Abstract
BMS-284756 is a novel quinolone that lacks the six-position fluorine typical of existing compounds. Despite this structural change, BMS-284756 maintains potent antibacterial activity against gram-negative and gram-positive aerobic and anaerobic pathogens. The objective of this study was to define the pharmacodynamic profile of BMS-284756 against Streptococcus pneumoniae. Protein binding in mice was assessed by the ultrafiltration method. For pharmacodynamic studies, neutropenic ICR mice were used, as well as an immunocompetent mouse species, CBA/J, in order to evaluate the impact of white blood cells on infection outcome. Mice were infected with 105 to 106 CFU per thigh, and therapy was initiated after 2 h. Animals received BMS-284756 orally over a range of 1.25 to 100 mg/kg/day divided into one to four doses. At 0 and 24 h postinfection, thighs were harvested for bacterial density measurement. Survival was assessed during 96 h of therapy and again at 3 days after therapy. Pharmacokinetic studies were also conducted with infected mice. Protein binding was determined to be 80%. The MICs for clinical isolates (n = 8) ranged from 0.03 to 2 μg/ml. The change in bacterial density and survival was correlated with the pharmacodynamic parameters percentage of time that the drug concentration in serum remains above the MIC, AUC (area under the concentration-time curve)/MIC ratio, and peak/MIC ratio, and the best predictor of response was the AUC/MIC ratio for both outcome measures. Total AUC/MIC ratios of 100 to 200 appear to result in maximal bactericidal effects. While a total AUC/MIC ratio exposure value of 100 (free AUC/MIC ratio, ∼20) resulted in nearly 100% survival at the conclusion of therapy, a total AUC/MIC ratio of 200 (free AUC/MIC ratio, ∼40) was required to ensure survival at 3 days posttherapy. These data demonstrate (i) the in vivo bactericidal activity of BMS-284756 against S. pneumoniae, (ii) that protein binding has a profound impact on the in vivo pharmacodynamic assessment of BMS-284756, and (iii) that an AUC/MIC ratio of 200 (free AUC/MIC ratio, ∼40) appears to best characterize the required dynamic exposure for optimization of bactericidal activity and maximal survival.
BMS-284756 is a new quinolone that lacks the six-position fluorine present on others in its class, but even with this structural difference, it has strong antibacterial activity against gram-positive and gram-negative aerobic and anaerobic pathogens. A debate exists as to the magnitude of the pharmacodynamic parameter AUC (area under the concentration-time curve)/MIC ratio, which is required to ensure effective bacterial eradication with the fluoroquinolones. While it has been suggested that an AUC/MIC ratio of 125 is required to produce acceptable clinical results, this value is derived from data on gram-negative organisms in patients with nosocomial pneumonia (3). More recently, in vitro studies evaluating the relationship of the AUC/MIC ratio with the effectiveness of fluoroquinolones against Streptococcus pneumoniae demonstrated that ratios of 30 to 64 are sufficient to provide rapid and sustained bactericidal activity (6-8). The in vitro results appear to substantiate the estimates of in vivo exposure in humans and its relationship to clinical and microbiologic cure rates observed with the fluoroquinolones in clinical trials (1, 11).
The present study was undertaken to describe the pharmacodynamic relationship between BMS-284756 exposure and outcome as assessed by bacterial density and survival in the pneumococcal thigh infection model. Additionally, an immunocompetent model was used to evaluate the impact of white blood cells on infection outcome.
MATERIALS AND METHODS
Antimicrobial test agents.
Analytical-grade standard BMS-284756 was obtained for in vitro testing from Bristol-Myers Squibb, Princeton, N.J. For all in vivo studies, laboratory standard BMS-284756 [lot 807t-3(A); expiration date, 31 July 2000] was obtained from the manufacturer, reconstituted on the basis of weight, and administered via the oral route by the oral gavage method with a feeding tube.
Bacterial isolates and susceptibilities.
Eight clinical isolates of S. pneumoniae were included in this study. The MIC of BMS-284756 was determined in duplicate by the microdilution method. The MICs were determined in cation-adjusted Mueller-Hinton broth (20 to 25 mg of calcium per liter, 10 to 12.5 mg of magnesium per liter) with 5% lysed horse blood in ambient air. Trypticase soy agar with 5% sheep blood was used as the growth medium.
Protein binding studies.
Protein binding studies were conducted by using an ultrafiltration method. An aqueous stock solution of BMS-284756 was prepared, and dilutions were made in fresh ICR mouse serum to yield final concentrations of 1, 10, and 100 μg/ml. All spiked serum samples were placed in a 37°C shaking water bath for 10 min. Serum samples (0.9 ml) were then transferred to Centrifree Micropartition devices (30,000-molecular-weight cutoff filter; Millipore, Bedford, Mass.). These filters were spun at 1,000 × g and 10°C for 15 min. Protein binding studies were conducted in triplicate at each concentration. Concentrations of BMS-284756 in both the initial serum solutions and filtrates were determined by a validated high-performance liquid chromatography (HPLC) method (see description of HPLC methods below).
Thigh infection model.
Specific-pathogen-free ICR mice weighing approximately 25 g were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, Ind.), and used throughout the experiment. Mice were rendered transiently neutropenic by intraperitoneal injection of cyclophosphamide on days −4 (150 mg/kg) and −1 (100 mg/kg) before treatment began. This regimen has been shown to induce neutropenia in the model for 5 days (2, 5, 12).
Isolates to be inoculated into the thighs were previously frozen at −80°C in skim milk. From the fresh isolate, approximately five colonies were transferred to tubes containing 6 ml of cation-adjusted Mueller-Hinton broth with 5% lysed horse blood. The tubes were placed into an incubator at 37°C for 10 h to obtain logarithmic growth of a 107- to 109-CFU/ml suspension and subsequently diluted to an inoculum of 105 to 107 CFU/ml. Final inoculum concentrations were confirmed by serial dilution and plating techniques. Thigh infection with each of the test isolates was produced by intramuscular injection of 0.1 ml of the inoculum into each thigh of the mice 2 h prior to the initiation of antimicrobial therapy (Fig. 1). In addition, similar inoculum-ranging studies were performed without cyclophosphamide-induced neutropenia with a second mouse species, CBA/J, and one pneumococcal isolate. This murine strain is susceptible to pneumococcal infection in the presence of this host defense (13).
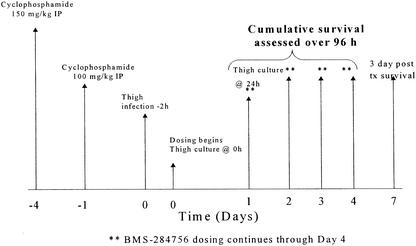
FIG. 1.
Study schematic for BMS-284756 thigh infection experiments. IP, intraperitoneal.
Pharmacokinetic studies.
Studies were undertaken to determine the pharmacokinetic profile of BMS-284756 in infected ICR mice. The mice received intraperitoneal injections of cyclophosphamide as previously described. Mice were administered a single oral BMS-284756 dose of 25, 50, or 100 mg/kg. Animals were euthanized by CO2 exposure, followed by cervical dislocation prior to intracardiac puncture. Blood was obtained from six mice per time point per group. Blood draws for the 25-mg/kg dose were performed at 0.5, 2, 4, 6, 8, and 10 h. For the 50-mg/kg dose, blood was obtained at 1, 2, 4, 6, 8, and 10 h. For the 100-mg/kg dose, blood was obtained at 0.5, 4, 12, 16, 18, and 24 h. The blood was centrifuged at 1,000 × g for 10 min; the serum was transferred into a polypropylene tube and stored at −80°C until analyzed.
Concentrations of BMS-284756 in murine serum were determined by using a validated HPLC procedure. Sample treatment involved the addition of 50 μl of a 0.5-μg/ml solution of an internal standard (moxifloxacin; Bayer Pharmaceuticals, West Haven, Conn.) and 500 μl of acetonitrile to a 200-μl sample contained in a polypropylene tube. After vortexing and centrifugation, the acetonitrile layer was evaporated to dryness and reconstituted with 200 μl of 0.01 N HCl. The aqueous layer was injected into the HPLC system with a Waters autosampler (model 717 plus; Waters Associates, Milford, Mass.) coupled to a Waters chromatographic pump (model M515). Chromatographic separations for both drugs were achieved on a reversed-phase 10-μm C18 column (250 by 4.6 nm; Nucleosil; Allteck Associates Inc. Deerfield, Ill.). Sample detection was done with a fluorescence detector (model 980; Applied Biosystems, Ramsey, N.J.) with the excitation and emission wavelengths set at 290 and 418 nm, respectively. Chromatograms were registered on an integrator (EZChrom Elite Chromatography Data System; Scientific Software, San Ramon, Calif.). The mobile phase was delivered at a flow rate of 1.3 ml/min and consisted of 20% acetonitrile and 80% buffer. The buffer was prepared with 4.14 g of sodium phosphate monobasic, 10.188 g of tetrabutylammonium hydrogen sulfate, and 3,000 ml of HPLC grade water.
The assay was linear over a range of 0.2 to 10 μg/ml. The intraday coefficients of variation of the low (0.5 μg/ml)- and high (8 μg/ml)-concentration quality control samples were 5.1 and 2.8%. The interday coefficients of variation of the low (0.5 μg/ml)- and high (8 μg/ml)-concentration quality control samples were 3.5 and 3.8%, respectively.
Analysis of BMS-284756 pharmacokinetic data was performed by using the nonlinear, mixed-effect model (NONMEM, double precision, version IV, level 1.0). A two-compartment structural model with first-order absorption and elimination from the central compartment was used. The basic parameters were elimination clearance (CL), volume of distribution of the central compartment (Vc), volume of distribution of the peripheral compartment (Vp), intercompartment clearance, and absorption constant (PREDPP subroutines ADAVA 4, TRANS 4). The elimination constant was defined as CL/Vc. A proportional-error model was used to describe the interindividual variability in pharmacokinetic parameters. The residual variability was described with an additive-error model. The first-order conditional estimation process was employed in NONMEM analyses.
After the NONMEM analyses, the resulting population pharmacokinetic parameter estimates were used to simulate the pharmacokinetic profiles of various dosing regimens by using the WinNonlin program (WinNonlin Pro, version 3.0; Pharsight Corporation, Mountain View, Calif.). The AUC, the maximum concentration of the drug in serum (Cmax), the time to Cmax (Tmax), and the half-life (t1/2) were then calculated from the simulated profiles, respectively.
Efficacy as assessed by bacterial density.
Animals prepared as described above were inoculated with seven S. pneumoniae isolates for which the MICs ranged from 0.03 to 2.0 μg/ml. Treatment was initiated at 2 h post thigh infection with an orally administered BMS-284756 regimen. The concentration-and-treatment regimen was varied to simulate a range of drug exposures. The final dosage ranges varied from 1.25 to 160 mg/kg/day divided into one to four doses; however, not all of the doses were the same for all of the isolates. For all of the isolates, at least five different treatment regimens (alteration of dose and/or dosing interval) were tested, except isolate 21, for which 10 treatment regimens were tested. Each treatment regimen contained eight thighs. Control animals (six thighs) received water orally in the same volume and on the same schedule as BMS-284756. Untreated control mice were sacrificed at the initiation of therapy or after 24 h. Treated mice were sacrificed after 24 h of therapy. Animals were euthanized by CO2 exposure, followed by cervical dislocation.
After sacrifice, both thighs were removed and individually homogenized in normal saline. Serial dilutions were placed on Trypticase soy agar with 5% sheep blood for CFU determinations. Efficacy (change in bacterial density) was calculated by subtracting the mean log number of CFU per thigh of the control mice obtained 2 h post bacterial inoculation from the log number of CFU per thigh of the BMS-284756-treated or untreated control mice at the end of therapy (24 h).
Efficacy as assessed by survival.
Groups of mice were similarly infected with each test strain for evaluation of survival during 96 h of therapy and again at 3 days posttherapy. BMS-284756 therapy was initiated at a time corresponding to 2 h post thigh inoculation. Similarly to density studies, BMS-284756 was administered in various regimens ranging from 1.25 to 200 mg/kg/day divided into one to four doses, producing 35 distinctly different regimens that were used for the test isolates over the 96-h treatment period. Control animals received water orally in the same volume and on the same schedule as the active drug. Although death has historically been used as an endpoint for studies of this type, that endpoint is no longer suitable in the present era of animal research. Therefore, our study method was modified to contemporary standards, which have been recently used in studies conducted at our institution (4). The term mortality has been used as an endpoint for this study; however, it should be clearly understood that, when possible, every attempt was made to minimize the pain and suffering of the animals. Animals were euthanized before they naturally succumbed to infection if symptoms of impending death were observed, including substantial alterations in posture (e.g., abnormal posture or tucking of the head into the abdomen), coat, exudate around the eyes and/or nose, and breathing or movement. In this study, whether an animal died because of the natural process or was euthanized, both were considered the same endpoint for experimental and statistical purposes.
Analysis of data.
Spearman's rank correlation coefficient was used to evaluate the relationship between mortality and the percentage of time that the drug concentration in serum remains above the MIC (%T>MIC), the peak/MIC ratio, and the AUC/MIC ratio for BMS-284756. This test was also used to evaluate the relationship between the change in the number of CFU after 24 h of therapy and the three pharmacodynamic parameters listed above. Additionally, the goodness of fit for each of these relationships was characterized and evaluated by using the sigmoid Emax model.
RESULTS
Eight clinical isolates of S. pneumoniae were used; the MICs of BMS-284756 and penicillin are displayed in Table 1. Organisms were selected to represent a range of susceptibilities to BMS-284756, thus allowing an opportunity for pharmacodynamic profiling over a wide range of exposures.
TABLE 1.
MIC of BMS-284756 and penicillin for S. pneumoniae isolates used in this study
| Isolate | MIC (μg/ml)
|
|
|---|---|---|
| Penicillin | BMS-284756 | |
| 85 | 1.0 | 0.03 |
| 75 | 1.0 | 0.06 |
| 59 | 2-4 | 0.06 |
| 77 | 1.0 | 0.06 |
| 21 | 0.03 | 0.125 |
| 113 | NAa | 0.5 |
| 92b | 0.25-0.5 | 2.0 |
| 114c | NA | 2.0 |
NA, not available.
Isolate used only in bacterial density studies.
Isolate used only in survival studies.
Protein binding and pharmacokinetic studies.
Protein binding studies were done in triplicate with ICR mice. The mean percentages of drug binding ± the standard error of the mean in ICR mice measured at concentrations of 1, 10, and 100 μg/ml were 77.4 ± 0.75 (coefficient of variation [CV], 0.97%), 80.90 ± 0.50 (CV, 0.62%), and 81.82 ± 1.12 (CV, 1.36%), respectively. The protein binding in mice appears to be similar to the range (79 to 89%) observed in other species, including humans. The population pharmacokinetic estimates of CL, Vc, intercompartment clearance, Vp, and absorption constant were 0.0798 (standard error [SE], 0.0159) liters/h, 0.0696 (SE, 0.0467) liters, 0.255 (SE, 0.0676) liters/h, 0.529 (SE, 0.0166) liters, and 1.53 (SE, 0.811) liters/h, respectively. This analysis proved quite robust, as shown by the plot of the observed concentration versus the predicted concentration of BMS-284756 in the infected animals, where the overall r2 was in excess of 0.89, indicting that the fit of the model to the data was reasonably good. The calculated AUC, Cmax, Tmax, and t1/2 based on these estimates are presented in Table 2.
TABLE 2.
Pharmacokinetic profile of BMS-284756 in infected neutropenic mice
| Dose (mg/kg) | Cmax (μg/ml) | Tmax (h) | AUC (h · μg/ml) | t1/2 (h) |
|---|---|---|---|---|
| 25 | 1.70 | 0.44 | 7.74 | 5.96 |
| 50 | 3.40 | 15.47 | ||
| 100 | 6.79 | 30.94 |
Bacterial density studies.
ICR mice received 0.1 ml of 105 to 107 CFU of S. pneumoniae per ml in each thigh. Time zero cultures at 2 h post thigh inoculation (prior to initiation of therapy) revealed good bacterial recovery for tissue, as the median log CFU count was 5.70 (range, 5.25 to 6.36). Organisms also grew well in untreated control animals over the 24-h study period, as the median log change in the CFU count in control animals was 2.67 (range, 2.00 to 3.09).
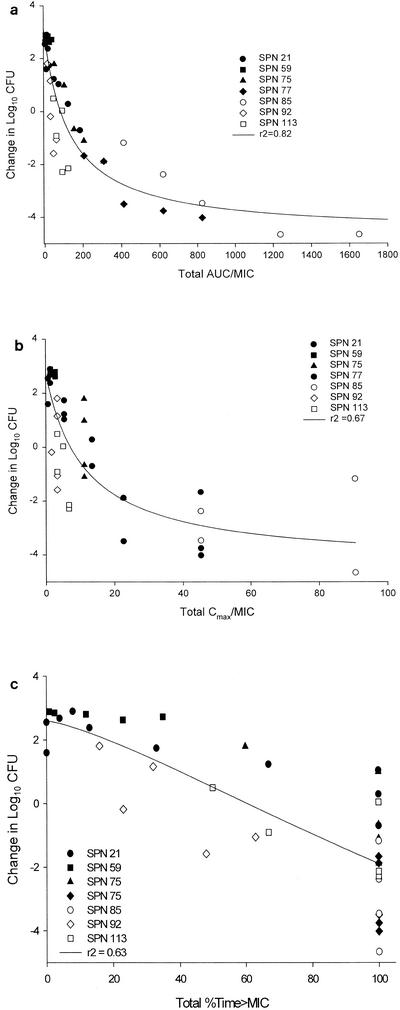
Significant correlations (P < 0.001) were observed for the relationships between the change in the CFU count and the total AUC/MIC ratio (r2 = 0.8230), the change in the CFU count and the total %T>MIC (r2 = 0.6314), and the change in the CFU count and the total peak/MIC ratio (r2 = 0.6702) for BMS-284756 (Fig. 2). Total AUC/MIC ratios of approximately 200 (free AUC/MIC ratio, ∼40) result in substantial bactericidal killing.
FIG. 2.
Relationship between changes in bacterial density and total drug concentrations for the pharmacodynamic parameters AUC/MIC ratio (r2 = 0.8230) (a), peak/MIC (r2 = 0.6702) (b), and %T>MIC (r2 = 0.6314) (c). Each datum point represents the mean of six to eight thighs. SPN, S. pneumoniae.
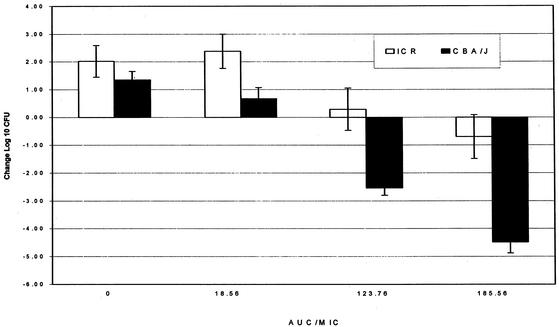
A BMS-284756 efficacy comparison was done by determining the change in bacterial density in ICR (neutropenic) and CBA/J (nonneutropenic) mice subjected to the same treatment regimens with S. pneumoniae isolate 21 (Fig. 3). The final dosage ranges varied from 2.5 to 75 mg/kg/day divided into one to three doses. Good growth was noted in control animals at 24 h regardless of the immunocompetency of the mice; ICR mice had 2-log or greater growth, and CBA/J mice demonstrated 1.4-log growth (P = 0.076). All CBA/J mouse groups demonstrated significantly (P ≤ 0.05) enhanced killing of the isolate, compared with that of the ICR mice that were correspondingly treated with similar exposures, achieving AUC/MIC ratios of 18.56, 123.76, and 185.56. The most profound effect of the immunocompetency of the CBA/J mice was noted when the ICR mouse bacterial density was nearly static, corresponding to a total AUC/MIC ratio of 123 or a free AUC/MIC ratio of approximately 25.
FIG. 3.
Comparison of the changes in the log CFU count versus the AUC/MIC ratio for ICR (neutropenic) and CBA/J (nonneutropenic) mice. Each group contains data from 6 to 10 thighs.
Survival studies.
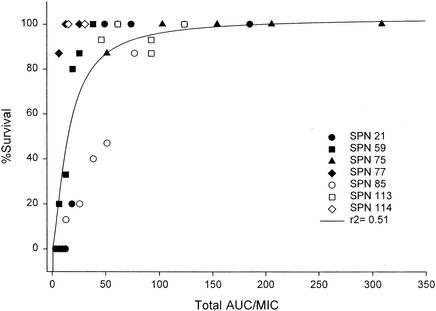
The overall mortality of untreated control mice after pneumococcal inoculation was 93%. Since the AUC/MIC ratio was determined to be the most predictive of outcomes on the basis of bacterial density, this parameter was evaluated in the survival studies. At the conclusion of therapy, an AUC/MIC ratio of 100 (free AUC/MIC ratio, ∼20) decreased variability in the data and provided nearly 100% survival (Fig. 4; r2 = 0.5069). Additionally, mortality at 3 days posttreatment was evaluated for the various treatment regimens. Survival at 3 days posttreatment was correlated with the AUC/MIC ratio (P < 0.001) (r2 = 0.3677). While a total AUC/MIC ratio of 100 (free AUC/MIC ratio, ∼20) provided nearly 100% survival on therapy, a higher AUC/MIC ratio (free AUC/MIC ratio, ∼40) was required to prevent mortality at 3 days posttherapy.
FIG. 4.
Relationship between percent survival at the end of therapy and the total AUC/MIC ratio (r2 = 0.5069). Each datum point represents 15 mice. SPN, S. pneumoniae.
A comparison of percent survival between ICR (neutropenic) and CBA/J (nonneutropenic) mice demonstrated increased efficacy of BMS-284756 in the immunocompetent group, at a total AUC/MIC ratio of 19 (free AUC/MIC ratio of 4) (Table 3). At this AUC/MIC ratio, the ICR group achieved 20% survival while the CBA/J group attained 70% survival. At total AUC/MIC ratios of ≤6, there was 100% mortality in both groups, and similarly, at total AUC/MIC ratios of ≥74, 100% survival was noted in both groups. At 3 days posttreatment, the difference between the two species of mice is more apparent, with the greatest effect noted at total AUC/MIC ratios of 50 to 74, corresponding to free AUC/MIC ratios of 10 to 15. At 3 days posttreatment, the CBA/J species reached 100% survival at total AUC/MIC ratios of ≥50 while the ICR mice reached a maximum of 93% survival at a total AUC/MIC ratio of 186.
TABLE 3.
Survival of ICRa and CBA/Jb mice after infection with S. pneumoniae 21 and 4 days of systemic exposure to BMS-284756
| AVC/MIC ratio | % Survival
|
||||
|---|---|---|---|---|---|
| Total | Free | At conclusion of therapy
|
3 days post-treatment
|
||
| ICR | CBA/J | ICR | CBA/J | ||
| 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 1 | 0 | 0 | 0 | 0 |
| 19 | 4 | 20 | 70 | 0 | 10 |
| 50 | 10 | 100 | 100 | 27 | 100 |
| 74 | 15 | 100 | 100 | 53 | 100 |
| 186 | 37 | 100 | 100 | 93 | 100 |
Neutropenic; n = 15.
Nonneutropenic; n = 10.
DISCUSSION
The goal of pharmacodynamic studies is to determine the interrelationships of the host-pathogen-drug triad in the hope of using these data to predict optimal regimens for patient care. While a variety of pharmacodynamic tools have been used to this end, one of the more common in vivo methods used is the thigh infection model. Our group has used this model to evaluate the dynamic response of S. pneumoniae to cefprozil exposure (10). These studies confirmed that the best-correlated parameter for the β-lactam cefprozil was %T>MIC. This study also demonstrated that animals infected with bacteria for which the MICs were ≤2 μg/ml survived the infection, whereas infection with bacteria for which the MICs were in excess of this value resulted in considerable mortality. Additionally, the breakpoint value of ≤2 μg/ml observed in the model appeared to be similar to that noted for otitis media in children undergoing cefprozil therapy. The latter data emphasize the potential utility of the thigh infection model in the design and support of drug exposures for in vivo human studies.
While the cefprozil work concentrated on the pharmacodynamics of cephalosporins, our present study provides new information to supplement the few published reports regarding the in vivo pharmacodynamics of quinolones against S. pneumoniae. In the present study, BMS-284756 demonstrated excellent in vivo bactericidal activity against S. pneumoniae. In addition, these data suggest that the AUC/MIC ratio best represents the dynamic response of S. pneumoniae to this novel quinolone as total AUC/MIC ratios of 100 to 200 (free AUC/MIC ratios, ∼20 to 40) resulted in maximal bactericidal effects.
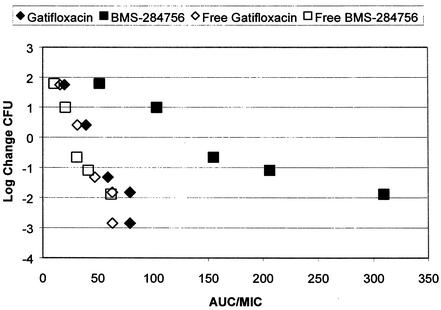
The protein binding of BMS-284756 is considerably higher (∼80%) than that of gatifloxacin (∼20%), which has been previous determined for the mouse species used in our in vivo experiments (9). It should be remembered, however, that as only the free drug has any effect on the organism, it is this free AUC/MIC ratio that should be considered in the comparison of fluoroquinolone effects. This issue is highlighted in Fig. 5, which displays the bactericidal activities of gatifloxacin and BMS-284756 against a single isolate of S. pneumoniae. It is evident from these data that the free drug is responsible for the detectable bactericidal activity, as the calculated free fractions of the two compounds produced essentially the same degree of pneumococcal killing for correspondingly similar AUC/MIC ratio exposure values.
FIG. 5.
Bactericidal effects of BMS-284756 and gatifloxacin on S. pneumoniae isolate 75 with respect to the free and total AUC/MIC ratio exposure values. The gatifloxacin data are from our previously published study, in which the same method was used (9).
The results of this study mirror our earlier work with gatifloxacin, which revealed that AUC/MIC ratios of at least 30 to 40 appear to optimize the dynamic response of bactericidal activity (9). In addition to data derived in our laboratory, in vivo studies conducted by Craig and Andes have found similar results with the same neutropenic-infection model (D. Andes and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. P-0191, 1999) and more recently with a nonneutropenic model (W. A. Craig and D. R. Andes, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. P-289, 2000). These studies determined that the AUC/MIC ratios required to optimize bacterial activity and survival with quinolones were similar to our findings of the free AUC/MIC ratios of 30 to 40 required by BMS-284756 to optimize our study outcomes. Moreover, previously published in vitro studies corroborate our findings that AUC/MIC ratios of 30 to 64 for the quinolones are required to produce effective bacterial eradication with S. pneumoniae, with the AUC/MIC ratio being the best predictor of our bacterial density and survival outcome (6, 7, 8). Our in vivo work also confirms a recent in vitro study with BMS-284756 that found that an AUC/MIC ratio of 30 produced good eradication of S. pneumoniae in their model (P. D. Lister and J. A. Black, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. P-295, 2000).
The issue of the minimally and maximally effective AUC/MIC ratios required to influence survival in this model was explored in our study through the use of two survival outcomes: survival assessed at the end of 96 h of therapy and survival assessed at 3 days posttherapy. It is assumed that survival at the end of therapy reflects a bacteriostatic condition, as the organism may still be present although not at a high enough concentration to cause death. Bactericidal conditions may be best reflected by 3-day posttreatment survival, as any remaining organism would have time to reach lethal concentrations prior to the second outcome measurement.
Through this study, we found that bacteriostatic conditions appear to be reached at exposures relating to a total AUC/MIC ratio of 100 (free AUC/MIC ratio, ∼20), while bactericidal effects occurred at ratios of 200 (free AUC/MIC ratio, ∼40). These data again support data obtained previously in our laboratory with gatifloxacin, which determined a beneficial effect on survival posttreatment to occur at AUC/MIC ratios of slightly greater than 20 (9).
Furthermore, the influence of white blood cells in this model was assessed by comparing neutropenic ICR mice with CBA/J mice that are inherently susceptible to some S. pneumoniae isolates without the need for cyclophosphamide. Again, the results of our present study reflect those of our previous study with gatifloxacin and the same pneumococcal isolate (S. pneumoniae 21). These findings suggest that immunocompetency had a profoundly beneficial effect on the immunocompromised mice in terms of bacterial density. Similar to that of the density studies, immunocompetent mice receiving the same exposures of BMS-284756 as the neutropenic animals had a significantly better survival rate at the conclusion of therapy and 3 days posttherapy.
In conclusion, we have determined that BMS-284756 displays excellent bactericidal activity against pneumococci in vivo. Moreover, the AUC/MIC ratio of BMS-284756 appears to best characterize its bactericidal effect and achievement of a ratio of 200 (free AUC/MIC ratio, 40) results in optimal pneumococcal killing and preserves survival after a lethal bacterial inoculation.
REFERENCES
- 1.Ambrose, P. G., D. M. Grasela, T. H. Grasela, J. A. Passarell, H. B. Mayer, P. F. Pierce. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Gross, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamm, T. E. 1995. Proposed institutional animal care and use committee guidelines for death as an end point in rodent studies. AALAS Contemp. Top. 34:69-71. [Google Scholar]
- 5.Joly-Guillou, M.-L., M. Wolff, J.-J. Pocidalo, F. Walker, and C. Carbon. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacy, M. K., W. Lu, X. Xu, P. R. Tessier, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob. Agents Chemother. 43:672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J. Antimicrob. Chemother. 43:79-86. [DOI] [PubMed] [Google Scholar]
- 8.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 43:1118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattoes, H. M., M. A. Banevicius, D. Li, C. Turley, D. Xuan, C. H. Nightingale, and D. P. Nicolau. 2001. Pharmacodynamic assessment of gatifloxacin against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:2092-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolau, D. P., C. O. Onyeji, M Zhong, P. R. Tessier, M. A. Banevicius, and C. H. Nightingale. 2000. Pharmacodynamic assessment of cefprozil against Streptococcus pneumoniae: implications for breakpoint determinations. Antimicrob. Agents Chemother. 44:1291-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolau, D. P. 1999. Using pharmacodynamic/pharmacokinetic surrogate markers in clinical practice: optimizing antibiotic therapy in respiratory tract infections. Am. J. Health-Syst. Pharm. 56(Suppl. 3):S16-S20. [DOI] [PubMed] [Google Scholar]
- 12.Onyeji, C. O., K. Q. Bui, R. C. Owens, Jr., D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Comparative efficacies of levofloxacin and ciprofloxacin against Streptococcus pneumoniae in a mouse model of experimental septicemia. Intern. J. Antimicrob. Agents 12:107-114. [DOI] [PubMed] [Google Scholar]
- 13.Tateda, K., K. Takashima, H. Miyazaki, T. Matsumoto, T. Hatori, and K. Yamaguchi. 1996. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob. Agents Chemother. 40:1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]