Abstract
Proper T cell function relies on the integration of signals delivered by Ag, cytokine, and costimulatory receptors. In this study, the interactions between IL-2, CD27, and its ligand CD70 and their effects on human T cell function were examined. Unstimulated CD8+T cells expressed relatively low levels of CD70 and high levels of CD27. Incubation in vitro with high doses of IL-2 (3,000 IU/ml) or administration of IL-2 in vivo resulted in substantial up-regulation of CD70 expression and the concomitant loss of cell surface CD27 expression on CD8+ cells. Withdrawal of IL-2 from activated CD8+ T cells that had been maintained in IL-2 resulted in a reversal of the expression of these two markers, whereas reciprocal changes were seen following treatment of PBMCs with IL-2. The proliferation observed in cells stimulated with IL-2 primarily occurred in a subset of the CD70+CD8+ T cells that up-regulated IL-2 receptor expression but did not occur in CD70+CD8+ T cells. Blocking CD70 resulted in a significant reduction of T cell proliferation induced by high-dose IL-2, indicating that the interaction of CD70 with CD27 played a direct role in T cell activation mediated by IL-2. Finally, studies conducted on tumor-infiltrating lymphocyte (TIL) samples that were administered to melanoma patients indicated that the size of the pool of CD27+CD8+ T cells in bulk TILs was highly associated (p = 0.004) with the ability of these TILs to mediate tumor regression following adoptive transfer.
The cytokine IL-2 is a member of a family of cytokines that signal through the common γ-chain receptor in combination with unique receptors expressed on T cells. In addition to acting to promote the proliferation and effector function of CD8+ and CD4+ T cells, IL-2 has been shown to delay the contraction phase of T cells responding to viral infection (1) as well as play a role in the reversal of T cell anergy or tolerance (2, 3). The administration of IL-2 to patients with metastatic melanoma and renal cancer resulted in objective responses in 10–15% of treated patients (4–7). In addition, the adoptive transfer of tumor-infiltrating lymphocytes (TIL)3 cultured in vitro with IL-2 administered following lymphodepleting chemotherapy leads to objective clinical responses in ~50% of treated patients refractory to treatment with IL-2 alone (8, 9).
The engagement of costimulatory molecules on T cells with their ligands, found predominantly on APCs, also plays a critical role in enhancing T cell activation. The interaction of the costimulatory molecule CD27 with its ligand CD70 plays a key role in T lymphocyte activation, proliferation, survival, and differentiation (10–13). In both the human and mouse, CD27 is constitutively expressed on naive and memory T cells as well as on subsets of activated B cells, NK cells, and hemopoietic progenitor cells; however, expression of CD27 is down-regulated in late effector stage T cells (14–17). In contrast, little or no expression of CD70 is found on quiescent T, B, and dendritic cells; however, activation has been found to transiently up-regulate expression of this marker on these cell types (13, 18).
Signaling through CD27 can lead to a modest delay in the contraction phase of influenza-specific CD8+ T cells in peripheral blood and spleen after viral infection (10), and several studies indicate that the lack of costimulatory signals can lead to the induction of T cell anergy as well as apoptosis (19–21). Transfection of tumors with CD70 leads to enhanced anti-tumor immune responses (22, 23), and stimulation of CD27 through CD70 has been shown to support clonal expansion of tumor-specific T cells (24, 25). These observations suggest that engagement of CD27 may play an important role in regulating responses to a variety of antigenic stimuli.
The findings presented in this study provide evidence that signaling mediated by the interaction between the CD27 and CD70 molecules expressed on CD8+ T cells may play an important role in IL-2-mediated T cell activation. Examination of the expression of CD27 and CD70 on samples of tumor-reactive TILs that were adoptively transferred as part of a human clinical trial also suggested that the expression of these molecules on CD8+ T cells in TILs may play a role in the response to adoptive immunotherapy.
Materials and Methods
Cells
Samples of PBMCs were obtained from patients with metastatic melanoma after the administration of tumor-reactive TIL or IL-2 in clinical protocols approved by the Institutional Review Board of the National Cancer Institute. The in vitro cultured TIL cell lines used to treat patients were established from tumor fragments by culturing dissociated cells in 3,000 IU/ml rhIL-2 and then expanded using anti-CD3 Abs in the presence of PBMC feeder cells.
Cell culture and activation
All experiments were performed in X-VIVO 15 (Cambrex) containing 5% human serum medium in the presence or absence of IL-2. PBMC samples from normal blood donors or IL-2-treated patients were cultured in various concentrations of IL-2 for up to 12 days, and analysis was performed as indicated in Results. For the CD70 blocking experiment PBMCs were thawed or obtained directly from pheresis procedures on patients, the indicated concentrations of Ab and IL-2 were added into 106/ml T cells at the same time, and cell cycle analysis or FACS analysis was conducted at day 4. The separation of CD27+ and CD27− cells was performed by initially culturing TILs in medium without IL-2 for 2 days. Anti-CD27-PE bead staining and anti-PE bead staining were subsequentially performed, followed by passage through an MS separation column from Miltenyi Biotec. The CD27− cells were obtained from the cells that had passed through during CD27+ cell selection and were then passed through an LD column (Miltenyi Biotec). The peptide stimulation experiment was done by pulsing with indicated concentrations of MART-126–35 (27L) for 18 h, and cytokine release was measured by ELISA. The total number of CD27+CD8+ T cells in TIL samples were evaluated by washing cells twice with medium without IL-2 and culturing the TILs in IL-2-free medium for 2 days, followed by Ab staining and FACS analysis.
Antibodies and FACS analysis
Characterization of the expression of TCR β-chain variable region expression on TIL was conducted using β-chain variable region Abs obtained from Beckman Coulter and Pierce. Anti-CD27, anti-CD70, and anti-CD8 were purchased from BD Biosciences. For evaluation of the levels of IL-2 binding to T cells, cells were stained with streptavidin-FITC using reagents from the recombinant human IL-2 Fluorokine (rhIL-2F) kit (R&D Systems) (26). Blocking Ab for human CD70 (clone BU69) was purchased from Ancell. FACS analysis was performed by using CellQuest software (BD Biosciences).
Real-time RT-PCR
Total RNA was isolated from CD27+ purified and CD27− purified cells, respectively, using RNeasy columns (Qiagen). RT-PCR was performed using the ThermoScript RT-PCR system (Invitrogen Life Sciences). Four microliters of RT-PCR products were used for the quantitative PCR using a commercially available TaqMan kit and probes and primers for CD27 (Hs00386811) and CD28 (Hs01007422) (Applied Biosystems). A full-length TCR β-chain was used for the internal control for all the assays. The Cβ-specific primers were GAGGGTCTCGGCCACCTT and AGGCGA CAGTTCAGGTCAAGA, and the probe was 5′-FAM-TGGCAGAAC CCCCGCAACCAACCAC-TAMRA-3′. The samples were run on a Chromo 4 detector with a PTC-200 Peltier thermal cycler (MJ Research)
Cell cycle analysis
T cells (106/ml) under different conditions were washed and fixed in 70% ethanol for at least 1 h at 4°C, and the cells were washed twice with PBS. One milliliter of propidium iodide staining solution (50 μg/ml) was added to the cell pellet and mixed well, 50 μl of RNase A stock solution (10 μg/ml) was added, and cells were incubated for 3 h or longer at 4oC, followed by flow cytometric analysis. A lymphocyte gate and doublet discrimination gate was used, and data were analyzed using ModFit LT version 3.1 software (Verity Software House).
cDNA-microarray analysis of CD27+ and CD27− T cells from patients’ TILs
RNA was isolated from CD27+ and CD27− populations of patients’ TILs as described above in the paragraph titled Real-time RT PCR and indirectly labeled via a single round of linear amplification with the Amino Allyl MessageAmp antisense RNA kit (Ambion). The cyanine dye-labeled samples were combined and hybridized overnight to a 22,000 gene-long oligo array (version 2.0; Operon Biotechnologies) supplied by the Laboratory of Molecular Technology (National Cancer Institute, Frederick, MD). Data image files were obtained using a GenePix 4000B scanner (Axon) and imported into GeneSpring version 7.0 (Silicon Genetics) for data analysis.
Results
IL-2 induces up-regulation of cell surface CD70 expression on CD8+ cells in vitro and in vivo
To examine the influence of IL-2 on the expression of CD27 and CD70, naive PBMCs were initially cultured in relatively low-dose (300 IU/ml) or high-dose (3000 IU/ml) rhIL-2. Only a small percentage of CD8+ T cells from peripheral blood expressed CD70 prior to in vitro culture (Fig. 1A). The frequency of CD8+ cells expressing CD70 was progressively up-regulated over a period of 12 days of in vitro culture in IL-2 (Fig. 1A, upper row), which was paralleled by an increase in the level of CD70 expression on the CD8+ T cells (data not shown). The expression of CD70 on CD8+ T cells following IL-2 treatment increased in all 12 patients who were analyzed and was more notable in cultures conducted in relatively high doses of IL-2 than in those conducted with low-dose IL-2 (Fig. 1A, upper row). The up-regulation of CD70 expression on CD8+ cells in vitro was inversely related to the loss of CD27 expression (Fig. 1A, bottom row).
FIGURE 1.
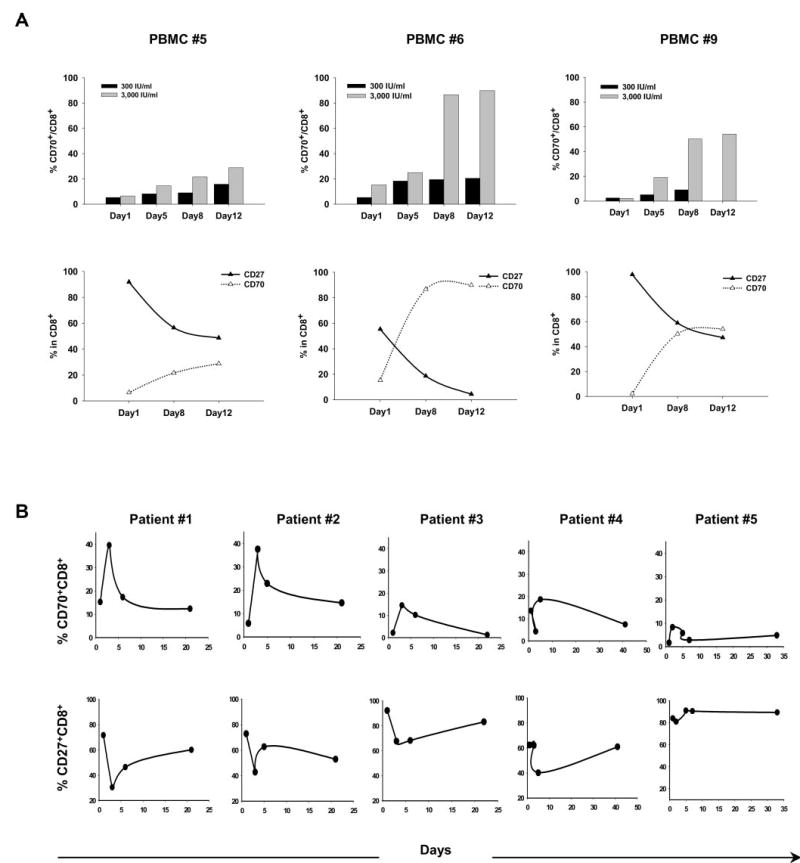
IL-2 induces up-regulation of cell surface CD70 expression on CD8+ cells from PBMCs in vitro and in vivo. A, The expression of CD70 and CD27 was examined on gated populations of CD8+ T cells from PBMCs derived from three melanoma patients obtained before any treatment in the Surgery Branch of the National Cancer Institute, National Institutes of Health. Cells were tested for CD70 and CD27 expression after culturing in medium with IL-2 (300 IU/ml or 3,000 IU/ml), and FACS analysis was conducted on days 1, 5, 8, and 12 after culture initiation. A time course of the effects of IL-2 on up-regulating CD70 expression on CD8+ cells (upper row) and the correlation between CD27 and CD70 expression on CD8+ cells during IL-2 culture (bottom row) are represented. B, The expression of CD70 and CD27 was examined on gated CD8+ T cells obtained from the peripheral blood of five patients either before treatment or following treatment with high-dose IL-2. The first IL-2 dose was administered on day 1. The time course demonstrating the modulation of CD70 expression is represented in the upper row, and the time course representing the modulation of CD27 expression is represented in the bottom row.
These observations led to attempts to determine whether similar changes in expression of CD27 and CD70 on CD8+ T cells occurred in circulating T cells present in melanoma patients who had been treated with high-dose IL-2. Before the administration of IL-2, patient peripheral CD8+ T cells expressed relatively low levels of CD70 in vivo; however, between 3 and 4 days following IL-2 administration, a significant increase in the percentage of CD8+ T cells that expressed CD70 was observed (Fig. 1B). In the peripheral blood of patients 1 and 2, expression of CD70 increased from ~15 and 5%, respectively, to nearly 40% of the CD8+ T cells in peripheral blood, whereas smaller increases were observed for the samples obtained from three additional patients. The maximum levels of CD70 expression were observed during a period of lymphopenia during which the absolute lymphocyte count dropped nearly 20-fold on average (from 1662 to 85 μl/ml) compared with the pretreatment level. Despite the large decrease in the absolute lymphocyte count, the absolute counts of CD70+CD8+ cells dropped only 2-fold (from 69 to 31 μl/ml) from pretreatment levels in peripheral blood. As the lymphocyte count started to recover in peripheral blood, the percentage of CD70-expressing CD8+ T cells declined to levels similar to those seen before treatment. The peak of CD70 expression correlated with a nadir in the expression of CD27 on the surface of CD8+ T cells during the lymphopenia that is routinely observed during IL-2 treatment (Fig. 1B, bottom row). This was followed by a gradual recovery of peripheral CD8+ T cells that expressed CD27 over a period of 3–6 weeks following the initial IL-2 infusion when CD70 expression levels were declining. Overall, these results indicated that there was an inverse relationship between the expression of CD70 and CD27 on CD8+ T cells and that IL-2 played a direct role in influencing the expression of these markers both in vitro and in vivo.
Effects of IL-2 on expression of CD27 and CD70 on activated CD8+ effector T cells
The observation that IL-2 treatment alone was capable of modulating the expression levels of CD70 and CD27 on CD8+ T cells in peripheral blood led to further studies examining the expression levels of these markers on in vitro cultured effector CD8+ from patients with melanoma. The majority of CD8+ T cells in TILs grown in IL-2 from 10 patients expressed significant levels of CD70, whereas only ~20% of the T cells expressed CD27 (data not shown). The withdrawal of IL-2 for a period of 2 days resulted in substantial up-regulation in CD27 expression on CD8+ T cells present in some TIL cultures. An example is shown in Fig. 2A. The overall increase in CD27 expression on TIL following IL-2 withdrawal for 2 days was statistically significant (p < 0.01, n = 10). The association between the expression of CD27 and CD70 on in vivo persistent T cells following adoptive transfer was then examined. Expression of CD27 progressively increased during a period of 2 mo on two dominant T cell clonotypes derived from TILs that persisted in the peripheral blood of two patients following adoptive transfer. Again, there appeared to be a negative correlation between CD27 and CD70 expression on the persisting T cells (Fig. 2B). These observations were consistent with the findings reported above indicating that in vitro culture in the absence of IL-2 resulted in up-regulation of CD27 expression and down-regulation of CD70 expression, because IL-2 levels present in peripheral blood are only elevated in vivo for a period of 2–3 days following TIL transfer when high-dose IL-2 was administered. Taken together, these results suggested that IL-2 plays a direct role in modulating the expression of both CD27 and CD70 on CD8+ T cells.
FIGURE 2.
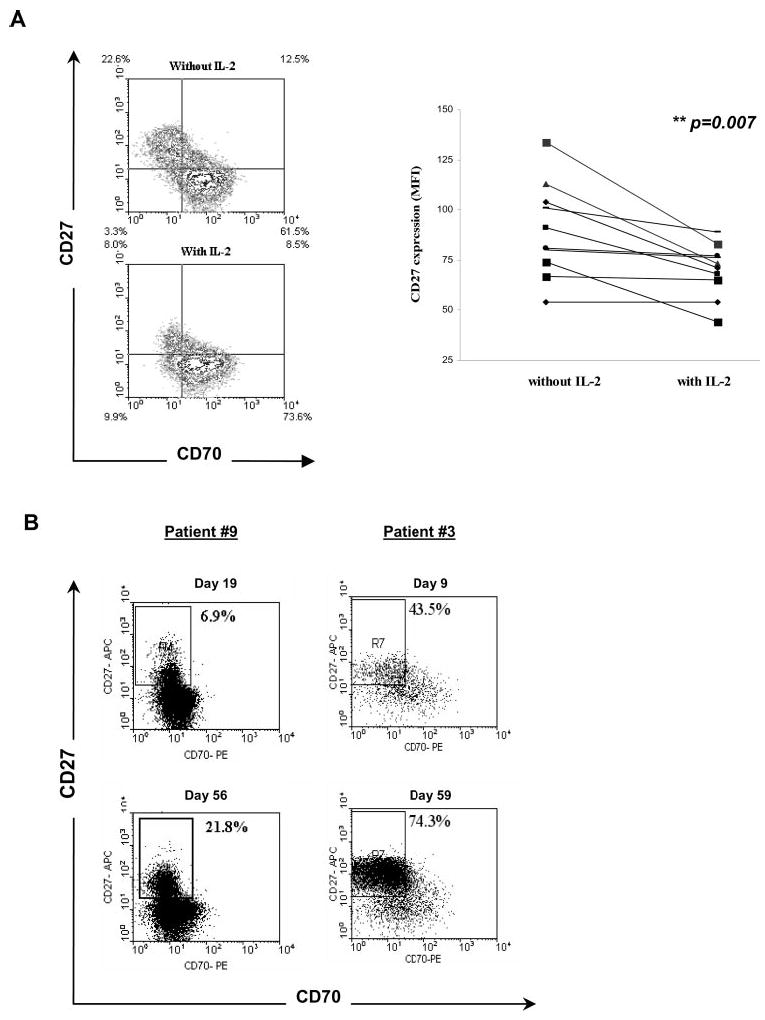
Effects of IL-2 on the expression of CD27 and CD70 on in vitro cultured TIL. A, The infused TIL from 10 melanoma patients were cultured in medium in the presence or absence of IL-2 for 2 days and costained with anti-CD27, anti-CD70, and anti-CD8 Abs. An example of a TIL culture is shown; the quadrant was based on isotype controls for gated populations of CD8+ T cells present within the TIL (left), and a summary analysis of the patients is shown (right). B, Flow cytometry of CD27 and CD70 on CD8+ cells present in the peripheral blood of two patients after adoptive TIL transfer. The gated populations, corresponding to dominant persistent clonotypes that were identified following adoptive transfer, were analyzed for expression of CD27 and CD70. For patient number 9, cells were gated using a tetramer of the dominant HLA-A2 MART-1 epitope, and for patient number 3 the population was gated using an anti-Vb16 Ab.
Preferential proliferation of a subset of CD8+ T cells that express CD70 and bind IL-2
The involvement of IL-2 in regulating expression of CD70 and CD27 was then investigated further by testing whether IL-2 binding correlated with the expression of these markers following in vitro culture of PBMCs in low-dose (300 IU/ml) or high-dose (3,000 IU/ml) IL-2 for 12 days. IL-2 has been shown to bind to IL-2Rα with low affinity, to the IL-2 Rβγ complex with intermediate affinity, and to the IL-2Rαβγ complex with high affinity (27, 28). Before the initiation of in vitro PBMC cultures, binding of rhIL-2F to CD8+ T cells was not detected (data not shown), and <10% of the CD8+ T cells from peripheral blood expressed CD70 at this time (Fig. 1A, upper row). Incubation of PBMCs with high-dose IL-2 for five days resulted in the generation of a subpopulation of T cells that was capable of binding rhIL-2F in four of the five samples that were examined (Fig. 3A), and this binding was correlated with the expression of CD25 (data not shown). High-dose IL-2 appeared to be more effective than low-dose IL-2 at enhancing the levels of IL-2 receptor expression on CD70+CD8+ cells, which is consistent with the observation that essentially all of the unstimulated peripheral CD8+ T cells expressed only the intermediate affinity IL-2βγ receptor complex. In addition, the CD70− T cells did not bind to IL-2, and only a subset of the CD70+ T cells bound detectable levels of IL-2. This heterogeneous response may result from differences in the susceptibility of naive vs memory cells to be activated by IL-2 alone, and differences between the responses of PBMCs obtained from different donors may reflect differences in the pool of activated vs naive cells present in these samples. When cell cycle analysis was conducted on gated populations of CD8+ T cells, the cells that demonstrated detectable binding to rhIL-2F contained a significantly higher percentage of cells in S phase than cells that failed to bind to IL-2 (p < 0.005; n = 5) (Fig. 3B).
FIGURE 3.
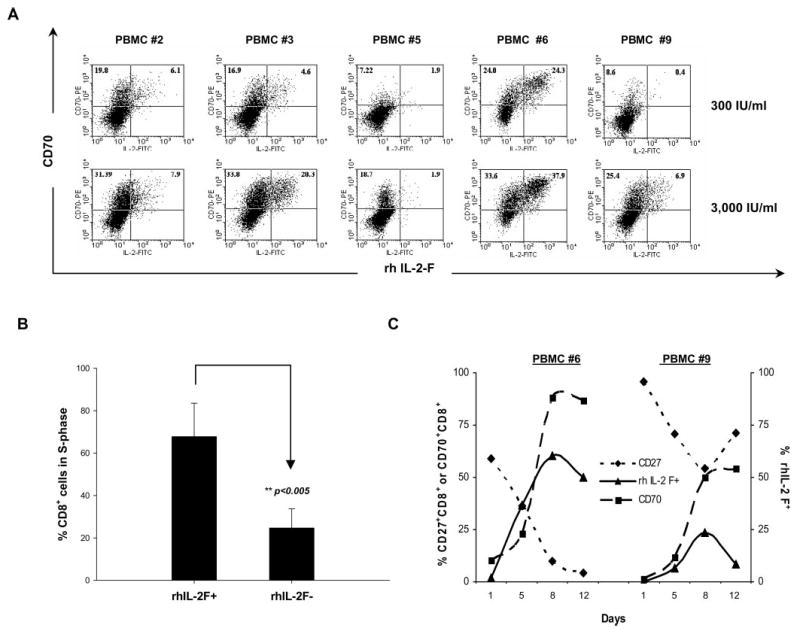
Binding of IL-2 to a subset of CD70+CD8+ T cells and association of binding with proliferation. A, The frequency of CD8+ cells that express CD70 and are bound to IL-2 was analyzed in PBMCs obtained from five patients following in vitro incubation with either low-dose (300 IU/ml) or high-dose (3,000 IU/ml) IL-2 for 5 days. B, Cell cycle analysis was conducted on samples of PBMCs that were cultured in 3,000 IU/ml IL-2 for 12 days. Cells that either bound to fluorescence-labeled rIL-2 (IL-2-FITC+) or did not bind detectable levels of IL-2 (IL-2-FITC−) were gated and analyzed for the percentage of cells in S phase. The mean ± SD of the percentage of gated populations from five PBMCs was calculated, and the significance of this difference was determined using the two-tailed Mann-Whitney U test. C, A kinetic analysis of the frequency of cells that bound to IL-2FITC in comparison to the frequency of cells that expressed CD27 and CD70 was conducted for two PBMC samples (from patients number 6 and number 9) that were incubated with high-dose IL-2 for a total of 12 days.
A time course study was then conducted to examine the effects of in vitro culture with IL-2 on the expression of CD27 and CD70. The results demonstrated that the percentage of CD70+CD8+ T cells that bound to rhIL-2F peaked on day 8 at a time when a significant loss in the expression of CD27 was observed (Fig. 3C). Twelve days following culture initiation, the levels of IL-2 binding had begun to decrease, and the expression of CD27 was either maintained at a low level or had begun to increase (Fig. 3C and data not shown).
IL-2 mediates T cell proliferation through the interaction of CD27 and CD70
It appeared that IL-2 preferentially bound to CD70+CD8+ T cells and promoted significant proliferation of CD8+ cells. To determine whether the interaction of CD70 with CD27 played a direct role in mediating T cell proliferation, TILs were incubated with a blocking Ab directed against CD70 in the presence of high-dose IL-2 (3,000IU/ml) for 2 days. Anti-CD70 Ab blocked CD70 expression (98.1%) and inhibited proliferation of the cultures by nearly 40%, (Table I); however, prolonged incubation with anti-CD70 led to significant apoptosis as compared with control Ab (data not shown). These results suggested that the ability of IL-2 to mediate T cell proliferation may be mediated in part through the interaction of CD27 with CD70.
Table I.
Blocking of CD70 expression on TIL-inhibited proliferation of CD8+ cells
| Anti-IgGa | Anti-CD70 (%) | Inhibitionb | pc | |
|---|---|---|---|---|
| CD70 expressiond | 41.8 ± 1.8 | 0.2 ± 0.0 | 98.1 ± 0.0 | <0.001 |
| Proliferatione | 36.0 ± 2.8 | 21.3 ± 0.7 | 37.9 ± 1.5 | <0.005 |
Patient TIL consisting of ~98% CD8+ T cells were cultured at a concentration of 1 × 106 cells/ml with 3,000 IU/ml IL-2 for 2 days with either an anti-CD70 or an isotype-matched control Ab at a concentration of 6.8 μg/ml.
Percentage of Inhibition = value(anti-IgG) minus; value(anti-CD70)/value(anti-IgG) × 100%.
The significance of differences was evaluated using a two-tailed Mann-Whitney test.
The expression of CD70 was analyzed by FACS analysis of gated CD8+ T cells.
Cell cycle analysis was carried out to determine the extent of proliferation of T cells cultures. Values indicate the percentage of CD8+ T cells in S phase.
Preferential secretion of IL-2 and proliferation by CD8+ T cells that express CD27
The ability of CD27+ and CD27− T cells to secrete IL-2 following Ag activation was then examined, because previous studies conducted with CD8+ T cells demonstrated preferential secretion of IL-2 by CD27+ T cells in response to a viral epitope (11). Cultures of TILs containing a high frequency of T cells reactive with the melanoma Ag MART-1 were incubated in the absence of IL-2 for 4 days, and the CD27+ cells and CD27− cells were then isolated. The results of a quantitative RT-PCR assay indicated that the T cells lacking cell surface expression of CD27 also contained low levels of mRNA encoding CD27 mRNA relative to CD27+ T cells (date not shown). Isolated CD27+ T cells secreted significantly higher levels of IL-2 than CD27− T cells following incubation with target cells pulsed with 0.1 μg/ml MART-1 peptide (Fig. 4A, left panel), whereas IFN-γ release was only slightly higher from CD27+CD8+ cells than from CD27−CD8+ cells with ≥1 μg/ml peptide (Fig. 4A, right panel). The analysis of cells present in S phase also indicated that, when cultured in the absence of exogenous IL-2 for 2 days, the percentage of cycling CD27+ TIL cells was significantly higher than the percentage of cycling CD27− T cells, whereas a significant difference was not observed between these populations when they were cultured in the presence of IL-2 (Fig. 4B).
FIGURE 4.
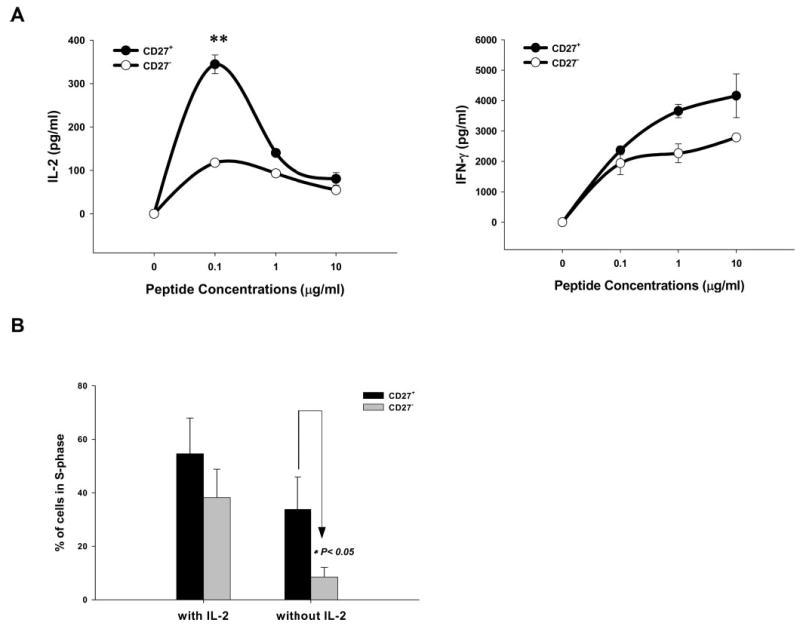
Preferential secretion of IL-2 and proliferation of CD8+ T cells that express CD27. A, CD27+ and CD27− T cells were isolated from a TIL culture that was maintained in medium without IL-2 for 4 days. The CD27+ and the CD27− T cells were cocultured with a T2 cell pulsed with the indicated concentrations of the MART-126–35 (27L) peptide for 18 h, and cytokine release was tested by ELISA. The data were representative of one of two patients whose TILs contained a high frequency of MART-1-positive T cells (>90%), and the frequency of MART-1-positive cells was equally distributed in CD27+ and CD27− populations. B, Four TILs were cultured in medium with (3,000 IU/ml) or without IL-2 for 2 days, and cell cycle analysis was conducted by determining the percentage of cells in the CD27+ and CD27− populations that were present in S phase, and the significance of this difference was determined using the two-tailed Mann-Whitney U test.
Microarray analysis was then conducted to determine whether there were significant differences in the gene expression profiles of the isolated CD27+ and CD27− T cells following IL-2 withdrawal. The results of this analysis indicated that more than one-third of the top 28 genes that were overexpressed in the CD27+ T cells relative to CD27− T cells were involved in either cell cycle regulation or DNA replication. Genes previously found to be over-expressed in tumor cells, such as the minichromosome maintenance proteins and proliferating cell nuclear Ags (29, 30), were also overexpressed in CD27+ T cells. In contrast, the majority of genes that were up-regulated in the CD27− population have been shown to be associated with activation and apoptosis, whereas genes associated with proliferation were not up-regulated in these cells (Table II), which was in line with previous studies showing that loss of expression of CD27 is a hallmark of late stage effector T cells.
Table II.
Genes differentially overexpressed in CD27+ or CD27− CD8+ cellsa
| CD27+/CD27− |
CD27−/CD27+ |
||||
|---|---|---|---|---|---|
| Gene Symbol | GenBank Association | Ratio | Gene Symbol | GenBank Association | Ratio |
| Cell cycle-related genes | |||||
| RRM2 | NM_001034 | 2.7 | |||
| TK1 | NM_003258 | 2.5 | |||
| FEN1 | NM_004111 | 2.4 | |||
| RAD51 | NM_002875 | 2.3 | |||
| MCM7 | NM_005916 | 2.3 | |||
| CDC451 | NM_003504 | 2.3 | |||
| MCM2 | NM_004526 | 2.2 | |||
| RFC2 | NM_002914 | 2.2 | |||
| DDAH2 | NM_013974 | 2.1 | |||
| TCF19 | S53374 | 2.1 | |||
| ASNS | NM_001673 | 2.1 | |||
| CAPG | NM_001747 | 2.1 | |||
| MCM4 | X74794 | 2.1 | |||
| MCM4 | X74794 | 2.1 | |||
| PCNA | NM_002592 | 2.0 | |||
| Anti-apoptosis gene | |||||
| REG-IV | NM_032044 | 3.3 | |||
| Cytokine production genes | |||||
| SSI-1 | NM_003745 | 2.5 | SCYA3 | NM_002983 | 4.8 |
| CISH | AF035947 | 2.5 | SCYA3L1 | D90145 | 2.9 |
| OSM | BC011589 | 2.5 | |||
| Activation and apoptosis genes | |||||
| TNFSF14 | NM_003807 | 2.1 | CD69 | NM_001781 | 9.2 |
| MAPK13 | NM_002754 | 2.1 | NR4A2 | NM_006186 | 8.8 |
| BID | NM_001196 | 2.1 | RGS1 | NM_002922 | 8.1 |
| IFNG | NM_000619 | 7.1 | |||
| PMAIP1 | NM_021127 | 5.2 | |||
| FOS | NM_005252 | 5.0 | |||
| TNFAIP3 | NM_006290 | 4.8 | |||
| FOSB | NM_006732 | 4.7 | |||
| EGR1 | NM_001964 | 4.3 | |||
| SGK | NM_005627 | 4.2 | |||
| NFKB1A | NM_020529 | 4.2 | |||
| RGS2 | NM_002923 | 3.7 | |||
| DUSP2 | NM_004418 | 3.6 | |||
| DAPK2 | NM_014326 | 2.8 | |||
| DUSP1 | NM_004417 | 2.8 | |||
The listed genes demonstrated 2-fold or greater differential expression between CD27+ and CD27− cells and more than three times background levels in either channel. Analysis was carried out on samples used in Fig. 4A.
Expression of CD27 and CD70 on TIL and relevance for therapy
To evaluate the potential relevance of these observations for adoptive immunotherapy, the expression levels of CD27 and CD70 were examined on CD8+ TIL cells that were administered to a series of melanoma patients following nonmyeloablative chemotherapy. Previous results demonstrated that ~50% of the patients treated in this trial responded to therapy (8, 9) and that the in vivo persistence of transferred TIL cells was associated with clinical response (31). As shown above, IL-2 can modulate CD27 and CD70 expression; thus, the majority of TIL samples expressed very low levels of CD27 when they were cultured in high-dose IL-2. Cultures of TILs were withdrawn from IL-2 for 2 days, and comparisons were then conducted between CD8+ T cell clono-types in the TILs that were previously shown to either persist or not persist in vivo in patients following TIL transfer (31). Persistent clonotypes expressed relatively higher levels of CD27+ cells than nonpersistent clonotypes in the same patient ( p < 0.05; n = 6) (Fig. 5), although considerable overlap existed between persisting and nonpersisting clonotypes among the patients. Bulk samples of TILs that were administered to patients who either did or did not respond to therapy were then withdrawn from IL-2 for 2 days, and the cellular phenotype was assessed. The results of this analysis indicated that the mean number of CD27+CD8+ and CD70+CD8+T cells in these IL-2-deprived TIL cultures was significantly higher in patients that showed an objective clinical response to cell transfer (p = 0.004 and p = 0.01, respectively; n = 33) (Table III). Neither the total number of infused T cells nor CD8+ T cells differed significantly between responders and non-responders (Table III), and no significant differences were observed between the expression of the costimulatory molecule CD28 and its corresponding ligand CD80 in the same TIL samples (data not shown). These observations suggest that analysis of the expression of CD27 and CD70 may be useful for the selection of effective TILs for use in adoptive immunotherapy.
FIGURE 5.
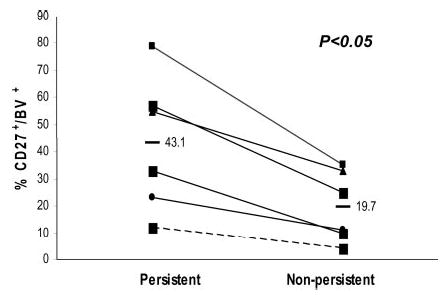
Frequency of CD27+ cells present in infusion TIL correlates with in vivo persistence of dominant TIL clonotypes after adoptively transfer. Six infusion TILs were cultured in medium without IL-2 for 2–4 days, and CD27 expression on dominant CD8+ clonotypes was analyzed by costaining with anti-TCR β-chain variable region-specific Abs as well as an anti-CD27 Ab. The frequency of CD27+ cells corresponding to each of the dominant clonotypes that were identified in a previous study is indicated (31). The significance of differences was determined by two-tailed Wilcoxon signed rank test.
Table III.
Total number of CD8+ and CD27+ CD8+ in TILs cultured without IL-2 for 2 daysa
| Patients | Total Cell Infused (×1010) | CD27+CD8+ (%) | CD70+CD8+ (%) | CD27+CD8+ (×1010) | CD70+CD8+ (×1010) | CD8+ (×1010) |
|---|---|---|---|---|---|---|
| Respondersb | ||||||
| 1 | 11.3 | 42.9 | 75.8 | 4.85 | 8.57 | 8.57 |
| 2 | 9.9 | 34.8 | 28.4 | 3.45 | 2.81 | 2.81 |
| 3 | 6.2 | 50.3 | 31.8 | 3.12 | 1.97 | 1.97 |
| 4 | 7.1 | 29.0 | 78.9 | 2.08 | 5.6 | 5.59 |
| 5 | 6.3 | 23.9 | 65.7 | 1.51 | 4.14 | 4.14 |
| 6 | 6.3 | 23.1 | 49.4 | 1.46 | 3.11 | 3.11 |
| 7 | 4.8 | 27.5 | 57.5 | 1.32 | 2.76 | 2.76 |
| 8 | 5.2 | 25.3 | 68.6 | 1.32 | 3.57 | 3.57 |
| 9 | 9.2 | 14.2 | 72.3 | 1.31 | 6.65 | 6.65 |
| 10 | 10.9 | 10.6 | 28.0 | 1.16 | 3.05 | 3.05 |
| 11 | 6.6 | 10.1 | 90.3 | 0.67 | 5.96 | 5.96 |
| 12 | 6.8 | 8.3 | 89.6 | 0.56 | 6.09 | 6.09 |
| 13 | 2.0 | 20.4 | 29.4 | 0.41 | 0.59 | 0.59 |
| 14 | 2.8 | 13.6 | 42.3 | 0.38 | 1.18 | 1.18 |
| 15 | 3.7 | 7.7 | 44.3 | 0.28 | 1.64 | 1.64 |
| 16 | 2.3 | 10.2 | 21.5 | 0.23 | 0.49 | 0.49 |
| Mean ± SEM | 6.3 ± 0.7 | 22 ± 3.2 | 54.6 ± 5.8 | 1.5 ± 0.3 | 3.62 ± 0.6 | 4.26 ± 0.6 |
| Nonresponders | ||||||
| 17 | 5.9 | 36.4 | 46.4 | 2.15 | 2.74 | 2.74 |
| 18 | 11.0 | 13.6 | 60.7 | 1.50 | 6.68 | 8.44 |
| 19 | 10.0 | 13.1 | 50.6 | 1.31 | 5.04 | 5.04 |
| 20 | 2.4 | 43.5 | 55.4 | 1.04 | 1.33 | 1.33 |
| 21 | 3.9 | 15.7 | 33.8 | 0.61 | 1.32 | 1.32 |
| 22 | 3.1 | 17.2 | 15.6 | 0.53 | 0.48 | 2.81 |
| 23 | 13.0 | 3.7 | 19.5 | 0.48 | 2.54 | 7.16 |
| 24 | 16.0 | 2.9 | 43.3 | 0.46 | 6.93 | 15.06 |
| 25 | 7.7 | 4.6 | 14.8 | 0.35 | 1.14 | 6.12 |
| 26 | 1.4 | 12.5 | 36.3 | 0.18 | 0.51 | 1.21 |
| 27 | 1.9 | 8.4 | 26.3 | 0.16 | 0.49 | 1.66 |
| 28 | 6.3 | 2.3 | 6.6 | 0.14 | 0.42 | 0.42 |
| 29 | 0.8 | 17.3 | 79.6 | 0.14 | 0.64 | 10.49 |
| 30 | 2.0 | 6.2 | 9.9 | 0.12 | 0.2 | 0.50 |
| 31 | 4.1 | 1.2 | 50.4 | 0.05 | 2.48 | 2.39 |
| 32 | 1.7 | 1.9 | 17.0 | 0.03 | 0.29 | 0.29 |
| 33 | 1.0 | 3.2 | 8.6 | 0.03 | 0.08 | 0.90 |
| Mean ± SEM | 6.1 ± 1.1 | 11 ± 2.9 | 34.4 ± 5.3 | 0.54 ± 0.1 | 2.44 ± 0.5 | 4.07 ± 1.1 |
| p value | 0.22 | 0.06 | 0.02 | 0.004 | 0.01 | 0.50 |
Samples from infusion bags were cultured without IL-2 for 2 days, and the expression of the indicated phenotypic markers were analyzed. The frequency of CD27+CD8+, CD70+CD8+, or CD8+ T cells, as well as the total number of cells corresponding to the indicated phenotype that would be present within each of the administered TIL under these conditions, was calculated based on the analysis of ungated samples. Quadrants were drawn based on staining obtained with IgG control antibodies. The significance of differences was determined using the two-tailed Wilcoxon rank sum test.
Response was defined as a 50% or greater decrease in the sum of perpendicular diameters of all measurable lesions for at least 1 mo with no increase in any lesions and no new lesions.
Discussion
Signaling mediated through the IL-2R, in conjunction with signals mediated through the T cell Ag receptor, promotes the proliferation and effector function of T cells (32). Some of the signals, such as up-regulation of the IL-2R α-chain, result from cooperative signals mediated both through the T cell Ag receptor as well as through the IL-2R itself; however, the relative role of signals delivered through these pathways has not been fully elucidated. Human tumor-reactive effector T cells have been shown to proliferate extensively in vitro in the presence of high-dose IL-2 alone (33). In addition, between 15 and 20% of melanoma and renal cancer patients treated with high-dose IL-2 alone respond to therapy (6), which may reflect the ability of IL-2 to maintain the proliferation of T cells that were activated by prior exposure to tumor Ags.
Interactions between the costimulatory receptor CD27 and its ligand, CD70, have also been found to play a key role in T lymphocyte activation, proliferation, survival, and differentiation (10–13). The intracytoplasmic domain of CD27 triggered by CD70 mediates these effects through activation of Jun kinase and NF-κB, which is required for optimal effector/memory CD8 T cell generation (34).
This study explores the effects of IL-2 on the expression of CD70 and its receptor, CD27, on CD8+ T cells, as well as the role of stimulation mediated through the CD27 pathway in regulating the proliferation and function of effector T cells. Incubation of PBMCs with IL-2 in vitro resulted in the up-regulation of CD70 expression on CD8+ cells and down-regulation of CD27 expression. This outcome did not appear to result from the selective death of CD27+ T cells, as significant levels of apoptosis were not observed in the CD27+ T cells that were present in these cultures (data not shown). Cells that possess a similar phenotype were observed in vivo in patients receiving high-dose IL-2 therapy at the time when IL-2 is present at detectable levels in peripheral blood. Expression of IL-2Rα (CD25) was not detected on CD8+ T cells before the up-regulation of CD70; thus, it appears that relatively high concentrations of IL-2 can up-regulate CD70 expression by signaling through the intermediate affinity IL-2Rβγ receptor, which has previously been shown to activate T cells (35–37).
Previous investigations demonstrated that CD27 expression is down-regulated on the surface of activated T cells following interaction with CD70. In CD70 transgenic mice that constitutively express CD70 on B cells, CD27 was down-regulated because of the continuous interaction of CD70 with CD27+ CD8+ T cells (10). In another report, the incubation of CD8+ T cell with anti-CD70 Ab interfered with the down-regulation of CD27 that was normally observed following Ag activation (11). The results of the present study suggest that down-regulation of CD27 expression on CD8+ T cells from peripheral blood following incubation with IL-2 may have resulted from interactions with CD70 expressed on T cells, which was associated with the acquisition of effector functions on those cells. This hypothesis was supported by the observation that the withdrawal of IL-2 from activated effector CD8+ T cells was associated with the up-regulation of CD27 and the down-regulation of CD70 expression. A similar phenotypic change was observed on CD8+ T cells present in the peripheral blood of IL-2-treated patients soon after the termination of IL-2 administration.
Additional experiments were then conducted to evaluate the nature of the interaction of CD27 with CD70 expressed on CD8+ T cells. The incubation of in vitro cultured effector CD8+ T cells that expressed CD70 with an anti-CD70 blocking Ab significantly inhibited the proliferation of these cells, even in the presence of high-dose IL-2, but did not alter the expression of markers such as CD25 (data not shown). These findings suggest that this Ab interfered with delivery of a signal through the CD27 molecule rather than delivering a direct signal by the cross-linking of CD70 molecules on the surface of T cells. Studies conducted on PBMCs that were cultured in vitro with IL-2 indicated that the subset of CD70+ cells that bound to rhIL-2F proliferated more extensively than cells that failed to bind to the rhIL-2F. The population of CD70+ CD8+ T cells that bound high levels of IL-2 also appeared to selectively down-regulate CD27 expression (data not shown), indicating that the delivery of a strong signal through the IL-2 receptor may play an important role in the down-regulation of CD27 expression by CD70. These observations suggest that engagement of CD27 by CD70 molecules expressed on T cells represents a down-stream mediator of the activation of T cells by IL-2.
The expression of CD27 was then examined on TILs that were adoptively transferred to patients, as this might provide an indication of the size of the effector/memory pool in these polyclonal populations of cells. Previous studies had demonstrated that the differentiation of T cells to end stage effector cells was associated with stable down-regulation of CD27 expression (14–17). The fact that IL-2, as well as TCR activation, transiently down-regulated CD27 expression on TILs hampered evaluation of the stage of differentiation of cells present in these polyclonal populations. However, the withdrawal of IL-2 from in vitro TIL cultures that had been grown continuously in the presence of IL-2 resulted in up-regulation of the expression of CD27 on a variable percentage of the T cells present in these cultures. The percentage of T cells that up-regulated CD27 expression varied widely between TILs, and for some cultures little or no up-regulation was observed. The minimal levels of CD27 expression that were observed on some TILs following IL-2 withdrawal may have resulted from the large percentages of end stage effector T cells present in these cultures.
Additional studies were then conducted to evaluate the properties of CD27+CD8+ T cell present within TILs. When TILs were cultured for 2 days in vitro in the absence of IL-2, CD27+CD8+ T cells proliferated to a greater extent than CD27−CD8+ T cells. Previous studies have demonstrated that central memory cells possess an enhanced ability to secrete IL-2 (38), and results presented in this study demonstrate that CD27−CD8+ cells secreted higher levels of IL-2 than CD27−CD8+ cells following Ag activation. These factors may help to account for the selective ability of CD27+CD8+ T cells to survive in vivo following adoptive transfer under conditions where common γ-chain cytokines such as IL-2 are limiting (17, 39). In this study, several genes that are selectively expressed in proliferating cells (29, 30) were up-regulated selectively in CD27+CD8+ T cells isolated from TILs. In contrast, the most highly expressed genes that were selectively up-regulated in CD27−CD8+ T cells included several genes involved with T cell activation and apoptosis, characteristics that have been associated with late stage effector T cells.
The potential relevance of these findings to clinical observations was then evaluated. Previous studies (31) suggested that the persistence of adoptively transferred T cells was associated with their proliferative capacity as well as with their ability to mediate tumor regression. Examination of individual clonotypes present within bulk TILs that persisted in patients’ peripheral blood following cell transfer revealed that these cells, when cultured before transfer in the absence of IL-2 for 2 days, expressed higher levels of CD27 than clonotypes that did not persist. An evaluation of the bulk TILs obtained from 33 patients treated with TILs following nonmyeloablative chemotherapy, including 16 responders and 17 nonresponders, demonstrated that the mean of total number of CD27+CD8+ T cells present in the TILs that were administered to responders were significantly higher than those administered to nonresponders. The expression of CD70 on cells cultured before transfer in the absence of IL-2 for 2 days also appeared to be significantly higher in TILs that were administered to responders than to nonresponders, implying that the pool of activated memory/effector cells present in TILs that were administered to responders was larger than in TILs that were administered to nonresponders. The expression of CD70 in vivo on other cells, such as B cells and dendritic cells that are present in patients that received adoptive TIL transfer, may also act to costimulate T cells that express CD27. The expression of CD70 on the transferred T cells alone, however, may provide a sufficient signal to promote the in vivo survival and proliferation of transferred CD8+ cells that are also capable of expressing CD27, as suggested by the in vitro studies presented in this report as well as by additional studies (40). In addition, in mouse model systems the expression of CD27 appeared to be associated with T cell accumulation at tissue effector sites and the survival of activated CD8+ T cells in vivo, whereas expression of CD28 was not associated with T cell migration (41). It is difficult to determine the relative roles of CD27 and CD70 expression in mediating the function of adoptively transferred T cells, but the expression of both the costimulatory receptor and ligand on T cells may enhance the in vivo function of these cells. These observations provide potential explanations for the association between CD27 expression in TILs and patient response to adoptive immunotherapy.
In summary, IL-2 is a crucial cytokine that modulates CD8+ T cell responses in immunotherapy. Our results suggested that IL-2 may function, at least in part, by promoting CD8+ T cell effector function through the interaction of CD27 with CD70. In addition, evaluation of the pool of T cells present in patients’ TILs that express CD27 and CD70 may facilitate the identification of effective TILs for use in patient treatment.
Acknowledgments
We thank Arnold Mixon and Shawn Farid for assistance with fluorescent cell analysis.
Footnotes
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations used in this paper: TIL, tumor infiltrating lymphocyte; rhIL-2F, recombinant human IL-2 Fluorokine.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 2.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto J, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 5.West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB, Oldham RK. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987;316:898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. J Am Med Assoc. 1994;271:907–913. [PubMed] [Google Scholar]
- 7.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphode-pleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arens R, Schepers K, Nolte MA, van Oosterwijk MF, van Lier RA, Schumacher TN, van Oers MH. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med. 2004;199:1595–1605. doi: 10.1084/jem.20031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochsenbein AF, Riddell SR, Brown M, Corey L, Baerlocher GM, Lansdorp PM, Greenberg PD. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. J Exp Med. 2004;200:1407–1417. doi: 10.1084/jem.20040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada S, Shinozaki K, Agematsu K. Involvement of CD27/CD70 interactions in antigen-specific cytotoxic T-lymphocyte (CTL) activity by per-forin-mediated cytotoxicity. Clin Exp Immunol. 2002;130:424–430. doi: 10.1046/j.1365-2249.2002.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Nolte MA, Arens R, van Os R, van Oosterwijk M, Hooibrink B, van Lier RA, van Oers MH. Immune activation modulates hematopoiesis through interactions between CD27 and CD70. Nat Immunol. 2005;6:412–418. doi: 10.1038/ni1174. [DOI] [PubMed] [Google Scholar]
- 15.Wiesmann A, Phillips RL, Mojica M, Pierce LJ, Searles AE, Spangrude GJ, Lemischka I. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, Robbins PF. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28:258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laouar A, Haridas V, Vargas D, Zhinan X, Chaplin D, van Lier RA, Manjunath N. CD70+ antigen-presenting cells control the proliferation and differentiation of T cells in the intestinal mucosa. Nat Immunol. 2005;6:698–706. doi: 10.1038/ni1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz RH. Acquisition of immunologic self-tolerance. Cell. 1989;57:1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 20.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 21.Daniel PT, Kroidl A, Cayeux S, Bargou R, Blankenstein T, Dorken B. Costimulatory signals through B7.1/CD28 prevent T cell apoptosis during target cell lysis. J Immunol. 1997;159:3808–3815. [PubMed] [Google Scholar]
- 22.Nieland JD, Graus YF, Dortmans YE, Kremers BL, Kruisbeek AM. CD40 and CD70 co-stimulate a potent in vivo antitumor T cell response. J Immunother. 1998;21:225–236. doi: 10.1097/00002371-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Couderc B, Zitvogel L, Douin-Echinard V, Djennane L, Tahara H, Favre G, Lotze MT, Robbins PD. Enhancement of antitumor immunity by expression of CD70 (CD27 ligand) or CD154 (CD40 ligand) costimulatory molecules in tumor cells. Cancer Gene Ther. 1998;5:163–175. [PubMed] [Google Scholar]
- 24.Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol. 1995;154:2612–2623. [PubMed] [Google Scholar]
- 25.Goodwin RG, Alderson MR, Smith CA, Armitage RJ, VandenBos T, Jerzy R, Tough TW, Schoenborn MA, Davis-Smith T, Hennen K, et al. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 1993;73:447–456. doi: 10.1016/0092-8674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 26.Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc Natl Acad Sci USA. 2000;97:9573–9578. doi: 10.1073/pnas.160099797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its α, β, and γc receptors. Science. 2005;310:1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 28.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 29.Tachibana KE, Gonzalez MA, Coleman N. Cell-cycle-dependent regulation of DNA replication and its relevance to cancer pathology. J Pathol. 2005;205:123–129. doi: 10.1002/path.1708. [DOI] [PubMed] [Google Scholar]
- 30.Munster PN, Norton L. Predictive factor for the response to adjuvant therapy with emphasis in breast cancer. Breast Cancer Res. 2001;3:361–364. doi: 10.1186/bcr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldmann T, Tagaya Y, Bamford R. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol. 1998;16:205–226. doi: 10.3109/08830189809042995. [DOI] [PubMed] [Google Scholar]
- 33.Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987;138:989–995. [PubMed] [Google Scholar]
- 34.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-κB activation pathways by NF-κB-inducing kinase. Immunity. 2004;21:477–489. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 36.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 37.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the α chain of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1997;94:3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herndler-Brandstetter D, Schwaiger S, Veel E, Fehrer C, Cioca DP, Almanzar G, Keller M, Pfister G, Parson W, Wurzner R, et al. CD25-expressing CD8+ T cells are potent memory cells in old age. J Immunol. 2005;175:1566–1574. doi: 10.4049/jimmunol.175.3.1566. [DOI] [PubMed] [Google Scholar]
- 39.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late stage effector T cells to CD27+CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 41.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. Vol. 176, No. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]


