Abstract
Purpose
Declines in physical functioning (PF) among elderly cancer patients threaten quality of life and the ability to maintain independence. Adherence to healthy lifestyle behaviors may prevent functional decline.
Patients and Methods
Project Leading the Way in Exercise and Diet (LEAD), an intervention development study of the Pepper Older Americans Independence Center, aimed to determine whether breast and prostate cancer survivors (age 65+ years) assigned to a 6-month home-based diet and exercise intervention experienced improvements in PF when compared with an attention control arm receiving general health information. An accrual target was set at 420, and PF (Short Form-36 subscale), physical activity (Community Healthy Activities Models Program for Seniors), and diet quality (index from 3-day recalls) were assessed at baseline and at 6 and 12 months (6 months after intervention).
Results
This developmental project did not achieve its accrual target (N = 182); however, PF change scores were in the direction and of the magnitude projected. Baseline to 6-month change scores in the intervention versus the control arms were as follows: PF, +3.1 v −0.5 (P = .23); physical activity energy expenditure, +111 kcal/wk v −400 kcal/wk (P = .13); and diet quality index, +2.2 v −2.9 (P = .003), respectively. Differences between arms diminished during the postintervention period.
Conclusion
These findings suggest that home-based diet and exercise interventions hold promise in improving lifestyle behaviors among older cancer survivors, changes that trend toward improved PF. Future studies should incorporate larger sample sizes and interventions that sustain long-term effects and also take into account secular trends; these efforts will require adequate planning and resources to overcome the numerous barriers to intervening in this difficult to reach yet vulnerable population.
INTRODUCTION
Currently, there are more than 10 million US cancer survivors, comprising 3% to 4% of the American population;1 61% are at least 65 years old. Given trends in aging coupled with increasing cure rates, unprecedented increases in the number of elderly cancer survivors are forecasted.1-5
Although survivorship is celebrated, the impact of cancer is significant and associated with several long-term health and psychosocial sequelae.2-6 Compared with others, cancer survivors are at greater risk for other cancers, cardiovascular disease, osteoporosis, diabetes, and accelerated functional decline.2-14
Baker et al7 compared 22,747 elderly cancer patients with an equal number of age-matched controls and found that individuals diagnosed with cancer had significantly poorer Short Form-36 (SF-36) health-related quality-of-life (QOL) scores, as well as poorer scores on each of the eight subscales (all P < .001). Chirikos et al9 also found significant differences in SF-36 scores among breast cancer survivors compared with age- and work-matched controls (n = 210), and they conclude their cost analyses by reporting “the economic consequence of functional impairment exacts an enormous toll each year on cancer survivors, their families, and the American economy at large.” Previous studies mirror these findings and provide consensus that cancer survivors experience long-term decrements in physical functioning (PF) that threaten their ability to live independently.10-13 Although the exact mechanisms behind decreased functional status among elders with cancer is unknown, the interaction of treatment, age, and lifestyle factors is hypothesized because (1) most cancer patients experience decreased function during treatment, but these losses appear temporary among the young and permanent among the old15; and (2) because functional status is significantly better among elderly survivors who are physically active and who adhere to a plant-based, low-fat diet.16
Lifestyle interventions that promote a healthy diet and exercise hold potential to positively reorient the trajectory of functional decline.16,17 A study of 988 breast and prostate cancer survivors found that most are sedentary and consume diets that are high in fat and low in fruits and vegetables (F&V), thus placing them at increased risk for comorbid disease.18 Despite poor habits, most survivors are interested in diet-related (85%) and exercise-related (83%) interventions;18 this interest is especially keen among newly diagnosed patients, and may represent a teachable moment.17,19 However, issues surrounding transportation are reported as a barrier to program participation, especially among older cancer survivors, thus establishing the need for home-based approaches.2,17,18
We explored whether a home-based diet and exercise program of telephone counseling and mailed materials would improve lifestyle behaviors among breast and prostate cancer survivors and whether these potential improvements ultimately enhanced PF. The study, Project Leading the Way in Exercise and Diet (LEAD), was an intervention development study of the Duke Pepper Older Americans Independence Center.20
PATIENTS AND METHODS
A description of the trial design was published previously.20 However, a brief summary follows.
Eligibility and Patient Accrual
Locoregionally staged breast and prostate cancer patients, who were aged ≥ 65 years old and within 18 months of diagnosis, were ascertained primarily from 13 hospital registries within North Carolina. Permission to contact patients was sought from oncology-care physicians, and letters of invitation were mailed to individuals approved for contact (Fig 1). Patients interested in participating were instructed to sign an enclosed consent form and complete a screening survey designed to exclude individuals who (1) had conditions that precluded unsupervised exercise (uncontrolled congestive heart failure or angina, recent myocardial infarction, or breathing difficulties requiring oxygen use or hospitalization; the use of a mobility aid other than a cane; or plans to have hip or knee replacement) or a high F&V diet (kidney failure or chronic warfarin use); (2) had progressive malignant disease or additional primary tumors; (3) were unable to participate fully in the telephone counseling or mailed material interventions (severe hearing or speaking impairments, inability to speak/write English, or mental incompetence); (4) reported less than two PF deficits,15,21 (unlikely to experience change in PF); or (5) were already routinely exercising or adhering to a low-fat, high F&V diet.
Fig 1.
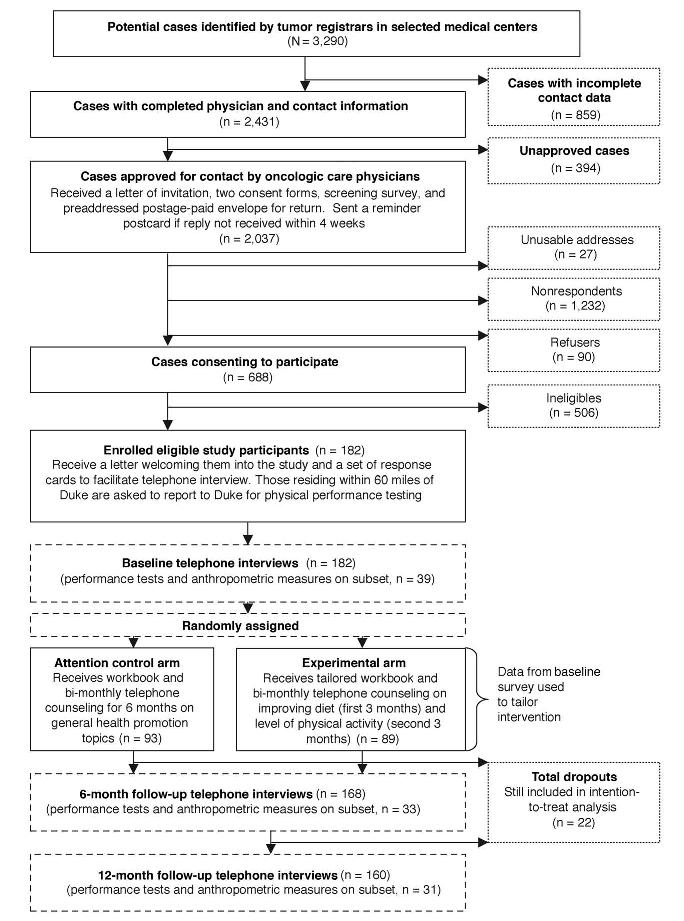
Study schema.
Baseline Measures
Eligible patients participated in a three-part computer-assisted telephone interview that ascertained the following: (1) Diet Quality Index from 3-day dietary recalls (NDS version 4.05-33; Nutrition Coordinating Center, Minneapolis, MN)22-24; (2) physical activity (Community Healthy Activities Models Program for Seniors [CHAMPS])25; (3) functional status (SF-36 Physical Function Subscale,21 with four appended items from Satariano et al;15); (4) QOL (Functional Assessment of Cancer Therapy Breast/Prostate)26,27; (5) perceived health28; (6) risk for depression (Center for Epidemiologic Studies of Depression Index)29; (7) comorbidity (Older Americans Resources and Services Index)30; (8) subjective and instrumental social support (Duke Social Support Index)31; (9) social desirability32; (10) self-efficacy and stage of readiness for dietary and exercise change33,34; and (11) sociodemographic factors. Patients residing within 60 miles of Duke were asked to report for clinically assessed heights, weights, and PF testing (further description in Appendix A).35
Random Assignment and Intervention
Eligible participants were block randomly assigned to study arms according to sex, race (white v nonwhite), and stage of readiness to pursue lifestyle change (precontemplation v contemplation/preparation).36-38 The treatment arm received telephone counseling and tailored print materials aimed at increased exercise and an improved overall diet (increased diet diversity with increased F&Vs and whole grains; decreased total fat, saturated fat, and cholesterol; and adequate iron and calcium), and the control arm received general health counseling and materials. Both interventions included 12 bimonthly 20- to 30-minute sessions over a 6-month period (see published methods article).20
Follow-Up Measures
Follow-up telephone surveys occurred at 6 and 12 months. Measures performed at baseline largely were repeated and appended with items assessing adverse events and process data.
Power Calculations and Statistical Analysis
A sample size of 420 participants (210 per arm) was established for this trial based on the following assumptions: (1) the attention control arm would experience no change in PF (primary end point) over time; (2) homogeneity of variances; and (3) the home-based intervention would achieve approximately half of the effect size observed by Morey et al39 in a more intensive, clinic-based intervention of similar content and conducted in a similar population. Two-tailed tests with α = .05 and 80% power were assumed with no adjustment for tests of multiple outcomes.
Mixed-model repeated measures analysis was used to assess differences between the change in the two arms over time controlling for the baseline value of the outcome of interest and marital status, smoking status, age at diagnosis, sex, educational attainment, and social desirability.40,41 Before analysis, the normality of the measures was assessed, and transformations were used if necessary. Correlations were explored between self-reported data (body mass index [BMI] and reported limitations to walking several blocks or walking over a mile [SF-36 items]) and in-person measures (BMI and 6-minute walk testing).
RESULTS
Over 3,000 prostate and breast cancer patients were identified by cancer registries for this study, of whom 74% had sufficient data to enable contact (Fig 1). Permission was granted to contact 84% of the patients, and most patient addresses were accurate (99%). Of the 2,010 contactable patients, consent forms and screeners were returned by 688 respondents (34% response rate). Respondents, compared with nonrespondents, were significantly younger (71.4 ∓ 5.0 v 73.0 ∓ 5.9 years, respectively; P < .0001), more proximal to diagnosis (10.8 ∓ 4.9 v 11.3 ∓ 5.8 months, respectively; P = .048), and more likely to be white (83% v 75%, respectively; P < .0001) and male (53% v 42%, respectively; P < .0001). Only 26% of respondents were eligible, with reasons for ineligibility as follows: conditions precluding unsupervised exercise (13%) or high intakes of F&Vs (19%) and/or conditions limiting the effectiveness of the intervention or ability to observe positive change in functional status, such as inability to read English or carry on normal telephone conversations (4%), current adherence to regular exercise (54%) or a healthy diet (11%), and/or reports of less than two PF limitations (31%). The cumulative effects of these factors resulted in our inability to meet the accrual target of 420 participants during the time and funding available for the study. To bolster accrual, we considered relaxing eligibility criteria to include exercisers and those with fewer functional limitations, but this strategy was dismissed because of threat of diminished effects.16,20
Of the 182 participants enrolled, most were of upper socioeconomic status, female, white, and married (Table 1). Although moderate numbers of comorbid conditions and functional limitations were reported, participants' perceived health and QOL tended to be good, and risk for depression was low. Few were current smokers; however, because of the selection criteria, most were sedentary and consumed suboptimal diets. No participants were underweight (BMI < 18.5), and 71% were overweight or obese (BMI ≥ 25.0). Most participants seemed ready to undertake changes in their diet and exercise behaviors and reported high levels of confidence in pursuing dietary and exercise goals, although readiness and confidence were higher for undertaking dietary change than for exercise (P = .0001/test for symmetry). At baseline, no significant differences were detected between study arms.
Table 1.
Characteristics of the Study Sample
| Intervention Arm (n = 89) |
Attention Control Arm (n = 93) |
|||
|---|---|---|---|---|
| Characteristic | No. | % | No. | % |
| Age, years | ||||
| Mean | 71.5 | 71.9 | ||
| SD | 4.4 | 5.6 | ||
| Range | 65-86 | 65-91 | ||
| Race | ||||
| White | 73 | 82.0 | 77 | 82.8 |
| African American | 13 | 14.6 | 14 | 15.0 |
| Other/unknown | 3 | 3.4 | 2 | 2.2 |
| Education | ||||
| < High school | 10 | 11.2 | 9 | 9.7 |
| High school graduate | 16 | 18.0 | 25 | 26.9 |
| > High school | 63 | 70.8 | 59 | 63.4 |
| Income < $12,000/year | 9 | 10.1 | 8 | 8.6 |
| Marital status: married | 58 | 65.2 | 71 | 76.3 |
| Type of cancer | ||||
| Breast | 51 | 57.3 | 53 | 57.0 |
| Prostate | 38 | 42.7 | 40 | 43.0 |
| No. of comorbid conditions | ||||
| Mean | 3.6 | 3.6 | ||
| SD | 2.1 | 2.1 | ||
| Perceived health | ||||
| Excellent/very good | 35 | 39.4 | 37 | 40.2 |
| Good | 34 | 38.2 | 43 | 46.7 |
| Fair/poor | 20 | 22.5 | 12 | 13.1 |
| Risk for depression score, CES-D | ||||
| Mean | 2.6 | 2.3 | ||
| SD | 3.3 | 3.2 | ||
| Quality of Life FACT-G score | ||||
| Mean | 88.2 | 90.4 | ||
| SD | 14.4 | 11.0 | ||
| Range | 46-108 | 39-108 | ||
| Smoking status: current smoker | 12 | 13.5 | 5 | 5.4 |
| BMI, kg/m2 | ||||
| Mean | 27.7 | 28.3 | ||
| SD | 5.3 | 5.3 | ||
| Exercise energy expenditure, kcal/wk | ||||
| Mean | 1,882 | 2,104 | ||
| SD | 1,916 | 1,735 | ||
| Stage of readiness for exercise* | ||||
| Precontemplation | 20 | 22.5 | 22 | 23.6 |
| Contemplation/preparation | 69 | 77.5 | 71 | 76.3 |
| Self-efficacy for exercise* | ||||
| Not at all/somewhat confident | 58 | 65.2 | 53 | 57.0 |
| Very/extremely confident | 31 | 34.8 | 40 | 43.0 |
| Diet Quality Index | ||||
| Mean | 67.6 | 67.5 | ||
| SD | 12.2 | 12.6 | ||
| Range | 34.1-91.3 | 27.2-92.7 | ||
| Stage of readiness to eat a healthy diet† | ||||
| Precontemplation | 9 | 10.1 | 9 | 9.7 |
| Contemplation/preparation/action | 80 | 89.9 | 84 | 90.3 |
| Self-efficacy to eat a healthy diet* | ||||
| Not at all/somewhat confident | 28 | 31.5 | 26 | 28.3 |
| Very/extremely confident | 61 | 68.5 | 66 | 71.7 |
| SF-36 Physical Function Subscale score | ||||
| Mean | 67.6 | 69.0 | ||
| SD | 22.3 | 20.0 | ||
| Range | 5-95 | 10-95 | ||
Abbreviations: SD, standard deviation; CES-D, Center for Epidemiologic Studies of Depression Index; FACT-G, Functional Assessment of Cancer Therapy–General; BMI, body mass index; SF-36, Short Form-36.
To exercise 30 minutes 3 times a week.
To follow a healthy diet low in fat and high in fruits and vegetables and whole grains (examples provided).
Print materials were distributed to all participants, and 168 completed all 12 telephone counseling sessions during the 6-month study period (7.7% dropout rate). Twelve-month follow-up data were obtained on 160 participants (cumulative 12.1% dropout rate). Reasons for dropout included lack of interest (n = 8), death (n = 6), illness (n = 5), and loss to follow-up (n = 3). No differences in attrition were observed between the study arms, and attrition was not related to age, race, or sex. No differences were noted between arms with regard to the number or level of adverse events.
Baseline and follow-up data for PF are depicted in Figure 2. Significant correlations were observed between the clinically administered 6-minute walk test and responses to the SF-36 items regarding difficulty in walking several blocks and walking over a mile (r = 0.54; all P < .0004), thus supporting the validity of self-reported data. Figure 2 also illustrates comparative change in diet and exercise behaviors and QOL. Baseline versus 6-month data suggest that the intervention was associated with a statistically significant improvement in diet quality (P < .003) and nonsignificant changes in other domains. Recidivism, although not significant (all P > .05), occurred from 6 to 12 months in each of these domains, except for QOL, where sustained increases in both arms were observed.
Fig 2.
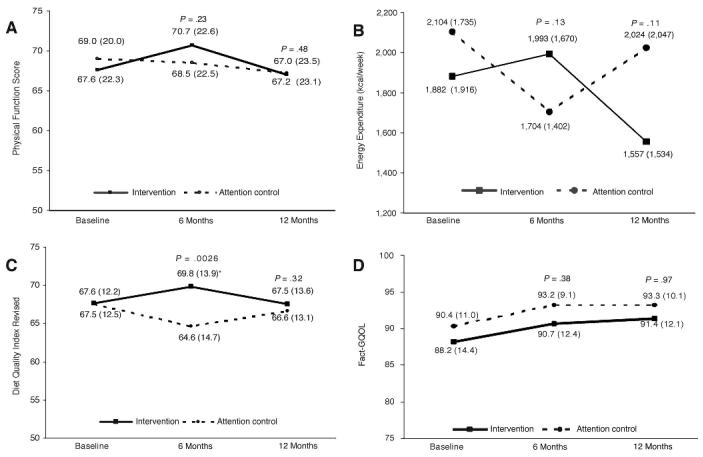
Baseline and follow-up data for physical functioning, change in diet and exercise behaviors, and quality of life. FACT-G, Functional Assessment of Cancer Therapy–General.
Baseline and follow-up data for other end points are listed in Table 2. The intervention arm experienced significant improvements in self-efficacy for exercise and exercise frequency tracked with weekly energy expenditure. No such changes were detected in readiness to exercise measures. Although overall diet quality improved with the intervention, no significant changes in specific food groups or dietary constituents were observed, and no significant changes were seen from high baseline levels of self-efficacy or stage of readiness to pursue a healthy diet.
Table 2.
Changes in Other Outcome Measures Over Time Between the Intervention and Attention Control Arms
| Intervention Arm |
Attention Control Arm |
P (intervention v attention control) |
||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Baseline (n = 89) | 6 Months (n = 82) | 12 Months (n = 77) | Baseline (n = 93) | 6 Months (n = 86) | 12 Months (n = 83) | 6 Months | 12 Months |
| Exercise frequency, sessions/wk | .22 | .94 | ||||||
| Mean | 9.9 | 10.9 | 9.7 | 11.5 | 10.1 | 10.3 | ||
| SD | 8.0 | 8.3 | 6.9 | 8.1 | 6.3 | 7.5 | ||
| Self-efficacy to exercise at least 30 minutes 3 times a week, % | .0074 | .46 | ||||||
| Not at all sure | 36.0 | 25.9 | 33.3 | 23.6 | 34.5 | 36.3 | ||
| A little sure | 14.6 | 7.4 | 14.7 | 24.7 | 17.9 | 11.3 | ||
| Somewhat sure | 14.6 | 17.3 | 14.7 | 9.0 | 10.7 | 11.3 | ||
| Very sure | 21.4 | 16.1 | 13.3 | 27.0 | 14.3 | 18.8 | ||
| Extremely sure | 13.5 | 33.3 | 24.0 | 15.7 | 22.6 | 22.5 | ||
| Readiness to exercise, % | .15 | .90 | ||||||
| Precontemplative | 22.5 | 23.2 | 39.0 | 23.6 | 36.1 | 44.6 | ||
| Contemplative | 5.6 | 2.4 | 6.5 | 4.3 | 5.8 | 2.4 | ||
| In preparation | 71.9 | 74.4 | 54.5 | 72.0 | 58.1 | 74.4 | ||
| % kcal from fat | .45 | .48 | ||||||
| Mean | 31.3 | 29.8 | 30.8 | 31.5 | 30.3 | 31.5 | ||
| SD | 7.2 | 6.8 | 6.7 | 7.0 | 6.9 | 7.5 | ||
| F&V, servings/d | .16 | .70 | ||||||
| Mean | 3.8 | 3.6 | 3.5 | 3.8 | 3.2 | 3.6 | ||
| SD | 2.0 | 2.0 | 2.1 | 2.2 | 1.8 | 2.1 | ||
| Self-efficacy to eat a healthy diet, % | .88 | .82 | ||||||
| Not at all sure | 4.5 | 1.3 | 2.6 | 2.2 | 1.2 | 1.2 | ||
| A little sure | 2.3 | 3.8 | 1.3 | 9.8 | 5.8 | 4.8 | ||
| Somewhat sure | 24.7 | 21.3 | 22.1 | 16.3 | 20.9 | 18.1 | ||
| Very sure | 43.8 | 55.0 | 57.1 | 46.7 | 51.2 | 54.2 | ||
| Extremely sure | 24.7 | 18.8 | 16.9 | 25.0 | 20.9 | 21.7 | ||
| Readiness to eat a healthy diet, % | .61 | .50 | ||||||
| Precontemplative | 10.1 | 9.8 | 19.5 | 9.7 | 15.1 | 16.9 | ||
| Contemplative | 84.3 | 89.0 | 72.7 | 79.6 | 79.1 | 79.5 | ||
| In preparation | 5.6 | 1.2 | 7.8 | 10.7 | 5.8 | 3.6 | ||
| BMI | .97 | .19 | ||||||
| Mean | 27.7 | 27.6 | 27.6 | 28.3 | 28.3 | 28.7 | ||
| SD | 5.3 | 5.3 | 5.2 | 5.3 | 5.0 | 5.2 | ||
| Depression, CES-D score | .55 | .74 | ||||||
| Mean | 2.6 | 2.2 | 2.2 | 2.3 | 2.2 | 1.7 | ||
| SD | 3.3 | 2.6 | 2.8 | 3.2 | 2.6 | 2.4 | ||
NOTE. All models use mixed models assuming continuous normal distribution and controlling for baseline value of the outcome and baseline marital status, smoking status, age at diagnosis, sex, race, educational attainment, and social desirability score.
Abbreviations: SD, standard deviation; F&V, fruits and vegetables; BMI, body mass index; CES-D, Center for Epidemiologic Studies of Depression Index.
Similar to QOL, which improved and stabilized at higher levels in both the intervention and control arms, scores for depression decreased in both arms and stabilized at lower levels, although differences did not reach significance. Weight status was fairly stable; the intervention arm reported a 0.1-unit decrease in BMI, and the attention control arm experienced a 0.4-unit increase during the study period. Excellent agreement was observed between BMIs calculated from self-reported weights and heights and those obtained via clinical assessments (interclass coefficient = 0.98).
DISCUSSION
To our knowledge, this is the first study to assess the impact of a home-based diet and exercise intervention on PF among elderly cancer survivors. In designing this study, we hypothesized that the intervention would achieve roughly half of the effect size observed by Morey et al39 in a more intensive, clinic-based study in a similar population. The forecasted effect size, a standard deviation (SD) of 0.23 in the change scores between the two arms, was close to that observed (SD = 0.19). This value is comparable to differences in PF scores observed between cancer patients and healthy age- and race-matched controls (SD = 0.22)7 and reported in other research as clinically significant (eg, abatement of a migraine headache, SD = 0.2142). However, we did not achieve our targeted accrual and were unable to declare this difference as statistically significant. Therefore, we join a host of underpowered trials, although our grounds (insufficient recruitment) differ from other trials (inaccurate projection).43-45 With a sample size of 182 participants, we would have required an SD of 0.35 to be 80% powered to declare statistical significance. Nonetheless, the strong trends observed in Project LEAD suggest that interventions that potentially improve PF warrant further exploration because functional decline exerts a major impact on older patients' QOL and health care costs related to supportive assistance.9 Thus, the findings of this study provide a basis by which to estimate statistical power for future research and provide preliminary evidence that home-based interventions may work within this hard to reach, vulnerable, and rapidly expanding population. The minimal attrition noted throughout this 12-month study also suggests that such interventions would likely be well received, although our low dropout rate and significant dietary change could be influenced by the socially advantaged and highly motivated nature of our sample.
This home-based intervention, which addressed dietary change in several domains, resulted in significant pre- to postintervention improvements in diet quality; these changes were not statistically significant for individual nutrients or food groups, but they cumulatively contributed to an overall improved diet. Although the intervention did not produce changes in physical activity that achieved statistical significance, it is possible that the CHAMPS instrument, which categorizes weekly activity into blocks of time, lacked sensitivity to detect modest increases in exercise. For example, an increase of two 20-minute exercise sessions per week over baseline would not be detected using the CHAMPS. Thus, future intervention studies may include CHAMPS categories of physical activity but also collect continuous (minutes of physical activity) data as well. Of note, a statistically significant increase in self-efficacy for exercise was observed among members of the intervention arm, suggesting that improvements in exercise behavior are mediated through this construct.33 Changes in self-efficacy or stage of readiness were not observed with respect to diet, which is a finding that may be explained by high baseline levels suggesting a ceiling effect. Given that our dietary intervention included both additive (encouragement to consume diverse diets with more F&Vs and whole grains) and reductive (encouragement to limit consumption of fat, saturated fat, and cholesterol) strategies to improve diet quality, our attempt to measure self-efficacy and stage of readiness for consuming a globally improved diet may have failed. Future studies may be better served by assessing these measures on individual domain-specific factors (eg, dietary fat, F&V, whole grains, and so on), rather than assessing healthy diet as a whole.
At the 12-month interview, differences between study arms diminished for PF, diet quality, and physical activity. Recidivism with fat-restricted diets (a large component of diet quality) and exercise interventions is a historic problem,43-45 and potential solutions usually involve increases in intervention intensity and frequency of contact. Previous studies also suggest that changes in lifestyle behaviors require continuous adherence for roughly 6 months before they become in-grained,34 thus extending the intervention period to allow for continued support once individuals have adopted new behaviors may increase the likelihood of durable effects. That being said, it may be unrealistic to expect stability over time46-48 because gradual declines in PF, physical activity, and diet quality are notable in longitudinal studies of aging populations.49-51 Therefore, the design of future trials should consider interim measures to control for secular trends.
Data related to QOL and depression were consistent and suggest that both interventions improved psychosocial well-being. Although these improvements may be an artifact of our highly motivated, socially advantaged sample, these results reinforce the need for an attention control when psychosocial outcomes are considered.
Perhaps the most valuable findings of this intervention development study relate to issues of feasibility and the potential for conducting such research on a larger scale. Lessons learned appear in the following paragraphs and may provide useful information to researchers who plan to pursue similar studies.
Patients ascertained from cancer registries often do not have complete data to allow for patient contact, especially if physician permission for contact is a proviso for institutional review board approval. In our experience, 26% of patients were not able to be contacted because of missing physician information.
Physician permission to contact patients was denied for 16% of patients, with concern regarding the Health Insurance Portability and Accountability Act as the most frequently cited reason for nonparticipation, even though the protocol met Health Insurance Portability and Accountability Act standards.52
A response rate of approximately 34% was noted for this home-based diet and exercise intervention that targeted newly diagnosed elderly breast and prostate cancer survivors. Levels of interest were greater among whites and males and those who were younger and more proximal to diagnosis. However, most of those expressing an interest already reported regular exercise (54%), and 11% followed healthy diets. With recent findings indicating that only 24.9% of elderly cancer survivors are physically active,53 it is clear that our recruitment efforts yielded a biased sample. Thus, strategies are necessary to increase receptivity for diet and exercise interventions among cancer survivors who need and could benefit from such interventions. Oncologists could provide valuable assistance by supporting healthful lifestyle change.17 Future trials also need to budget adequate resources to accrue this population, which is acknowledged as hard to reach.54
Most (68%) newly diagnosed breast and prostate cancer survivors interested in participating in home-based diet and exercise interventions report no contraindications to unsupervised physical activity or an F&V–rich diet. Furthermore, the lack of differences noted between arms regarding adverse events suggests that, with appropriate screening, such interventions are safe.
High levels of agreement were noted between self-reported and clinically assessed BMIs, and significant correlations existed between self-reported walking items of the SF-36 and clinically assessed 6-minute walk tests. These findings provide evidence that telephone interviews performed in elderly populations yield valid information.
The low rate of attrition suggests that home-based lifestyle intervention studies are well-accepted among elderly cancer survivors. However, the recidivism observed in behavioral end points suggests a need for further research in developing interventions that produce durable effects.
Thus, Project LEAD provides valuable information. First, its process data can help inform other intervention trials that target older cancer survivors. Second, data suggest that home-based diet and exercise interventions can be safely delivered and improve lifestyle behaviors, which ultimately may improve PF. Given that Project LEAD is an initial foray into home-based lifestyle interventions among elderly cancer survivors, its approach holds promise and beckons for more research in this area, research aimed at producing durable improvements in behavior and function and that is adequately resourced to ensure accrual of this vulnerable and difficult to reach population.
Supplementary Material
Acknowledgment
We thank Teresa Baker, Heather MacDonald, Cathie Ostrowski, Pamela Eberle-Wiley, Andrea Wilkinson, Rebecca Tesh, Diane Parham, Betty Ray, Miriam Nelles, Annie Langley, and Pamela Haines, PhD, and Boyd Switzer, PhD. We also are grateful to advisory board members and participating physicians and institutions (online only Appendix B).
Footnotes
Supported by Grants No. AG11268 and CA106919 from the National Institutes of Health, the Mary Duke Biddle Foundation, Grant No. NR07795 (E.C.C.), and the Susan G. Komen Foundation (W.D.-W.).
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 13-17, 2005; at the 2005 Annual Meeting of the American Dietetic Association, St. Louis, MO, October 22-25, 2005; at the 58th Annual Meeting of the Gerontological Society of America, New Orleans, LA, November 18-22; and at the 4th Annual Frontiers in Cancer Prevention Research Meeting of the American Association of Cancer Research, Baltimore, MD, October 30-November 2, 2005.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.National Cancer Institute http://cancercontrol.cancer.gov/ocs/prevalence Office of Cancer Survivorship: Estimated US cancer prevalence counts: Who are our cancer survivors in the US.
- 2.Aziz NM. Cancer survivorship research: Challenge and opportunity. J Nutr. 2002;132(suppl):3494S–3503S. doi: 10.1093/jn/132.11.3494S. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 4.Rowland JH, Aziz N, Tesauro G, et al. The changing face of cancer survivorship. Semin Oncol Nurs. 2001;17:236–240. doi: 10.1053/sonu.2001.27912. [DOI] [PubMed] [Google Scholar]
- 5.Yancik R, Ries LA. Cancer in older persons: An international issue in an aging world. Semin Oncol. 2004;31:128–136. doi: 10.1053/j.seminoncol.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA. Late effects of cancer and its treatment. Semin Oncol Nurs. 2001;17:241–248. doi: 10.1053/sonu.2001.27914. [DOI] [PubMed] [Google Scholar]
- 7.Baker F, Haffer S, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97:674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 8.Brown BW, Brauner C, Minnotte MC. Noncancer deaths in white adult cancer patients. J Natl Cancer Inst. 1993;85:979–997. doi: 10.1093/jnci/85.12.979. [DOI] [PubMed] [Google Scholar]
- 9.Chirikos TN, Russell-Jacobs A, Jacobsen PB. Functional impairment and the economic consequences of female breast cancer. Women Health. 2002;36:1–20. doi: 10.1300/J013v36n01_01. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the U.S.: Age, health and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 11.Mandelblatt JS, Edge SB, Meropol NJ, et al. Predictors of long-term outcomes in older breast cancer survivors: Perceptions versus patterns of care. J Clin Oncol. 2003;21:855–863. doi: 10.1200/JCO.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Silliman RA, Prout MN, Field T, et al. Risk factors for a decline in upper body function following treatment for early stage breast cancer. Breast Cancer Res Treat. 1999;54:25–30. doi: 10.1023/a:1006159720583. [DOI] [PubMed] [Google Scholar]
- 13.Williams ME. Identifying the older person likely to require long-term care services. J Am Geriatr Soc. 1987;35:761–766. doi: 10.1111/j.1532-5415.1987.tb06355.x. [DOI] [PubMed] [Google Scholar]
- 14.Wingo PA, Ries LA, Parker SL, et al. Long-term cancer patient survival in the United States. Cancer Epidemiol Biomarkers Prev. 1998;7:271–282. [PubMed] [Google Scholar]
- 15.Satariano WA, Ragheb NE, Branch LG, et al. Difficulties in physical functioning reported by middle-aged and elderly women with breast cancer: Case-control comparison. J Gerontol. 1990;45:M3–M11. doi: 10.1093/geronj/45.1.m3. [DOI] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Clipp EC, Morey M, et al. Physical function among elders with breast or prostate cancer: Associations with diet and exercise. Int J Behav Nutr Phys Act. 2004;1:16. doi: 10.1186/1479-5868-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W, Aziz N, Rowland J, et al. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demark-Wahnefried W, Peterson B, McBride C, et al. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 19.McBride CM, Clipp E, Peterson B, et al. Psychological impact of diagnosis and risk reduction among cancer survivors. Psycho-Oncol. 2000;9:418–427. doi: 10.1002/1099-1611(200009/10)9:5<418::aid-pon474>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Demark-Wahnefried W, Clipp EC, Morey M, et al. Leading the Way in Exercise and Diet (Project LEAD): Intervening to improve function among older breast and prostate cancer survivors. Control Clin Trials. 2003;24:206–223. doi: 10.1016/s0197-2456(02)00266-0. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Sherbourne CD. The MOS 36-item short-form heath survey (SF-36) Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 22.Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index revised: A measurement instrument for populations. J Am Diet Assoc. 1999;99:697–704. doi: 10.1016/S0002-8223(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 23.Conway JM, Ingwersen LA, Vinyard BT, et al. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food in-take in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–1178. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell D, Shacklock F. Computers in nutrition: The Minnesota nutrition database. Nutr Today. 1991;26:52–53. [Google Scholar]
- 25.Stewart AL, Mills KM, King AC, et al. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Brady MJ, Cella DF, Mo F. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality of life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 27.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-Prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 28.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 29.Kohout F, Berkman L, Evans D, et al. Two shorter forms of the CES-D Depression Symptoms Index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 30.Fillenbaum GG. Multidimensional Functional Assessment of Older Adults. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 31.Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34:61–69. doi: 10.1016/S0033-3182(93)71928-3. [DOI] [PubMed] [Google Scholar]
- 32.Crowne DP, Marlowe D. The Approval Motive. John Wiley; New York, NY: 1964. [Google Scholar]
- 33.Bandura A. Social Learning Theory. Prentice Hall; Englewood Cliffs, NJ: 1977. [Google Scholar]
- 34.Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 35.Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129–161. [Google Scholar]
- 36.Crespo CJ, Smit E, Andersen RE, et al. Race/ethnicity, social class and their relation to physical inactivity during leisure time: Results from the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Prev Med. 2000;18:46–53. doi: 10.1016/s0749-3797(99)00105-1. [DOI] [PubMed] [Google Scholar]
- 37.Gartside PS, Wang P, Glueck CJ. Prospective assessment of coronary heart disease risk factors: The NHANES I epidemiologic follow-up study (NHEFS) 16-year follow-up. J Am Coll Nutr. 1998;17:263–269. doi: 10.1080/07315724.1998.10718757. [DOI] [PubMed] [Google Scholar]
- 38.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 39.Morey MC, Schenkman M, Studenski S. Spinal-flexibility-plus-aerobic-only training: Effects of a randomized clinical trial on function in at risk older adults. Med Sciences. 1999;54A:335–342. doi: 10.1093/gerona/54.7.m335. [DOI] [PubMed] [Google Scholar]
- 40.Laird N, Ware J. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 41.Bryk A, Raudenbush S. Application of hierarchical linear models to assessing change. Psychol Bull. 1987;10:147–158. [Google Scholar]
- 42.QualityMetric Inc . Health Care Financing Administration, US Dept of Health and Human Services; Lincoln, RI: 2000. The SF-36 health survey: A summary of responsiveness to clinical interventions. Prepared for The Health Assessment Lab and the National Committee for Quality Assurance under Contract No. 500-97-P001. [Google Scholar]
- 43.Lilford R, Stevens AJ. Underpowered studies. Br J Surg. 2002;89:129–132. doi: 10.1046/j.0007-1323.2001.01989.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosoff PM. Can unpowered clinical trials be justified? IRB: Ethics Human Res. 2006;26:16–20. [PubMed] [Google Scholar]
- 45.Schultz KF, Grimes DA. Sample size calculations in randomized trials: Mandatory and mystical. Lancet. 2005;365:1348–1353. doi: 10.1016/S0140-6736(05)61034-3. [DOI] [PubMed] [Google Scholar]
- 46.Insull W, Jr, Henderson MM, Prentice RL, et al. Results of a randomized feasibility study of a low-fat diet. Arch Intern Med. 1990;150:421–427. [PubMed] [Google Scholar]
- 47.Marcus BH, Dubbert PM, Forsyth LH, et al. Physical activity behavior change: Issues in adoption and maintenance. Health Psychol. 2000;19(suppl 1):32–41. doi: 10.1037/0278-6133.19.suppl1.32. [DOI] [PubMed] [Google Scholar]
- 48.Stern MP, Farquhar JW, McCoby N, et al. Results of a two-year health education campaign on dietary behavior: The Stanford Three Community Study. Circulation. 1976;54:826–833. doi: 10.1161/01.cir.54.5.826. [DOI] [PubMed] [Google Scholar]
- 49.Maunsell E, Drolet M, Brisson J, et al. Dietary change after breast cancer: Extent, predictors, and relation with psychological distress. J Clin Oncol. 2002;20:1017–1025. doi: 10.1200/JCO.2002.20.4.1017. [DOI] [PubMed] [Google Scholar]
- 50.Nagi S. Disability concepts revisited: Implications for prevention. In: Pope A, Tarlov A, editors. Disability in America: Toward a National Agenda for Prevention. National Academy Press; Washington, DC: 1991. pp. 1309–1327. [Google Scholar]
- 51.Patterson RE, Neuhouser ML, Hedderson MM, et al. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103:323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 52.Kulynych J, Korn D. The new HIPAA (Health Insurance Portability and Accountability Act of 1996) medical privacy rule: Help or hindrance for clinical research? Circulation. 2003;108:912–914. doi: 10.1161/01.CIR.0000080642.35380.50. [DOI] [PubMed] [Google Scholar]
- 53.Bellizzi KM, Rowland JH, Jeffrey DD, et al. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8885–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 54.Kimmick GG, Peterson BL, Kornblith AB, et al. Improving accrual of older persons to cancer treatment trials: A randomized trial comparing an educational intervention with standard information. J Clin Oncol. 2005;23:2201–2207. doi: 10.1200/JCO.2005.01.222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


