Abstract
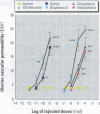
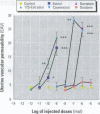
The in vivo effects of xenoestrogens are of interest in relation to their potential health risks and/or beneficial effects on humans and animals. However, the apparent in vivo potency of the examined response can be confounded by a short half-life, and the metabolism of estrogens is very dependent on the nature of conversion and/or inactivation. To minimize such variables, we examined the estrogenic potency of a range of xenoestrogens in an acute in vivo assay--the stimulation of increased uterine vascular permeability in ovariectomized mice 4 hr after subcutaneous administration. While estradiol (E 2 ) and estriol (E 3 ; a relatively weak natural estrogen) readily induced vascular responses [median effective dose (ED 50 ) <10 -9 mol], much higher amounts of xenoestrogens were required. Bisphenol A was about 10,000-fold less potent than E 2 and E 3 , and octylphenol and nonylphenol were about 100,000-fold less potent; dioctyl phthalate, benzyl butyl phthalate, dibutyl phthalate, and trichlorinated biphenol produced no effect. Coumestrol was the most active phytoestrogen, with an ED 50 between 10 -6 and 10 -7 mol; genistein was about 10-fold less potent than coumestrol, and neither daidzein nor formononetin produced any marked effect, even at doses up to 10 -5 mol. All increases in vascular permeability could be blocked by the pure antiestrogen ICI 182,780. There was no evidence that any of the compounds could act as an antiestrogen in this assay or that they could exert synergistic effects in combination. These results indicate that even short-term exposure to most of the xenobiotic estrogens can induce typical estrogenic effects in vivo , but their estrogenic potency is very weak even when assessed in an acute response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson N. G. Early oestrogen-induced changes in uterine albumin exchange in mice. Acta Physiol Scand. 1977 Jul;100(3):325–331. doi: 10.1111/j.1748-1716.1977.tb05957.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. E., Milligan S. R. Silastic implants for delivery of oestradiol to mice. J Reprod Fertil. 1993 Sep;99(1):219–223. doi: 10.1530/jrf.0.0990219. [DOI] [PubMed] [Google Scholar]
- El Samannoudy F. A., Shareha A. M., Ghannudi S. A., Gillaly G. A., El Mougy S. A. Adverse effects of phytoestrogens-7. Effect of beta-sitosterol treatment on follicular development, ovarian structure and uterus in the immature female sheep. Cell Mol Biol Incl Cyto Enzymol. 1980;26(3):255–266. [PubMed] [Google Scholar]
- HUGGINS C., JENSEN E. V. The depression of estrone-induced uterine growth by phenolic estrogens with oxygenated functions at positions 6 or 16: the impeded estrogens. J Exp Med. 1955 Sep 1;102(3):335–346. doi: 10.1084/jem.102.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995 Jun;103(6):582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. C., Eden J. A. A review of the clinical effects of phytoestrogens. Obstet Gynecol. 1996 May;87(5 Pt 2):897–904. [PubMed] [Google Scholar]
- Laws S. C., Carey S. A., Hart D. W., Cooper R. L. Lindane does not alter the estrogen receptor or the estrogen-dependent induction of progesterone receptors in sexually immature or ovariectomized adult rats. Toxicology. 1994 Sep 6;92(1-3):127–142. doi: 10.1016/0300-483x(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Markiewicz L., Garey J., Adlercreutz H., Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993 May;45(5):399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- Martin L., Pollard J. W., Fagg B. Oestriol, oestradiol-17beta and the proliferation and death of uterine cells. J Endocrinol. 1976 Apr;69(1):103–115. doi: 10.1677/joe.0.0690103. [DOI] [PubMed] [Google Scholar]
- Milligan S. R., Mirembe F. M. Intraluminally injected oil induces changes in vascular permeability in the 'sensitized' and 'non-sensitized' uterus of the mouse. J Reprod Fertil. 1985 May;74(1):95–104. doi: 10.1530/jrf.0.0740095. [DOI] [PubMed] [Google Scholar]
- Milligan S. R., Mirembe F. M. Time course of the changes in uterine vascular permeability associated with the development of the decidual cell reaction in ovariectomized steroid-treated rats. J Reprod Fertil. 1984 Jan;70(1):1–6. doi: 10.1530/jrf.0.0700001. [DOI] [PubMed] [Google Scholar]
- Milligan S. R. The sensitivity of the uterus of the mouse and rat to intraluminal instillation. J Reprod Fertil. 1987 Jan;79(1):251–259. doi: 10.1530/jrf.0.0790251. [DOI] [PubMed] [Google Scholar]
- Nagel S. C., vom Saal F. S., Thayer K. A., Dhar M. G., Boechler M., Welshons W. V. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997 Jan;105(1):70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudali G., Apiou F., Muel B. Mammary cancer produced in mice with estriol. Eur J Cancer. 1975 Jan;11(1):39–41. doi: 10.1016/0014-2964(75)90035-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz R., Brown N. G., Allen D. L., Bigsby R. M., Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997 May;138(5):1780–1786. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]