Abstract
Host extracellular matrix (ECM) components represent ideal microbial adhesion targets that many pathogens use for colonization of tissues and initiation of infection. This study investigated the interaction of the spirochete Treponema pallidum with the ECM component laminin. To identify candidate laminin-binding adhesins, the T. pallidum genome was analyzed to predict open reading frames that encode putative outer membrane proteins, as these proteins interact directly with host ECM components. Subsequent recombinant expression of these proteins and analysis of their laminin-binding potential identified one protein, Tp0751, that demonstrated specific attachment to laminin. Tp0751 attached to laminin in a dose-dependent, saturable manner but did not attach to the ECM component collagen type I or IV or to the negative control proteins fetuin or bovine serum albumin. Sodium metaperiodate treatment of laminin reduced the Tp0751-laminin interaction in a concentration-dependent manner, suggesting that oligosaccharides play a role in this interaction. In addition, Tp0751-specific antibodies were detected in serum samples collected from both experimental and natural syphilis infections, indicating that Tp0751 is expressed in vivo during the course of infection. Collectively, these experiments identified Tp0751 as a laminin-binding protein that is expressed during infection and may be involved in attachment of T. pallidum to host tissues.
Syphilis, which is caused by the spirochete bacterium Treponema pallidum subsp. pallidum, is a chronic bacterial infection that remains a public health concern worldwide. In 1995, the World Health Organization's global estimate of annual new syphilis infections was 12 million, with a global syphilis burden of at least 25 million cases (26). Syphilis is a chronic infection that fluctuates between symptomatic stages and prolonged asymptomatic stages. If left untreated, syphilis can cause serious late sequelae that may involve any organ system and lead to loss of function and destruction of tissue.
As observed for other pathogenic bacteria, the critical first steps in the establishment of a T. pallidum infection are attachment to and colonization of epithelial cell surfaces. Numerous studies have confirmed that T. pallidum attaches to host cells (3, 4, 16-20, 34, 64, 67, 77), although neither the host cell nor the T. pallidum components mediating such attachment have been clearly defined. Nonpathogenic T. phagedenis biotype Reiter does not adhere to cultured mammalian cells (8, 67), suggesting that attachment to host cells is a function unique to pathogenic treponemes. Previous studies have demonstrated that adherence appears to contribute to the prolonged survival, motility, and virulence of T. pallidum (18, 19). Results from experimentally induced T. pallidum infections (12, 59) and in vitro studies (67) have shown that T. pallidum adheres to and invades epithelial surfaces, traverses the tissue barrier, and enters the circulation by invading the tight junctions between endothelial cells. Treponemal invasion results in widespread bacterial dissemination, which in turn sets the stage for establishment of chronic infection. Similarly, in a natural syphilis infection, T. pallidum is believed to attach to and enter mucosal surfaces or epidermal abrasions, become widely disseminated throughout the tissues of the body, and establish a chronic infection (44, 45, 72). The invasive nature of T. pallidum is well illustrated by the fact that invasion of the central nervous system occurs in greater than 40% of syphilis patients (43) and that syphilis can be transmitted by an infected woman to her fetus in utero. Host cell attachment is critical to this ability of T. pallidum to invade and establish infection in many diverse tissues. These observations demonstrate the importance of treponemal attachment to host cells, not only in the initial stages of infection but also throughout the course of T. pallidum infection.
Adherence to and colonization of epithelial surfaces are the primary events in the pathogenesis of many bacterial infections (5, 52). This process involves the interaction of specific bacterial surface structures, known as adhesins, with host components. Extracellular matrix (ECM) molecules represent ideal microbial adhesion targets that many pathogens use for colonization of tissues and initiation of infection. The term MSCRAMM (microbial surface components recognizing adhesive matrix molecules) has been introduced to describe microbial molecules that recognize ECM components, including fibronectin, fibrinogen, collagens, laminins, vitronectin, and heparan sulfate (52). Bacterial adhesins that specifically recognize ECM components have been characterized in many pathogens, including the spirochetes Borrelia burgdorferi (10, 30, 31, 56), B. garinii (38), Leptospira interrogans (47), and T. denticola (14, 15, 74).
Similarly, numerous studies have documented the specific interaction of T. pallidum with ECM components. Previous literature suggests that fibronectin and laminin are ECM molecules that are central to treponemal cytoadherence. T. pallidum has been shown to attach to fibronectin- and laminin-coated surfaces (20, 34). Attachment is not a nonspecific association, as shown by the inability of heat-killed T. pallidum (20) and nonpathogenic T. phagedenis biotype Reiter (54, 75) to bind to these ECM components. T. pallidum has been reported to bind to cultured mammalian cells via the C-terminal cell-binding domain of fibronectin (64). Pretreatment of host cells with antiserum to fibronectin, but not pretreatment with control irrelevant antiserum, inhibits attachment of T. pallidum (34, 54, 64). Likewise, the immunoglobulin G (IgG) fraction from immune serum blocks treponemal attachment to both fibronectin and laminin (20).
Laminins are large multidomain glycoproteins that are important constituents of condensed, polymer-like aggregates of the ECM termed basement membranes (68). Since the discovery of laminin in 1979 (70), a growing number of pathogens have been shown to bind specifically to this ECM component, including Helicobacter pylori (76), Mycobacterium leprae (51, 60), Enterococcus faecalis (50, 79), Trichomonas vaginalis (11), Streptococcus pyogenes (35), Escherichia coli (62), and T. denticola (14). The results reported herein confirm and extend the studies performed by Fitzgerald et al. (20) documenting T. pallidum attachment to laminin by describing the identification of a T. pallidum laminin-binding protein.
MATERIALS AND METHODS
ECM proteins.
Collagen types I and IV, fibronectin, fibrinogen, and laminin isolated from the Engelbreth-Holm-Swarm murine sarcoma, as well as the control proteins bovine serum albumin (BSA) and fetuin, were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Purification of T. pallidum.
T. pallidum subsp. pallidum (Nichols strain) was propagated in New Zealand White rabbits as described elsewhere (42). All animal studies were approved by the local institutional review boards and conducted in accordance with standard accepted principles. Treponemes were purified to remove contaminating rabbit proteins by the protocol described by Hanff et al. (33). Briefly, treponemes were extracted in saline and subjected to two 400 × g spins to remove gross cellular debris, and 4 × 108 T. pallidum cells in a total volume of 10 ml were gently overlaid onto a 20-ml cushion of 43% Percoll (Sigma). Treponemes were purified by density centrifugation at 34,800 × g for 30 min, followed by removal of the visible treponeme band and resuspension in 25 ml of saline. The treponemes were subsequently separated from the Percoll by centrifugation at 100,000 × g for 1 h. Percoll-purified treponemes were shown by dark-field microscopy to be viable and fully motile.
Treponemal adherence assay.
Adherence assays were performed on the basis of previously published protocols (20, 53, 54, 64, 66). Briefly, 4 μg of attachment matrices, including fibronectin, collagens I and IV, fibrinogen, laminin, and the attachment-negative control proteins fetuin and BSA, were coated in phosphate-buffered saline (PBS) on Lab-Tek II chamber slides (Nunc, Rochester, N.Y.) by incubation for 16 h at room temperature. After being washed with PBS, slides were blocked for 2 h with 3% BSA, washed a second time, and then incubated for 2 h at 34°C under anaerobic conditions with 108 Percoll-purified T. pallidum cells in saline. After gentle washing with saline (10 times for 5 min each time), the attached spirochetes were visualized by dark-field microscopy and quantitative attachment was determined by calculating the number of attached treponemes per field. The assay was blinded, and a total of six fields were read for each attachment condition. Statistical analyses were performed by using the Student two-tailed t test.
Computer analyses.
Computer analyses were performed on the published T. pallidum genome (22) to identify putative outer membrane proteins. Such proteins have the potential to reside on the bacterial surface and thus are the most likely to possess cellular functions such as host cell attachment. T. pallidum open reading frames (ORFs) were retrieved from the sexually transmitted disease sequence database (http://stdgen.lanl.gov/stdgen/) and used for sequence analyses. The PSORT program (49) (http://psort.nibb.ac.jp) was used to predict the cellular localization of proteins encoded by the ORFs. Briefly stated, PSORT assigns a cellular localization based on the presence or absence of a signal sequence; the nature of the signal sequence, if one is present (i.e., cleavable or uncleavable); the presence or absence of hydrophobic transmembrane segments; and the presence of characteristic amino acid compositions (49) (http://psort.nibb.ac.jp/helpwww.html). By using these criteria, PSORT correctly classifies 83% of gram-negative bacterial proteins (n = 106) into one of the four possible localization sites (outer membrane, inner membrane, periplasmic space, cytoplasm) (http://psort.nibb.ac.jp/helpwww.html).
T. pallidum translated ORFs with a greater than 50% calculated likelihood of outer membrane localization, as identified through PSORT analysis, were subjected to additional sequence analyses. These included screening of the ORFs against the 3D-PSSM database (36) (http://www.sbg.bio.ic.ac.uk/∼3dpssm/) to identify homologous proteins with known tertiary structures, as well as the PROSITE database (http://www2.ebi.ac.uk/ppsearch) for identification of characteristic outer membrane protein motifs.
Recombinant expression.
ORFs encoding computer-predicted putative outer membrane proteins were PCR amplified from T. pallidum subsp. pallidum (Nichols strain) genomic DNA by using primers designed from the coding sequence of each gene in Table 1. To ensure optimal expression of each recombinant molecule within E. coli, DNA sequences encoding predicted N-terminal signal sequences were excluded from the primer design and, as a result, from the expressed recombinant proteins. The positive control laminin-binding protein (SwissProt accession number 069174) was amplified from Staphylococcus aureus genomic DNA by using the primers listed in Table 1. Following amplification, PCR products (except Tp0326 and Tp0856 [see below]) were digested with BamHI and either HindIII (positive control laminin-binding protein) or EcoRI (all other products), ligated to a similarly digested pRSETc T7 expression vector (Invitrogen, Carlsbad, Calif.), and transformed first into E. coli XL-1 Blue and then into E. coli expression strain BL21(DE3)/pLysS. ORFs Tp0326 and Tp0856 were directly cloned into the pBAD TOPO TA expression vector and expressed in E. coli strain TOP10 (both from Invitrogen). The sequence and reading frame of the expression constructs were verified by DNA sequencing with vector-specific primers, as well as internal primers based on each of the ORFs. Expression of the constructs and purification of the resulting six-histidine-tagged recombinant proteins were performed as previously described (6). The expressed proteins were renatured by dialysis on the basis of the renaturation protocol described by Qi et al. (57) by using the dialysis modification described by Zhang et al. (80). This procedure has been shown to produce recombinant proteins that closely resemble native T. pallidum proteins (80). Briefly, the detergent Zwittergent 3-12 (Calbiochem, San Diego, Calif.) was added to the expressed proteins to a final concentration of 0.5% prior to dialysis against 100 mM Tris (pH 8.0)-200 mM NaCl-10 mM EDTA. Quantitation of each of the recombinant proteins was performed with the BCA Protein Assay kit (Pierce, Rockford, Ill.).
TABLE 1.
ORF-specific primers used to amplify fragments for recombinant expression
| ORF | Sense primer(s)a | Antisense primer(s)a | Amplicon details
|
|
|---|---|---|---|---|
| Size (bp) | Portion of ORF | |||
| Tp0155 | 5′-ggatccaaccattgacacctgcc | 5′-gaattctgcagctgaattatagaac | 428 | 5′ end |
| 5′-ggatccttaacacgccgtcttcttc | 5′-gaattccggaagggtacgcatac | 630 | 3′ end | |
| Tp0316 (TprF) | 5′-ggatcctatgcaggcgtactcactccg | 5′-gaattctcagcaagcaccccctgttcc | 1,062 | Internal |
| Tp0326 (Tp92) | 5′-caggcaaacgacaattgg | 5′-caaattatttaccgtgaacg | 2,448 | Full length |
| Tp0453 | 5′-ggatcccgtggaaggcatcagtag | 5′-gaattccgaacttccctttttggag | 758 | Full length |
| Tp0483 | 5′-ggatccacgcggcgctcaaaaccg | 5′-gaattcgttatgaaagcgatagccg | 590 | 3′ end |
| Tp0557 | 5′-ggatccatccgcacgtttttatccgcac | 5′-gaattcgggggcagtgtagcgcagg | 636 | Full length |
| Tp0620 (TprI) | 5′-ggatcccgactcaccctcgaacca | 5′-gaattcggtgagcaggtgggtgtag | 633 | Internal |
| Tp0751 | 5′-ggatccgggacaccgccgcacac | 5′-gaattccttgcggtgtgtgtgcgc | 360 | 5′ end |
| Tp0856 | 5′-gcggcgaagactcgctc | 5′-cttgcgtccgagcagg | 1,086 | Full length |
| Tp0952 | 5′-ggatcctttccaagaagtcttcgaagtg | 5′-gaattcagcgcccccgttaaaggg | 502 | 5′ end |
| 5′-ggatcctgttgggacactcgatggg | 5′-gaattcattcgaaatgttttgctcacag | 542 | 3′ end | |
| S. aureus LBPb | 5′-ggatcctgccaattattacagatg | 5′-aagcttttatttatctaagttatagaa | 1,304 | Full length |
Restriction sites incorporated into the primers are underlined.
LBP, laminin-binding protein.
Laminin-binding adherence assays.
To test for adherence of the recombinant proteins to laminin, enzyme-linked immunosorbent assay (ELISA)-based assays were performed. ELISA plates (Nunc-Immuno Plate MaxiSorp Surface; Nalge Nunc International, Rochester, N.Y.) were coated with 0.5 μg of either laminin or the negative control protein BSA in PBS and incubated for 1.5 h at 37°C. The specificity profile of the laminin-binding recombinant protein was determined by coating with the ECM components collagens I and IV and the negative control proteins fetuin and BSA under identical conditions. To test for the dose-dependent attachment of laminin-binding recombinant proteins to laminin, wells were coated with 100 μl of various laminin concentrations ranging from 0 to 5 μg/ml in PBS. Wells were washed three times with PBS-0.05% Tween 20 (PBST) and blocked for 30 min at 37°C with 1% BSA, followed by addition of either the recombinant T. pallidum proteins or the positive control laminin-binding protein from S. aureus. For the adherence assays, 100 μl of the recombinant proteins was added per well at a constant concentration of 20 μg/ml in PBS, while for determination of the dose-dependent binding of Tp0751 to immobilized laminin, a range of recombinant Tp0751 concentrations of 0 to 5,000 nM in PBS was used. After incubation for 1.5 h at 37°C, wells were washed six times with PBST. Adherent recombinant proteins were detected with nickel-labeled horseradish peroxidase (Ni-HRP; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) in accordance with the manufacturer's instructions. Briefly, wells were treated for 5 min at room temperature with 2% sucrose and dried for 1 h at 37°C. A 1:2,000 dilution of Ni-HRP in 1% BSA was added to the wells, and the plate was incubated for 30 min at room temperature. Wells were washed six times with PBST and developed with the TMB peroxidase substrate system (Kirkegaard & Perry Laboratories). The plates were read at 600 nm with an ELISA plate reader (Bio-Tek Instruments, Winooski, Vt.). Statistical analyses were performed with the Student two-tailed t test.
Laminin chemical oxidation.
Chemical oxidation of laminin was performed with sodium metaperiodate as previously described (78). Briefly, ELISA plates were coated with 0.5 μg of laminin in 50 mM sodium acetate buffer, pH 5.0, and incubated for 1.5 h at 37°C. Wells were washed three times with 50 mM sodium acetate buffer, pH 5.0, prior to exposure to various concentrations of periodate (0 to 100 mM) for 1 h at room temperature in the dark. Wells were washed three times with 50 mM sodium acetate buffer, pH 5.0, and blocked for 30 min at 37°C with 1% BSA. To assess the attachment of the recombinant laminin-binding protein to periodate-treated laminin, 100 μl of the recombinant protein was added to the wells at a constant concentration of 20 μg/ml in PBS. After incubation for 1.5 h at 37°C, wells were washed six times with PBST and developed with a 1:2,000 dilution of Ni-HRP as outlined above.
Syphilis serum reactivity.
The reactivity of human and rabbit syphilitic serum samples against the T. pallidum recombinant protein was tested with an ELISA-based assay. Human serum samples (kindly provided by Bruno Schmidt, Ludwig Bolzman Institut for Dermato-Venercological Serodiagnosis, Vienna, Austria) were collected from individuals with confirmed syphilis infections and corresponded to various stages of infection, including primary syphilis, secondary syphilis, early latent infection, and neurosyphilis (n = 43). Uninfected human serum samples (n = 15) were obtained from laboratory personnel in Seattle, Wash. Sequential rabbit serum samples were collected at days 3 through 90 postinfection from New Zealand White rabbits that had been infected with T. pallidum subsp. pallidum (Nichols strain) as described elsewhere (42). Briefly, ELISA plates were coated at 4°C with the T. pallidum recombinant protein at a concentration of 4 μg/ml in PBS and incubated overnight. Wells were blocked for 2.5 h at room temperature with 4% milk powder-0.2% Triton X-100 and washed four times with Tris-buffered saline with 0.05% Tween 20 (TTBS). Wells were incubated with a 1:50 dilution of either human or rabbit syphilitic serum (1:50 dilution in blocking reagent), washed four times with TTBS, and incubated for 1 h at room temperature with a 1:2,000 dilution of either goat anti-human IgG (γ chain specific) F(ab′)2-peroxidase or goat anti-rabbit IgG (whole molecule) F(ab′)2 fragment-peroxidase (both from Sigma). Wells were washed four times with TTBS and developed with the TMB peroxidase substrate system. Optical densities were read at 600 nm with an ELISA plate reader. For statistical analyses, the cutoff for assignment of a negative or positive result was standardized from the absorbance values obtained from the 15 uninfected control serum samples. Negative serum samples were defined as those that yielded absorbance values less than the mean plus two times the standard deviation of the absorbance values of the uninfected serum samples, while positive serum samples were defined as those that gave absorbance values greater than this cutoff. All human studies were approved by the local institutional review boards and conducted in accordance with standard accepted principles.
RESULTS
Analysis of T. pallidum attachment to ECM components.
To confirm and extend previous studies investigating the attachment of T. pallidum to ECM components (1, 20, 34, 53, 54, 64-66, 75), attachment assays were performed with purified T. pallidum preparations and various ECM components. For these studies, T. pallidum was purified by Percoll density centrifugation (33) to minimize contaminating rabbit proteins. Treponemes purified in this manner are viable and motile and have been demonstrated by Hanff et al. (33) to remain fully virulent. This purification step was performed because treponemes freshly harvested from rabbit testicles are extensively coated with host proteins that may themselves contribute to ECM attachment. By use of a purified source of T. pallidum, the contribution of treponemal proteins to ECM attachment can be more accurately determined.
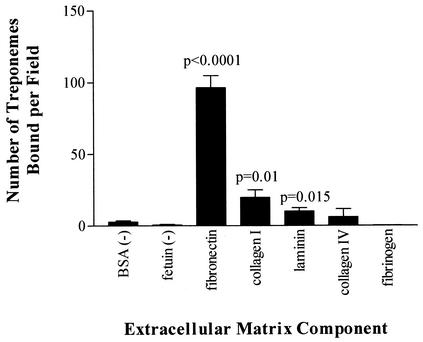
As demonstrated in Fig. 1, high numbers of purified T. pallidum bacteria were attached to fibronectin-coated surfaces (P < 0.0001). In addition, statistically significant numbers of organisms were attached to collagen I (P = 0.01) and laminin (P = 0.015). No significant level of attachment to the other two ECM components tested, collagen IV (P = 0.5663) and fibrinogen (P = 0.1574), or to the attachment-negative control proteins fetuin and BSA was observed. These results suggest that T. pallidum possesses adhesins that bind specifically to fibronectin, collagen I, and laminin. The identities of the treponemal adhesins that mediate binding to the ECM components fibronectin and collagen I are the focus of separate studies. The present investigation focused on the identification of the T. pallidum adhesin(s) that mediates attachment to the ECM component laminin.
FIG. 1.
T. pallidum attachment to various ECM components. Each bar represents the average number of treponemes bound ± the standard deviation for six microscopic fields, and the results are representative of three independent experiments. For statistical analyses, attachment to each ECM component was compared to attachment to the negative control BSA by the Student two-tailed t test.
Computer prediction of potential T. pallidum adhesins.
The release of the T. pallidum genome sequence (22) has provided readily accessible information on the complete repertoire of ORFs contained within the genome. To identify potential T. pallidum adhesins, computer analyses of the published T. pallidum genome (http://www.stdgen.lanl.gov/stdgen/) were performed to identify ORFs predicted to encode outer membrane proteins. Such proteins may reside on the bacterial surface and thus are most likely to possess cellular functions such as host cell attachment. These analyses identified 27 T. pallidum ORFs that have the potential to encode outer membrane proteins and thus may encode proteins involved in host attachment. Ten ORFs were given priority for expression on the basis of either their strong PSORT predictions for potential outer membrane location (≥69%; Table 2), the predicted structural similarity of these proteins to known β-barrel-containing proteins (3D-PSSM analysis), or the presence of motifs characteristic of outer membrane proteins (PROSITE analysis).
TABLE 2.
Recombinant expression of the T. pallidum ORFs
| T. pallidum ORF | PSORT outer membrane predictiona | Portion(s) of ORF expressed
|
|
|---|---|---|---|
| Amino acid residues | Fragment of protein | ||
| 0155 | 86 | 46-187 | N terminal |
| 162-371 | C terminal | ||
| 0316 (TprF) | 93 | 16-369 | Internal |
| 0326 (Tp92) | 85 | 22-837 | Full length |
| 0453 | 78 | 30-287 | Full length |
| 0483 | 69 | 179-374 | C terminal |
| 0557 | 72 | 26-237 | Full length |
| 0620 (TprI) | 92 | 259-469 | Internal |
| 0751 | 92 | 54-173 | N terminal |
| 0856 | 94 | 33-394 | Full length |
| 0952 | 87 | 34-199 | N terminal |
| 166-345 | C terminal | ||
Percent probability that the protein resides in the outer membrane.
Recombinant expression.
As shown in Table 2, the selected 10 of the 27 T. pallidum ORFs encoding potential outer membrane proteins have been expressed as recombinant proteins in E. coli. Where possible, the entire ORF was expressed; however, in many cases, toxicity prevented expression of the full-length ORF and, instead, fragments were expressed as outlined in Table 2. In addition, a laminin-binding protein from S. aureus was expressed in parallel as a positive control. The expressed proteins (except TprF and TprI) were renatured by dialysis.
Laminin-binding adherence assay.
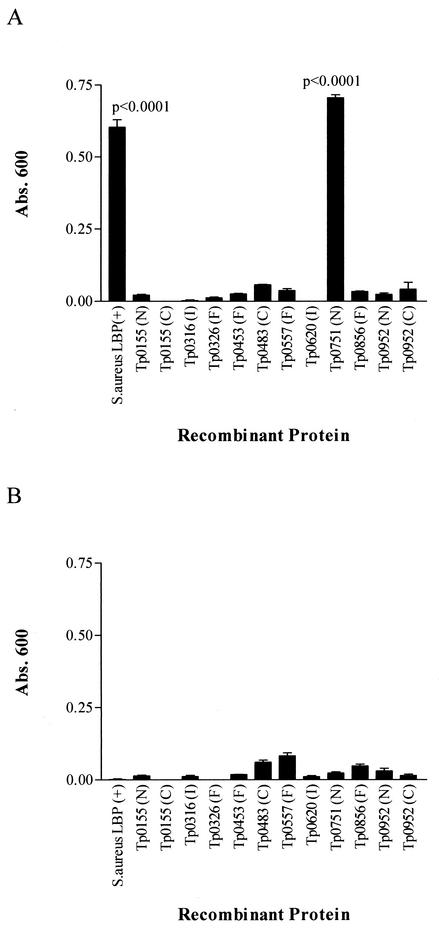
The recombinant S. aureus laminin-binding protein and the 10 T. pallidum ORFs expressed as recombinant proteins were examined for the ability to bind to laminin with an ELISA-based assay. As observed in Fig. 2A, the S. aureus laminin-binding protein demonstrated a significant level of attachment to laminin (P < 0.0001). Of the panel of expressed T. pallidum ORFs tested for attachment to laminin, one protein, Tp0751, exhibited a significant degree of laminin binding (P < 0.0001). In each case, the statistical significance of attachment was determined by comparison with the ability of the recombinant protein to attach to BSA. As shown in Fig. 2B, in all cases, the panel of expressed proteins exhibited minimal binding to BSA.
FIG. 2.
Binding of recombinant proteins to human laminin (A) and BSA (B). Each bar represents the mean absorbance (Abs.) value at 600 nm ± the standard deviation for three wells, and the results are representative of three independent experiments. For statistical analyses, the attachment of each of the recombinant proteins to laminin was compared to the attachment of the protein to BSA by the two-tailed t test. LBP, laminin-binding protein; F, full-length protein; N, N-terminal fragment; C, C-terminal fragment; I, internal fragment.
Specificity of recombinant Tp0751 attachment to laminin.
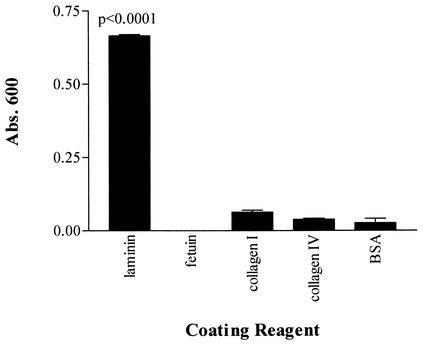
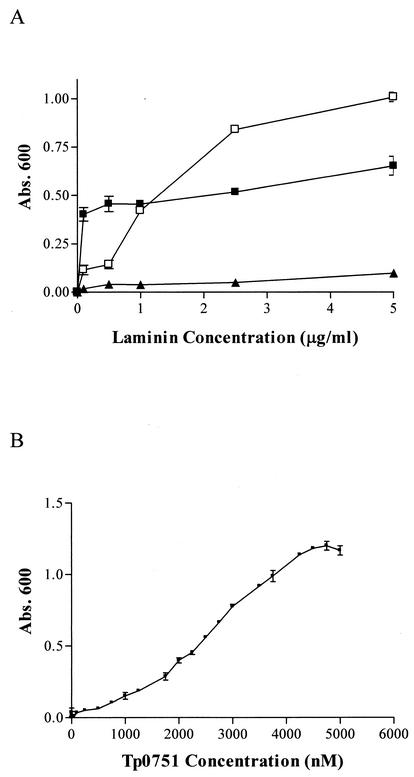
The attachment of recombinant T. pallidum protein Tp0751 to laminin was investigated in several experiments. The specificity profile of Tp0751 was determined by testing attachment to the ECM components laminin and collagens I and IV and the negative control proteins fetuin and BSA. As shown in Fig. 3, Tp0751 showed a statistically significant level of attachment to laminin (P < 0.0001) but did not demonstrate significant attachment to either collagen I, collagen IV, fetuin, or BSA. These results demonstrate the attachment specificity of Tp0751 for laminin. To further investigate the Tp0751-laminin interaction, ELISA analyses were performed to assess the binding of recombinant Tp0751 to immobilized laminin as a function of both varying laminin concentrations and varying recombinant Tp0751 concentrations. As shown in Fig. 4A, Tp0751 bound to increasing concentrations of laminin in a dose-dependent, saturable manner, compared to the minimal level of laminin attachment observed for the negative control expressed T. pallidum ORF, Tp0557. The positive control S. aureus laminin-binding protein also bound to laminin at a consistently higher level than the negative control recombinant protein. Further, the saturated nature of the positive control binding curve suggests that the dose-dependent attachment of this recombinant protein to laminin is outside the laminin concentration range used for optimal attachment of Tp0751. In addition, as shown in Fig. 4B, increasing concentrations of recombinant Tp0751 bound to immobilized laminin in a dose-dependent, saturable manner.
FIG. 3.
Specificity profile of attachment of recombinant Tp0751 to various coating reagents. Each bar represents the mean absorbance (Abs.) value at 600 nm ± the standard deviation for three wells, and the results are representative of three independent experiments. For statistical analyses, attachment to each component was compared to attachment to the negative control BSA by the Student two-tailed t test.
FIG. 4.
Binding of recombinant Tp0751 (□) to immobilized laminin as a function of varying laminin concentrations (A) and varying Tp0751 concentrations (B). Each datum point represents the mean absorbance (Abs.) value at 600 nm ± the standard deviation for three wells, and the results are representative of three independent experiments. Positive and negative control recombinant proteins tested in panel A were the S. aureus laminin-binding protein (▪) and T. pallidum ORF Tp0557 (▴), respectively.
Contribution of carbohydrate moieties to Tp0751-laminin attachment.
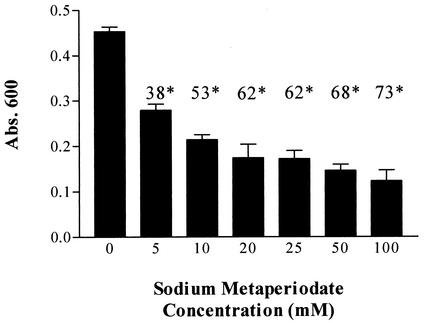
The oxidative effect of sodium metaperiodate was exploited to determine the contribution of carbohydrate moieties to the interaction of laminin with recombinant Tp0751. Sodium metaperiodate treatment of laminin was conducted under mild conditions previously shown to cleave carbohydrate vicinal hydroxyl groups without altering the polypeptide chain structure (78). As shown in Fig. 5, recombinant Tp0751 exhibited decreased attachment to metaperiodate-treated laminin. Attachment decreased in a statistically significant manner as a function of increasing sodium metaperiodate concentration (Fig. 5), with a reduction of 73% of Tp0751 attachment observed at the highest metaperiodate concentration (100 mM). These results suggest that laminin carbohydrate groups play a role in the interaction of Tp0751 with laminin.
FIG. 5.
Contribution of laminin carbohydrate moieties to recombinant Tp0751-laminin interaction. Shown is a bar graph representing Tp0751 attachment to untreated laminin (0 mM bar) and laminin treated with various concentrations of sodium metaperiodate (5 to 100 mM). Each bar represents the mean absorbance (Abs.) value at 600 nm ± the standard deviation for three wells, and the results are representative of three independent experiments. Shown above each bar is the percent reduction in recombinant Tp0751 attachment to sodium metaperiodate-treated laminin as a function of the sodium metaperiodate concentration. The data are expressed as percent reduction in attachment compared to the level of attachment to untreated laminin. In each case (*), the P value, as measured by the Student two-tailed t test, was ≤0.0009.
Syphilis serum reactivity to recombinant Tp0751.
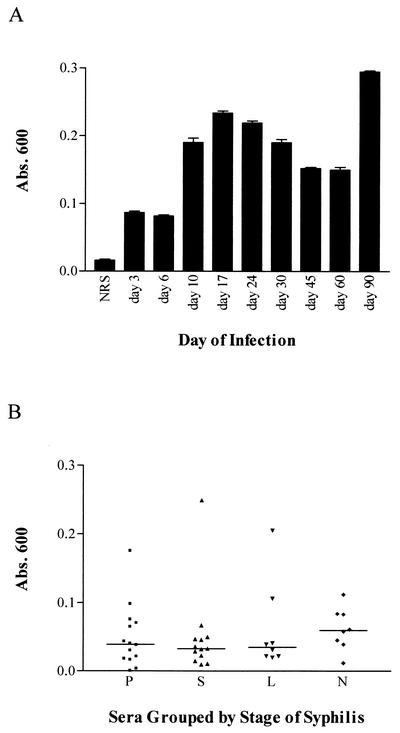
The antibody reactivity of serum samples collected from syphilis patients and experimentally infected rabbits against recombinant Tp0751 was determined by ELISA analysis. Sequential rabbit serum samples collected from experimentally infected rabbits demonstrated reactivity against recombinant Tp0751 (Fig. 6A). Reactivity first appeared at day 10 postinfection, and consistent levels of antibody reactivity were observed through day 90 postinfection. Similarly, serum samples from individuals with syphilis infections (n = 43) and a control group of uninfected individuals (n = 15) were analyzed for reactivity against recombinant Tp0751. While none of the serum samples from the control group of uninfected individuals demonstrated reactivity to Tp0751 (data not shown), serum samples from 42% of syphilis patients demonstrated Tp0751 reactivity (Fig. 6B). The reactivity did not appear to correlate with the stage of infection at which serum was collected (Fig. 6B). The presence of antibodies against Tp0751 in serum samples collected from both experimental and natural syphilis infections is indicative of expression of the protein in vivo during the course of infection.
FIG. 6.
Reactivity of syphilitic serum samples to recombinant Tp0751. (A) Reactivity of sequential rabbit serum samples collected from experimentally infected rabbits. The serum samples on the x axis represent pools of serum samples collected from rabbits infected with T. pallidum for the indicated time periods. NRS, normal rabbit serum. (B) Reactivity of human serum samples from different stages of syphilis infection. The serum samples on the x axis are grouped by stages of syphilis: P, primary (n = 14); S, secondary (n = 13); L, latent (n = 8); N, neurosyphilis (n = 8). The overall mean absorbance (Abs.) of each group is represented by a horizontal line.
DISCUSSION
The critical first step in the establishment of infection by pathogenic bacteria is attachment to host components. Laminins are major constituents of basement membranes and, as such, are widely distributed throughout the host. Specifically, laminins are associated with basement membranes that underlie epithelial and endothelial cell layers and surround muscle cells and peripheral nerves (69). Attachment to the ECM component laminin could be an important portal for the initiation of T. pallidum infection, since tissue injury that leads to the degradation of the epithelial layer would expose the laminin-rich basement membrane. In addition, T. pallidum attachment to laminin may also play a role in spirochetal dissemination and tissue invasion. In this respect, it is relevant that in infected tissues T. pallidum localizes to perivascular areas and that spirochetes have been shown by phase-contrast and scanning electron microscopy to attach to isolated basement membranes of retinal and kidney tissues (20).
Attachment to laminin has been suggested to play a role in the pathogenesis of a number of microorganisms, including various species of bacteria (14, 35, 50, 55, 62, 76), parasites (11, 25, 27, 39), and fungi (40, 46, 71). Previous investigations have studied the attachment of T. pallidum to several ECM components, including laminin (20), but the identity of the spirochete molecule(s) mediating this attachment was not determined. In this report, we have identified T. pallidum ORF Tp0751 as encoding a protein that attaches to immobilized laminin. The binding of Tp0751 to laminin is characteristic of a specific receptor-ligand interaction: (i) it is dependent on the concentration of both laminin and Tp0751, (ii) it is saturable, and (iii) it is specific for laminin and is not observed with other highly glycosylated proteins.
Several reports have suggested, on the basis of immunological cross-reactivity, the evolutionary conservation of at least some sequences in both prokaryotic and eukaryotic laminin-binding proteins (41, 48). In general, however, laminin-binding proteins vary widely in molecular size and amino acid sequence, and to date, no consensus laminin-binding motif has been identified within the documented laminin adhesins. Along these lines, the Tp0751 sequence demonstrates no shared homology with sequences contained within the protein databases, including the predicted ORFs from the related spirochete B. burgdorferi. In addition, a search of the unfinished T. denticola genome (http://tigrblast.tigr.org/ufmg/) identified a low-specificity match to a translated ORF contained within the genome (16% identity, 29% similarity). These results suggest that Tp0751 may be unique to Treponema.
Laminin is a highly glycosylated molecule that possesses about 40 N-linked oligosaccharides with repeating units of poly-N-acetyllactosaminyl side chains attached to the trimannosyl core portion of bi-, tri-, and tetra-antennary complex-type oligosaccharides (2, 23, 37). These complicated N-linked oligosaccharides result in many different structures, some of which are unique to laminin (63). For the majority of pathogenic organisms that demonstrate attachment to laminin, glycosylation plays a key role in the laminin-pathogen interaction. Specifically, carbohydrate groups have been shown to be important for the interaction of laminin with H. pylori (76), M. leprae (61), L. donovani (27), S. aureus (7), T. vaginalis (11), and E. coli (62). In addition, the interaction of an α6/β1 integrin, designated gp120/140, with laminin is also dependent on N-linked oligosaccharides (9). Consistent with these findings, reduced attachment of Tp0751 to metaperiodate-treated laminin was observed, indicating that carbohydrate groups present on laminin are important for the Tp0751-laminin interaction. This finding is also consistent with previous investigations showing reduced attachment of T. pallidum to cultured mammalian cells pretreated with periodate (1).
Within the context of mammalian cells, the interaction of both integrin (28) and non-integrin (24) receptors with laminin has been correlated with the invasive ability of tumor cells. In each case, the receptor-laminin interactions have been shown to result in signal transduction and subsequent synthesis and release of proteases by tumor cells. These proteases promote the invasion process of tumor cells by degrading ECM components. A similar scenario may exist for T. pallidum, in which attachment to laminin may induce the production of proteases and, in this way, increase the invasive capabilities of this pathogen. Consistent with this hypothesis are the observations that tissue degradation is a well-documented phenomenon associated with syphilis infection and that previous investigations have documented the morphological destruction of cultured cells upon attachment of T. pallidum (21). In addition, the related oral spirochete T. denticola has been shown to both attach to laminin (14, 32) and produce proteases that result in basement membrane degradation and treponemal invasion (29, 58, 73). Further, in T. denticola the protein identified as mediating attachment to laminin, the major surface protein (14, 32), has been shown to be closely associated in vivo with the chymotrypsin-like protease implicated in basement membrane degradation (13). The questions of whether a similar situation exists for T. pallidum and whether this correlation parallels the method of tumor invasiveness await further experimentation.
In conclusion, this report details the identification of a T. pallidum adhesin that exhibits specific attachment to laminin. Although the complete picture of T. pallidum attachment to host cells remains to be determined, it is likely that spirochete attachment and infection depend on complex interactions of several bacterial proteins with various ECM, and perhaps other host cell, components.
Acknowledgments
We are grateful to Susannah Weyte, Lynn Barrett, Elizabeth Brown, Michael Myint, and Tina Davis for assistance with recombinant expression; Wesley Van Voorhis and Eileen Sun for assistance with T. pallidum genomic analysis; and Barbara Molini and Sheila Lukehart for the gift of T. pallidum and sequential rabbit serum samples. We also thank Bruno Schmidt for the gift of human syphilis serum samples.
This work was supported by Public Health Service grants AI-51334 and AI-43456 from the National Institutes of Health, University of Washington faculty awards (Royalty Research Fund and STD New Investigator Award AI-31448), and the Canadian Institutes of Health Research.
Editor: D. L. Burns
REFERENCES
- 1.Alderete, J. F., and J. B. Baseman. 1980. Surface characterization of virulent Treponema pallidum. Infect. Immun. 30:814-823. [DOI] [PMC free article] [PubMed]
- 2.Arumugham, R. G., T. C. Hsieh, M. L. Tanzer, and R. A. Laine. 1986. Structures of the asparagine-linked sugar chains of laminin. Biochim. Biophys. Acta 883:112-126. [DOI] [PubMed] [Google Scholar]
- 3.Baseman, J. B., and J. F. Alderete. 1983. The parasitic strategies of Treponema pallidum, p. 229-239. In R. Schell and D. Musher (ed.), Pathogenesis and immunology of Treponema infections. Marcel Dekker, Inc., New York, N.Y.
- 4.Baseman, J. B., and E. C. Hayes. 1980. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J. Exp. Med. 151:573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J. Infect. Dis. 143:325-345. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, C. E., S. A. Lukehart, C. Castro, B. Molini, C. Godornes, and W. C. Van Voorhis. 2000. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J. Infect. Dis. 181:1401-1413. [DOI] [PubMed] [Google Scholar]
- 7.Carneiro, C. R., E. Postol, C. Boilesen, and R. R. Brentani. 1993. Participation of glycosylation sites in the binding of Staphylococcus aureus to laminin. Braz. J. Med. Biol. Res. 26:689-697. [PubMed] [Google Scholar]
- 8.Carranza, N., Jr., G. R. Riviere, K. S. Smith, D. F. Adams, and T. Maier. 1997. Differential attachment of oral treponemes to monolayers of epithelial cells. J. Periodontol. 68:1010-1018. [DOI] [PubMed] [Google Scholar]
- 9.Chammas, R., S. S. Veiga, S. Line, P. Potocnjak, and R. R. Brentani. 1991. Asn-linked oligosaccharide-dependent interaction between laminin and gp120/140: an α6/β1 integrin. J. Biol. Chem. 266:3349-3355. [PubMed] [Google Scholar]
- 10.Coburn, J., W. Chege, L. Magoun, S. C. Bodary, and J. M. Leong. 1999. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol. Microbiol. 34:926-940. [DOI] [PubMed] [Google Scholar]
- 11.Crouch, M. L., and J. F. Alderete. 1999. Trichomonas vaginalis interactions with fibronectin and laminin. Microbiology 145:2835-2843. [DOI] [PubMed] [Google Scholar]
- 12.Cumberland, M. C., and T. B. Turner. 1949. Rate of multiplication of Treponema pallidum in normal and immune rabbits. Am. J. Syph. 33:201-212. [PubMed] [Google Scholar]
- 13.Fenno, J. C., P. M. Hannam, W. K. Leung, M. Tamura, V. J. Uitto, and B. C. McBride. 1998. Cytopathic effects of the major surface protein and the chymotrypsinlike protease of Treponema denticola. Infect. Immun. 66:1869-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenno, J. C., K. H. Muller, and B. C. McBride. 1996. Sequence analysis, expression, and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J. Bacteriol. 178:2489-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenno, J. C., M. Tamura, P. M. Hannam, G. W. Wong, R. A. Chan, and B. C. McBride. 2000. Identification of a Treponema denticola OppA homologue that binds host proteins present in the subgingival environment. Infect. Immun. 68:1884-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald, T. J., P. Cleveland, R. C. Johnson, J. N. Miller, and J. A. Sykes. 1977. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J. Bacteriol. 130:1333-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald, T. J., R. C. Johnson, J. N. Miller, and J. A. Sykes. 1977. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect. Immun. 18:467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald, T. J., R. C. Johnson, J. A. Sykes, and J. N. Miller. 1977. Interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells: effects of oxygen, reducing agents, serum supplements, and different cell types. Infect. Immun. 15:444-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald, T. J., J. N. Miller, and J. A. Sykes. 1975. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect. Immun. 11:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald, T. J., L. A. Repesh, D. R. Blanco, and J. N. Miller. 1984. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br. J. Vener. Dis. 60:357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald, T. J., L. A. Repesh, and S. G. Oakes. 1982. Morphological destruction of cultured cells by the attachment of Treponema pallidum. Br. J. Vener. Dis. 58:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, C. Fujii, S. Garland, B. Hatch, K. Horst, L. Watthey, J. Weidman, H. O. Smith, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara, S., H. Shinkai, R. Deutzmann, M. Paulsson, and R. Timpl. 1988. Structure and distribution of N-linked oligosaccharide chains on various domains of mouse tumour laminin. Biochem. J. 252:453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulop, T., and A. Larbi. 2002. Putative role of 67 kDa elastin-laminin receptor in tumor invasion. Semin. Cancer Biol. 12:219-229. [DOI] [PubMed] [Google Scholar]
- 25.Furtado, G. C., M. Slowik, H. K. Kleinman, and K. A. Joiner. 1992. Laminin enhances binding of Toxoplasma gondii tachyzoites to J774 murine macrophage cells. Infect. Immun. 60:2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerbase, A. C., J. T. Rowley, D. H. Heymann, S. F. Berkley, and P. Piot. 1998. Global prevalence and incidence estimates of selected curable STDs. Sex. Transm. Infect. 74(Suppl. 1):S12-S16. [PubMed] [Google Scholar]
- 27.Ghosh, A., K. Bandyopadhyay, L. Kole, and P. K. Das. 1999. Isolation of a laminin-binding protein from the protozoan parasite Leishmania donovani that may mediate cell adhesion. Biochem. J. 337(Pt. 3):551-558. [PMC free article] [PubMed] [Google Scholar]
- 28.Giannelli, G., S. Astigiano, S. Antonaci, M. Morini, O. Barbieri, D. M. Noonan, and A. Albini. 2002. Role of the α3β1 and α6β4 integrins in tumor invasion. Clin. Exp. Metastasis 19:217-223. [DOI] [PubMed] [Google Scholar]
- 29.Grenier, D., V. J. Uitto, and B. C. McBride. 1990. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect. Immun. 58:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Höök. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 31.Guo, B. P., S. J. Norris, L. C. Rosenberg, and M. Höök. 1995. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect. Immun. 63:3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haapasalo, M., K. H. Muller, V. J. Uitto, W. K. Leung, and B. C. McBride. 1992. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect. Immun. 60:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanff, P. A., S. J. Norris, M. A. Lovett, and J. N. Miller. 1984. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex. Transm. Dis. 11:275-286. [DOI] [PubMed] [Google Scholar]
- 34.Hayes, N. S., K. E. Muse, A. M. Collier, and J. B. Baseman. 1977. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect. Immun. 17:174-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hytönen, J., S. Haataja, D. Gerlach, A. Podbielski, and J. Finne. 2001. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol. Microbiol. 39:512-519. [DOI] [PubMed] [Google Scholar]
- 36.Kelley, L. A., R. M. MacCallum, and M. J. Sternberg. 2000. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J. Mol. Biol. 299:499-520. [DOI] [PubMed] [Google Scholar]
- 37.Knibbs, R. N., F. Perini, and I. J. Goldstein. 1989. Structure of the major concanavalin A reactive oligosaccharides of the extracellular matrix component laminin. Biochemistry 28:6379-6392. [DOI] [PubMed] [Google Scholar]
- 38.Kopp, P. A., M. Schmitt, H. J. Wellensiek, and H. Blobel. 1995. Isolation and characterization of fibronectin-binding sites of Borrelia garinii N34. Infect. Immun. 63:3804-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, E., W. G. Yang, T. Zhang, and S. L. Stanley, Jr. 1995. Interaction of laminin with Entamoeba histolytica cysteine proteinases and its effect on amebic pathogenesis. Infect. Immun. 63:4150-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopes, J. D., M. C. Moura-Campos, A. P. Vicentini, J. L. Gesztesi, W. de Souza, and Z. P. Camargo. 1994. Characterization of glycoprotein gp43, the major laminin-binding protein of Paracoccidioides brasiliensis. Braz. J. Med. Biol. Res. 27A:2309-2313. [PubMed]
- 41.Lopez-Ribot, J. L., M. Casanova, C. Monteagudo, P. Sepulveda, and J. P. Martinez. 1994. Evidence for the presence of a high-affinity laminin receptor-like molecule on the surface of Candida albicans yeast cells. Infect. Immun. 62:742-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukehart, S. A., S. A. Baker-Zander, and S. Sell. 1980. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J. Immunol. 124:454-460. [PubMed] [Google Scholar]
- 43.Lukehart, S. A., E. W. Hook III, S. A. Baker-Zander, A. C. Collier, C. W. Critchlow, and H. H. Handsfield. 1988. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann. Intern. Med. 109:855-862. [DOI] [PubMed] [Google Scholar]
- 44.Mahoney, J. F., and K. K. Bryant. 1933. Contact infection of rabbits in experimental syphilis. Am. J. Syph. 17:188-193. [Google Scholar]
- 45.Mahoney, J. F., and K. K. Bryant. 1934. Time element in penetration of genital mucosa by Treponema pallidum. J. Vener. Dis. Infect. 15:1-5. [Google Scholar]
- 46.McMahon, J. P., J. Wheat, M. E. Sobel, R. Pasula, J. F. Downing, and W. J. Martin. 1995. Murine laminin binds to Histoplasma capsulatum: a possible mechanism of dissemination. J. Clin. Investig. 96:1010-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merien, F., J. Truccolo, G. Baranton, and P. Perolat. 2000. Identification of a 36-kDa fibronectin-binding protein expressed by a virulent variant of Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol. Lett. 185:17-22. [DOI] [PubMed] [Google Scholar]
- 48.Mota, G. F., C. R. Carneiro, L. Gomes, and J. D. Lopes. 1988. Monoclonal antibodies to Staphylococcus aureus laminin-binding proteins cross-react with mammalian cells. Infect. Immun. 56:1580-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakai, K., and M. Kanehisa. 1991. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins 11:95-110. [DOI] [PubMed] [Google Scholar]
- 50.Nallapareddy, S. R., X. Qin, G. M. Weinstock, M. Höök, and B. E. Murray. 2000. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 68:5218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng, V., G. Zanazzi, R. Timpl, J. F. Talts, J. L. Salzer, P. J. Brennan, and A. Rambukkana. 2000. Role of the cell wall phenolic glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell 103:511-524. [DOI] [PubMed] [Google Scholar]
- 52.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Höök. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 53.Peterson, K., J. B. Baseman, and J. F. Alderete. 1987. Molecular cloning of Treponema pallidum outer envelope fibronectin binding proteins, P1 and P2. Genitourin. Med. 63:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson, K. M., J. B. Baseman, and J. F. Alderete. 1983. Treponema pallidum receptor binding proteins interact with fibronectin. J. Exp. Med. 157:1958-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plotkowski, M. C., J. M. Tournier, and E. Puchelle. 1996. Pseudomonas aeruginosa strains possess specific adhesins for laminin. Infect. Immun. 64:600-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Probert, W. S., and B. J. Johnson. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30:1003-1015. [DOI] [PubMed] [Google Scholar]
- 57.Qi, H. L., J. Y. Tai, and M. S. Blake. 1994. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect. Immun. 62:2432-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Que, X.-C., and H. K. Kuramitsu. 1990. Isolation and characterization of the Treponema denticola prtA gene coding for chymotrypsinlike protease activity and detection of a closely linked gene encoding PZ-PLGPA-hydrolyzing activity. Infect. Immun. 58:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raiziss, G. W., and M. Severac. 1937. Rapidity with which Spirochaeta pallida invades the bloodstream. Arch. Dermatol. Syphilol. 35:1101-1109. [Google Scholar]
- 60.Rambukkana, A., J. L. Salzer, P. D. Yurchenco, and E. I. Tuomanen. 1997. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-α2 chain. Cell 88:811-821. [DOI] [PubMed] [Google Scholar]
- 61.Rambukkana, A., H. Yamada, G. Zanazzi, T. Mathus, J. L. Salzer, P. D. Yurchenco, K. P. Campbell, and V. A. Fischetti. 1998. Role of α-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science 282:2076-2079. [DOI] [PubMed] [Google Scholar]
- 62.Tanskanen, J., S. Saarela, S. Tankka, N. Kalkkinen, M. Rhen, T. K. Korhonen, and B. Westerlund-Wikstrom. 2001. The gaf fimbrial gene cluster of Escherichia coli expresses a full-size and a truncated soluble adhesin protein. J. Bacteriol. 183:512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanzer, M. L., S. Chandrasekaran, J. W. Dean III, and M. S. Giniger. 1993. Role of laminin carbohydrates on cellular interactions. Kidney Int. 43:66-72. [DOI] [PubMed] [Google Scholar]
- 64.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1985. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J. Exp. Med. 161:514-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1985. Putative Treponema pallidum cytadhesins share a common functional domain. Infect. Immun. 49:833-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas, D. D., J. B. Baseman, and J. F. Alderete. 1986. Enhanced levels of attachment of fibronectin-primed Treponema pallidum to extracellular matrix. Infect. Immun. 52:736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas, D. D., M. Navab, D. A. Haake, A. M. Fogelman, J. N. Miller, and M. A. Lovett. 1988. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl. Acad. Sci. USA 85:3608-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timpl, R. 1996. Macromolecular organization of basement membranes. Curr. Opin. Cell Biol. 8:618-624. [DOI] [PubMed] [Google Scholar]
- 69.Timpl, R., and M. Dziadek. 1986. Structure, development, and molecular pathology of basement membranes. Int. Rev. Exp. Pathol. 29:1-112. [PubMed] [Google Scholar]
- 70.Timpl, R., H. Rohde, P. G. Robey, S. I. Rennard, J. M. Foidart, and G. R. Martin. 1979. Laminin—a glycoprotein from basement membranes. J. Biol. Chem. 9933-9937. [PubMed]
- 71.Tronchin, G., K. Esnault, G. Renier, R. Filmon, D. Chabasse, and J. P. Bouchara. 1997. Expression and identification of a laminin-binding protein in Aspergillus fumigatus conidia. Infect. Immun. 65:9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turner, T. B. 1970. Syphilis and the treponematoses, p. 346-390. In S. Mudd (ed.), Infectious agents and host reactions. The W. B. Saunders Co., Philadelphia, Pa.
- 73.Uitto, V. J., Y. M. Pan, W. K. Leung, H. Larjava, R. P. Ellen, B. B. Finlay, and B. C. McBride. 1995. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect. Immun. 63:3401-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Umemoto, T., Y. Nakatani, Y. Nakamura, and I. Namikawa. 1993. Fibronectin-binding proteins of a human oral spirochete Treponema denticola. Microbiol. Immunol. 37:75-78. [DOI] [PubMed] [Google Scholar]
- 75.Umemoto, T., and I. Namikawa. 1994. Binding of host-associated treponeme proteins to collagens and laminin: a possible mechanism of spirochetal adherence to host tissues. Microbiol. Immunol. 38:655-663. [DOI] [PubMed] [Google Scholar]
- 76.Valkonen, K. H., T. Wadström, and A. P. Moran. 1997. Identification of the N-acetylneuraminyllactose-specific laminin-binding protein of Helicobacter pylori. Infect. Immun. 65:916-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong, G. H. W., B. Steiner, and S. Graves. 1983. Effect of syphilitic rabbit sera taken at different periods after infection on treponemal motility, treponemal attachment to mammalian cells in vitro, and treponemal infection in rabbits. Br. J. Vener. Dis. 59:220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woodward, M. P., W. W. Young, Jr., and R. A. Bloodgood. 1985. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J. Immunol. Methods 78:143-153. [DOI] [PubMed] [Google Scholar]
- 79.Xiao, J., M. Höök, G. M. Weinstock, and B. E. Murray. 1998. Conditional adherence of Enterococcus faecalis to extracellular matrix proteins. FEMS Immunol. Med. Microbiol. 21:287-295. [DOI] [PubMed] [Google Scholar]
- 80.Zhang, H. H., D. R. Blanco, M. M. Exner, E. S. Shang, C. I. Champion, M. L. Phillips, J. N. Miller, and M. A. Lovett. 1999. Renaturation of recombinant Treponema pallidum rare outer membrane protein 1 into a trimeric, hydrophobic, and porin-active conformation. J. Bacteriol. 181:7168-7175. [DOI] [PMC free article] [PubMed] [Google Scholar]