E. canis 28-kDa proteins are encoded on multigene loci consisting of 25 alleles. Expression of multiple 28-kDa proteins could provide an explanation for multiple bands ranging from 23 to 30 kDa observed on previous blots. In contrast, these are outer membrane proteins with numerous cysteine resides (five to seven) that may be involved in disulfide bond formation leading to changes in conformation and, ultimately, electrophoretic mobility. In previous studies in which antioxidants were not routinely used, these bonds have the potential to reoxidize during electrophoresis, leading to variations in molecular mass of any disulfide bond-containing protein. In our work, the inclusion of antioxidants in the electrophoresis buffer may explain the consistent single major immunoreactive band at 28 kDa. It is possible that multiple bands in previous blots are due to disulfide bond oxidation in the 28-kDa proteins, causing variations in the electrophoretic mobility of these proteins.
The major immunoreactive antigens identified in this study most likely have linear B-cell epitopes, although some conformational epitopes may also be retained during SDS-PAGE. The epitope type could result in differences in the immunoreactivity of a particular antigen by this methodology. Therefore, it is conceivable that some antigens with dominant conformational epitopes might not exhibit strong immunoreactivity by Western blotting or be weakly reactive; conversely, both linear and conformational epitopes could be located on the same protein. Thus, inclusion of conformational epitopes could potentially strengthen the conclusions. The immunoreactive MAP2 orthologs in E. canis and E. chaffeensis have been reported to differ in immunoreactivity on Western blotting, although both have been shown to be highly immunoreactive in conformational forms when used in ELISA (1, 2). A conformationally dependent epitope on major surface protein 5 (MSP5) of the related organism Anaplasma marginale has also been reported (26) and confirms that these epitopes play an important role in the immunoreactivity of certain proteins.
Evidence exists to suggest that some of the major immunoreactive proteins identified in this study, including the 140- and 28-kDa proteins, may be immunoprotective. The 140-kDa protein ortholog A. marginale MSP1a protected cattle when used as a subunit vaccine (35, 36); antibodies against the E. chaffeensis 28-kDa protein have provided protection against lethal infection in a SCID mouse model (44); and E. ruminantium MAP1, a p28 ortholog, has been shown to provide partial protection in mice (31). Conversely, the 19-kDa major immunoreactive protein, which is likely a highly conserved ortholog of E. ruminantium MAP2 and A. marginale MSP5, has been reported not to provide protection in cattle immunized with native or recombinant MSP5 (37). Protection afforded by E. ruminantium MAP2 has not been evaluated, but the antigen is capable of eliciting strong Th1-type cellular responses (28, 29). Further studies are needed to identify immunodominant T-cell epitopes and determine whether or not these orthologous proteins of Anaplasma and Ehrlichia spp. are immunoprotective.
The isotype of the antibody response in acute- and convalescent-phase sera against E. canis whole-cell lysates was exclusively of the IgG2 subclass in most dogs irrespective of the route of inoculation. These findings are consistent with another recent report, which found the predominant IgG2 isotype in all dogs naturally or experimentally infected with E. canis regardless of the disease phase (13). Notably in one study, antibodies effective in eliminating E. chaffeensis infection were exclusively IgG, with IgG2a being the most efficient (18). In humans and mice, a Th1 response and the corresponding production of gamma interferon (IFN-γ) are associated with isotype switching to IgG2 subclass antibodies. The IgG2 subclass is associated with a Th1-like immune response and indicates that this isotype switch may be a result of a high IFN-γ production by T cells. Consistent with our observations, a polarized IgG2 response has also been previously reported against E. canis and the closely related organism E. ruminantium (8, 13). Numerous studies with E. ruminantium have demonstrated a biased Th1 response to antigens in vivo and in vitro, including MAP1 and MAP2 (27, 29, 40). Furthermore, IgG2 antibodies were produced after immunization of calves with outer membranes from another related intracellular bacterium, A. marginale, and are associated with protection (6), and T-cell lines derived from calves immunized with A. marginale outer membranes or MSP1 exhibit high IFN-γ responses consistent with a Th1-type response to multiple major surface proteins (5). MSP1a appears to be an ortholog of the gp120 and gp140 of E. chaffeensis and E. canis, respectively, and the strong immunoreactivity of the gp140 in this study would suggest that important T-cell epitopes are present.
Interestingly, IgM subclass responses to E. canis were not observed in most dogs. A low level of IgM antibody was observed by ELISA in four dogs on day 28, and the antibody response determined by IFA and Western blotting utilizing a secondary antibody capable of detecting IgM confirmed the negative results on days 7 and 14. Studies investigating antibody responses to Ehrlichia spp. have not been routinely inclusive of IgM; therefore, this is the first study to report the absence of a significant IgM response in the acute phase of canine ehrlichiosis. In humans with monocytic ehrlichiosis, IgM has also been detected concurrently with increases in IgG, leading to questions regarding the usefulness of IgM serology for diagnosis (D. H. Walker, personal communication). All dogs were serologically negative at the beginning of this study and were laboratory raised. A previous exposure to Ehrlichia spp. could explain the anamnestic type response we observed, but there is no evidence to support this hypothesis. It is possible that the IgM response was very short in duration and that in the 7 days between sample collections the response had peaked and then declined to undetectable levels. A previous study with E. ruminantium found IgM 4 days after infection, but IgM was undetectable by day 7 (30).
The reactivity of gp140 with sera from healthy dogs has been reported previously (22). gp140 typically is reactive with 60% of normal dog sera, suggesting that natural antibodies may be directed against a carbohydrate epitope on a gp140 glycan. Natural antibodies are produced to carbohydrates of bacteria and parasites and are known to cross-react with other antigens (10). Although reactivity against the recombinant protein was observed, similar reactivity was not observed with the native gp140 on the E. canis whole-cell lysate immunoblots. gp140 appears to be a minor protein in quantity compared to other proteins in the whole-cell blots, and the amount of recombinant gp140 protein used is substantially higher. Thus, detection of antibodies with the recombinant gp140 may be related to sensitivity and may be a function of the quantity of antigen. Interestingly, gp200 did not react with antibodies from normal dog sera, which suggests that if carbohydrate epitopes are involved, gp140 has a distinct epitope not found on gp200. An increased antibody response to gp140 above background levels was evident, indicating that a specific immune response was directed at gp140, and this reactivity was confirmed by the E. canis whole-cell lysate immunoblots.
Acknowledgments
We thank Nahed Ismail for helpful discussions and Thomas Bednarek for assistance with digital imaging.
This work was supported by funding from the Clayton Foundation for Research.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alleman, A. R., A. F. Barbet, M. V. Bowie, H. L. Sorenson, S. J. Wong, and M. Belanger. 2000. Expression of a gene encoding the major antigenic protein 2 homolog of Ehrlichia chaffeensis and potential application for serodiagnosis. J. Clin. Microbiol. 38:3705-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alleman, A. R., L. J. McSherry, A. F. Barbet, E. B. Breitschwerdt, H. L. Sorenson, M. V. Bowie, and M. Belanger. 2001. Recombinant major antigenic protein 2 of Ehrlichia canis: a potential diagnostic tool. J. Clin. Microbiol. 39:2494-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob. Agents Chemother. 42:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouqui, P., J. S. Dumler, D. Raoult, and D. H. Walker. 1992. Antigenic characterization of ehrlichiae: protein immunoblotting of Ehrlichia canis, Ehrlichia sennetsu, and Ehrlichia risticii. J. Clin. Microbiol. 30:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, W. C., G. H. Palmer, H. A. Lewin, and T. C. McGuire. 2001. CD4+ T lymphocytes from calves immunized with Anaplasma marginale major surface protein 1 (MSP1), a heteromeric complex of MSP1a and MSP1b, preferentially recognize the MSP1a carboxyl terminus that is conserved among strains. Infect. Immun. 69:6853-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhles, W. C. J., D. L. Huxsoll, and M. Ristic. 1974. Tropical canine pancytopenia: clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J. Infect. Dis. 130:357-367. [DOI] [PubMed] [Google Scholar]
- 8.Byrom, B., M. Obwolo, A. F. Barbet, and S. M. Mahan. 2000. A polarized Th1 type immune response to Cowdria ruminantium infection is detected in immune DBA/2 mice. J. Parasitol. 86:983-992. [DOI] [PubMed] [Google Scholar]
- 9.Codner, E. C., and L. L. Farris-Smith. 1986. Characterization of the subclinical phase of ehrlichiosis in dogs. J. Am. Vet. Med. Assoc. 189:47-50. [PubMed] [Google Scholar]
- 10.Galili, U., R. E. Mandrell, R. M. Hamadeh, S. B. Shohet, and J. M. Griffiss. 1988. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 56:1730-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaunt, S. D., R. E. Corstvet, C. M. Berry, and B. Brennan. 1996. Isolation of Ehrlichia canis from dogs following subcutaneous inoculation. J. Clin. Microbiol. 34:1429-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrus, S., T. Waner, I. Aizenberg, J. E. Foley, A. M. Poland, and H. Bark. 1998. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J. Clin. Microbiol. 36:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrus, S., T. Waner, D. Strauss-Ayali, H. Bark, F. Jongejan, G. Hecht, and G. Baneth. 2001. Dynamics of IgG1 and IgG2 subclass response in dogs naturally and experimentally infected with Ehrlichia canis. Vet. Parasitol. 99:63-71. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal, Z., W. Chaichanasiriwithaya, and Y. Rikihisa. 1994. Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J. Clin. Microbiol. 32:1658-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuehn, N. F., and S. D. Gaunt. 1985. Clinical and hematologic findings in canine ehrlichiosis. J. Am. Vet. Med. Assoc. 186:355-358. [PubMed] [Google Scholar]
- 16.Lewis, G. E. J., S. L. Hill, and M. Ristic. 1978. Effect of canine immune serum on the growth of Ehrlichia canis within nonimmune canine macrophages. Am. J. Vet. Res. 39:71-76. [PubMed] [Google Scholar]
- 17.Lewis, G. E. J., and M. Ristic. 1978. Effect of canine immune macrophages and canine immune serum on the growth of Ehrlichia canis. Am. J. Vet. Res. 39:77-82. [PubMed] [Google Scholar]
- 18.Li, J. S., F. Chu, A. Reilly, and G. M. Winslow. 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169:1419-1425. [DOI] [PubMed] [Google Scholar]
- 19.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 20.Long, S. W., X.-F. Zhang, H. Qi, S. Standaert, D. H. Walker, and X.-J. Yu. 2002. Antigenic variation of Ehrlichia chaffeensis resulting from differential expression of the 28-kilodalton protein gene family. Infect. Immun. 70:1824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride, J. W., J. E. Comer, and D. H. Walker. Identification of novel immunoreactive Ehrlichia canis and Ehrlichia chaffeensis glycoprotein orthologs. Ann. N. Y. Acad. Sci., in press. [DOI] [PubMed]
- 22.McBride, J. W., R. E. Corstvet, E. B. Breitschwerdt, and D. H. Walker. 2001. Immunodiagnosis of Ehrlichia canis infection with recombinant proteins. J. Clin. Microbiol. 39:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBride, J. W., X. Yu, and D. H. Walker. 2000. A conserved, transcriptionally active p28 multigene locus of Ehrlichia canis. Gene 254:245-252. [DOI] [PubMed] [Google Scholar]
- 24.McBride, J. W., X.-J. Yu, and D. H. Walker. 1999. Molecular cloning of the gene for a conserved major immunoreactive 28-kilodalton protein of Ehrlichia canis: a potential serodiagnostic antigen. Clin. Diagn. Lab. Immunol. 6:392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride, J. W., X.-J. Yu, and D. H. Walker. 2000. Glycosylation of homologous immunodominant proteins of Ehrlichia chaffeensis and Ehrlichia canis. Infect. Immun. 68:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munodzana, D., T. F. McElwain, D. P. Knowles, and G. H. Palmer. 1998. Conformational dependence of Anaplasma marginale major surface protein 5 surface-exposed B-cell epitopes. Infect. Immun. 66:2619-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mwangi, D. M., S. M. Mahan, J. K. Nyanjui, E. L. N. Taracha, and D. J. McKeever. 1998. Immunization of cattle by infection with Cowdria ruminantium elicits T lymphocytes that recognize autologous, infected endothelial cells and monocytes. Infect. Immun. 66:1855-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mwangi, D. M., D. J. McKeever, and S. M. Mahan. 1998. Cellular immune responses of cattle to Cowdria ruminantium. Dev. Biol. Stand. 92:309-315. [PubMed] [Google Scholar]
- 29.Mwangi, D. M., D. J. McKeever, J. K. Nyanjui, A. F. Barbet, and S. M. Mahan. 1998. Major antigenic proteins 1 and 2 of Cowdria ruminantium are targets for T-lymphocyte responses of immune cattle. Ann. N. Y. Acad. Sci. 849:372-374. [DOI] [PubMed] [Google Scholar]
- 30.Neitz, A. W., G. J. Viljoen, J. D. Bezuidenhout, P. T. Oberem, L. Visser, and N. M. Vermeulen. 1986. Detection of Cowdria ruminantium antigen and antibody during the course of heartwater disease in sheep by means of an enzyme-linked immunosorbent assay. Onderstepoort J. Vet. Res. 53:205-207. [PubMed] [Google Scholar]
- 31.Nyika, A., S. M. Mahan, M. J. Burridge, T. C. McGuire, F. Rurangirwa, and A. F. Barbet. 1998. A DNA vaccine protects mice against the rickettsial agent Cowdria ruminantium. Parasite Immunol. 20:111-119. [DOI] [PubMed] [Google Scholar]
- 32.Nyindo, M., I. Kakoma, and R. Hansen. 1991. Antigenic analysis of four species of the genus Ehrlichia by use of protein immunoblot. Am. J. Vet. Res. 52:1225-1230. [PubMed] [Google Scholar]
- 33.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohashi, N., A. Unver, N. Zhi, and Y. Rikihisa. 1998. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J. Clin. Microbiol. 36:2671-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer, G. H., A. F. Barbet, G. H. Cantor, and T. C. McGuire. 1989. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect. Immun. 57:3666-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer, G. H., A. F. Barbet, W. C. Davis, and T. C. McGuire. 1986. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science 231:1299-1302. [DOI] [PubMed] [Google Scholar]
- 37.Palmer, G. H., and T. F. McElwain. 1995. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet. Parasitol. 57:233-253. [DOI] [PubMed] [Google Scholar]
- 38.Reddy, G. R., and C. P. Streck. 2000. Variability in the 28-kDa surface antigen protein multigene locus of isolates of the emerging disease agent Ehrlichia chaffeensis suggests that it plays a role in immune evasion. Mol. Cell. Biol. Res. Commun. 1:167-175. [DOI] [PubMed] [Google Scholar]
- 39.Rikihisa, Y., S. A. Ewing, and J. C. Fox. 1994. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J. Clin. Microbiol. 32:2107-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Totte, P., D. McKeever, F. Jongejan, A. Barbet, S. M. Mahan, D. Mwangi, and A. Bensaid. 1998. Analysis of cellular responses to native and recombinant proteins of Cowdria ruminantium. Ann. N. Y. Acad. Sci. 849:155-160. [DOI] [PubMed] [Google Scholar]
- 41.Troy, G. C., and S. D. Forrester. 1990. Canine ehrlichiosis, p. 404-418. In C. E. Green (ed.), Infectious diseases of the dog and cat. W. B. Saunders Co., Philadelphia, Pa.
- 42.Unver, A., M. Perez, N. Orellana, H. Huang, and Y. Rikihisa. 2001. Molecular and antigenic comparison of Ehrlichia canis isolates from dogs, ticks, and a human in Venezuela. J. Clin. Microbiol. 39:2788-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss, E., J. C. Coolbaugh, and J. C. Williams. 1975. Separation of viable Rickettsia typhi from yolk sac and L cell host components by Renografin density gradient centrifugation. Appl. Microbiol. 30:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winslow, G. M., E. Yager, K. Shilo, E. Volk, A. Reilly, and F. K. Chu. 2000. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect. Immun. 68:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu, X. J., P. Crocquet-Valdes, and D. H. Walker. 1997. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene 184:149-154. [DOI] [PubMed] [Google Scholar]
- 46.Yu, X.-J., J. W. McBride, C. M. Diaz, and D. H. Walker. 2000. Molecular cloning and characterization of the 120-kilodalton protein gene of Ehrlichia canis and application of the recombinant 120-kilodalton protein for serodiagnosis of canine ehrlichiosis. J. Clin. Microbiol. 38:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu, X.-J., J. W. McBride, and D. H. Walker. 1999. Genetic diversity of the 28-kilodalton outer membrane protein gene in human isolates of Ehrlichia chaffeensis. J. Clin. Microbiol. 37:1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, X. J., J. W. McBride, X. F. Zhang, and D. H. Walker. 2000. Characterization of the complete transcriptionally active Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family. Gene 248:59-68. [DOI] [PubMed] [Google Scholar]