Abstract
To evaluate the pharmacokinetic effect of adding delavirdine mesylate to the antiretroviral regimens of human immunodeficiency virus (HIV)-infected patients stabilized on a full dosage of ritonavir (600 mg every 12 h), 12 HIV-1-infected subjects had delavirdine mesylate (400 mg every 8 h) added to their current antiretroviral regimens for 21 days. Ritonavir pharmacokinetics were evaluated before (day 7) and after (day 28) the addition of delavirdine, and delavirdine pharmacokinetics were evaluated on day 28. The mean values (± standard deviations) for the maximum concentration in serum (Cmax) of ritonavir, the area under the concentration-time curve from 0 to 12 h (AUC0-12), and the minimum concentration in serum (Cmin) of ritonavir before the addition of delavirdine were 14.8 ± 6.7 μM, 94 ± 36 μM · h, and 3.6 ± 2.1 μM, respectively. These same parameters were increased to 24.6 ± 13.9 μM, 154 ± 83 μM · h, and 6.52 ± 4.85 μM, respectively, after the addition of delavirdine (P is <0.05 for all comparisons). Delavirdine pharmacokinetic parameters in the presence of ritonavir included a Cmax of 23 ± 16 μM, an AUC0-8 of 114 ± 75 μM · h, and a Cmin of 9.1 ± 7.5 μM. Therefore, delavirdine increases systemic exposure to ritonavir by 50 to 80% when the drugs are coadministered.
Combination antiretroviral therapy for the treatment of human immunodeficiency virus (HIV) infection with various combinations of the four currently licensed categories of agents (nucleoside analogs, nonnucleoside reverse transcriptase inhibitors, protease inhibitors, and fusion inhibitors) may be used in patients, and drug interactions between these agents should be evaluated in order to determine the optimal doses. Ideally, this information should be obtained from patient studies to maximize the external validity of the results. However, there is an increasing sense that any intervention must be consistent with patient care standards in order to be ethical. Since these objectives may be inconsistent with scientific principles of the design of drug interaction studies, an increasing number of studies are conducted with HIV-negative volunteers. Assuming that the pharmacokinetic results of studies conducted with volunteers are consistent with the results in HIV patients, these studies are valid. However, some examples now exist in the literature of studies in which data generated from healthy volunteers are inconsistent with those from patient studies. In one trial, the coadministration of delavirdine and adefovir resulted in lower saquinavir concentrations in plasma than those in subjects receiving adefovir without delavirdine (8). The mechanisms underlying this unexpected observation remain unclear, but the findings underscore the complexity of designing regimens when the combinations to be compared have not previously been examined with HIV-infected subjects.
Delavirdine is a bisheteroarylpiperazine, nonnucleoside reverse transcriptase inhibitor approved for use as a component of combination therapy for HIV infection. Clinical studies of delavirdine combined with dual nucleoside reverse transcriptase inhibitors have examined its clinical efficacy for the treatment of HIV-1 infection (for example, see reference 9), and delavirdine is an alternative regimen for the treatment of HIV infection (http://www.hivatis.com). Delavirdine is metabolized by CYP3A4 into an inactive, N-dealkylated metabolite (Rescriptor package insert, Agouron Pharmaceuticals, San Diego, Calif.). In contrast to the other commercially available nonnucleoside reverse inhibitors (nevirapine and efavirenz), which are known to be enzyme inducers, delavirdine inhibits CYP3A4 (20). Ritonavir is an HIV protease inhibitor which is also metabolized by CYP3A4. A potent inhibitor of CYP3A4, ritonavir was originally prescribed at doses of 600 mg every 12 h when it was introduced. However, patient intolerance of full doses led to its primary use as a pharmacologic enhancer to increase the concentrations in plasma of a second protease inhibitor to improve the convenience of antiretroviral regimens by extending the dosing interval, reducing pill burden, and/or eliminating food-induced reductions in pharmacokinetic exposure (13; A. Hsu, G. R. Granneman, A. Japour, G. Cao, C. Locke, and L. Carothers, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-57, 1997; D. Kempf, A. Hsu, P. Jiang, R. Rode, K. Hertogs, and B. Larder, 8th Conf. Retrovir. Opportunistic Infect., abstr. 523, 2001; D. Kempf, A. Hsu, and J. Isaacson, 2nd Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 7.3, 2001). As part of early pharmacokinetics studies of delavirdine, we evaluated the influence of delavirdine on ritonavir in patients stabilized on ritonavir-containing regimens.
MATERIALS AND METHODS
Study design.
This study followed a single-group, multiple-dose experimental design. To qualify for enrollment in this study, HIV-infected patients had to have been stabilized on antiretroviral therapy that included 600 mg of ritonavir (six 100-mg capsules) twice daily as the sole protease inhibitor for a period of at least 14 days prior to enrollment. Patients were provided with ritonavir from a study supply on day 1, and adherence to the regimen was monitored (through patient interviews and pill counts) for 7 days prior to a baseline, 12-h pharmacokinetic assessment of ritonavir alone (day 7). Immediately after the ritonavir pharmacokinetic assessment, patients began receiving concomitant delavirdine at a dosage of 400 mg (four 100-mg tablets) three times daily for 21 days. However, due to the different regimens (thrice daily versus twice daily), the times of the remaining daily doses did not coincide. On day 28, a 12-h pharmacokinetic assessment of ritonavir and delavirdine was conducted. Levels of CD4 and HIV RNA were also evaluated and safety lab tests were performed at screening, on day 7, and on day 28. Written informed consent was obtained prior to any screening procedures, and all subjects were paid for their participation in the study.
All patients were required to meet the following criteria in order to enroll in the study: infection with HIV-1 confirmed, age between 18 and 55 years, and weight within 15% of the predicted ideal weight. Patients had to have the ability to comprehend the consent form and the willingness to sign it, and they had to have acceptable screening lab results (severity below grade 1 according to the AIDS Clinical Trials Group toxicity scale) within 30 days of study entry. Patients were eligible if they also had a Karnofsky performance status of at least 80, were able to swallow many large tablets without difficulty, were willing to abstain from alcohol for 48 h prior to study participation and for the duration of the study, and had acceptable medical histories, physical exams, electrocardiograms, and chest X rays during screening prior to entering the drug-dosing period of the study. In addition, the results of urine tests had to be negative for illegal drugs (both at screening and during the study). All patients had agreed to practice adequate birth control to prevent pregnancy, and their sera were found to be negative for beta human chorionic gonadotropin within 15 days prior to study entry (for women). Tobacco use was permitted during the study, but patient usage patterns could not change during the study treatment period.
Patients were excluded if they had participated in any other investigational trials or received prohibited concurrent medications (rifampin, rifabutin, astemizole, loratidine, terfenadine, amiodarone, bepridil, buproprion, cisapride, clozapine, encainide, flecanide, meperidine, piroxicam, propafenone, propoxyphene, carbamazepine, phenytoin, phenobarbital, or quinidine) within 21 days of initial study medication dosing. Patients did not have histories of clinically significant nervous system or muscle diseases, seizure disorders, AIDS dementia, or psychiatric disorders that might have impaired their adherence to the study regimen. Patients also did not have clinically significant medical problems. The numbers of concurrent medications used are summarized in Table 1. Five subjects were receiving clarithromycin; however, no medications were added or discontinued during the entire study period.
TABLE 1.
Demographic characteristics of patients participating in delavirdine-ritonavir interaction studya
| Subject | Age (yr) | Race | HIV riskb | Wt (kg) | Smoking status | Duration of ritonavir treatment (mos) | Nucleosidesc | No. of concurrent medications |
|---|---|---|---|---|---|---|---|---|
| 1 | 33 | Black | Heterosexual | 78 | Never | 5 | 3TC, ZDV | 2 |
| 2 | 39 | Black | Homosexual | 75 | Smoker | 11 | 3TC, ZDV | 6 |
| 3 | 44 | White | Homosexual, IVDU | 100 | Smoker | 12 | 3TC, d4T | 6 |
| 4 | 40 | White | Heterosexual | 62 | Smoker | 2 | 3TC, d4T | 3 |
| 5 | 38 | White | Homosexual | 67 | Smoker | 7 | ddI, d4T | 2 |
| 6 | 50 | White | Homosexual | 61 | Smoker | 10 | 3TC, ZDV | 3 |
| 7 | 32 | White | Homosexual, IVDU | 67 | Smoker | 12 | 3TC, d4T | 9 |
| 8 | 54 | White | Homosexual | 86 | Never | 4 | 3TC, ZDV | 2 |
| 9 | 41 | White | Blood transfusion recipient | 108 | Never | 7 | 3TC, ZDV | 6 |
| 10 | 47 | Hispanic | Homosexual | 90 | Never | 12 | 3TC, d4T | 7 |
| 13 | 34 | White | Homosexual | 67 | Smoker | 1 | None | 7 |
| 14 | 39 | White | Homosexual | 90 | Never | 2 | 3TC, d4T | 9 |
| Mean or total | 40.7 | 2 black, 9 white, 1 Hispanic | 7 homosexual, 2 homosexual; IVDUs, 2 heterosexual, 1 blood transfusion recepient | 79.0 | 7 smokers, 5 nonsmokers | 7 | 10 3TC, 5 ZDV, 6 d4T, 1 ddI user | 4.9 |
| SD | 6.51 | 14.4 | 4.3 | 2.5 |
All 12 patients who completed the study were male.
IVDU, intravenous drug used.
3TC, lamivudine; ZDV, zidovudine; d4T, stavudine; ddI, dideoxyinosine.
Drug administration.
All medications were administered as oral doses taken with 6 fluid ounces (180 ml) of room temperature water. Ritonavir was taken with a meal of the patient's choosing. Patients were allowed to select a breakfast to be consumed with ritonavir during each of the two pharmacokinetic evaluations in the study. The breakfasts for each subject were identical on the days of the pharmacokinetic assessments but differed among patients. During each pharmacokinetic assessment, blood specimens (5 ml) were collected predose and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, and 12 h postdose for pharmacokinetic analysis. For both the delavirdine and ritonavir assessments, venous whole blood was drawn via venipuncture or indwelling intravenous cannula into a sodium-heparin-containing vacuum tube at the designated times after drug administration. Plasma was harvested by centrifuging the specimens at 1,000 to 2,000 × g (∼3,000 rpm) for 10 min. After centrifugation, the upper plasma layer was carefully transferred to plastic storage vials and immediately frozen at −20°C.
Assay methodology. (i) Delavirdine and desalkyl-delavirdine.
Plasma samples were assayed for delavirdine and desalkyl-delavirdine concentrations by using validated, sensitive, and specific isocratic high-performance liquid chromatography (HPLC) methods. Delavirdine, desalkyl-delavirdine, and the internal standard (IS) were extracted from plasma by protein precipitation with acetonitrile; the supernatant was mixed with buffer and directly injected. Chromatographic separation was achieved by using a cyano guard column (Brownlee CN) and a cyano analytical column (DuPont Zorbax SB CN). The mobile phase consisted of 10 mM KH2PO4 (pH 6.0)-acetonitrile-methanol (20:7:7), which was run at a flow rate of 1.5 ml/min. The analytes were detected by fluorescence by using an excitation wavelength of 295 nm and an emission filter at 418 nm. The retention times of the primary analytes were ∼3.1 min (desalkyl-delavirdine), ∼8.3 min (IS), and ∼9.4 min (delavirdine). Calibration standard responses were linear by a weighted (1/concentration2) least-squares linear regression based on peak height ratios. Correlation coefficients were 0.9994 for delavirdine and desalkyl-delavirdine. The lower limit of quantitation for delavirdine and desalkyl-delavirdine was 25.0 ng/ml. For delavirdine, interday coefficients of variation (CV) for back calculated concentrations of calibration standards ranged from 1.3 to 6.3%, with mean accuracies within 99.7 to 100.4% of the nominal concentrations. Assay accuracies, expressed as the ratios of the mean calculated quality control (QC) standard concentrations to the nominal concentrations, were 93.1, 98.6, 98.1, and 97.5%, respectively, for the low (400 ng/ml), medium (4,000 ng/ml), high (20,000 ng/ml), and diluted (40,000 ng/ml, 2× dilution) concentrations of QC standards. Assay precision levels ranged from 2 to 3% across the three QC pools.
For desalkyl-delavirdine, the interday CV for back calculated concentrations of calibration standards ranged from 1.1 to 5.9%, with mean accuracies within 96.8 to 101.5% of the nominal concentrations. Assay accuracies, as indicated by QC standards, were 93.0, 98.6, and 96.6%, respectively, for the low (400 ng/ml), medium (4,000 ng/ml), and high (20,000 ng/ml) concentrations of QC standards. Assay precision levels for the high curve (CV of QC standards) were 2.6, 2.1, and 2.2%, respectively, for the low, medium, and high QC pools.
(ii) Ritonavir.
Plasma samples were assayed for ritonavir concentrations by using a validated, sensitive, and specific isocratic HPLC method (9a). Ritonavir and the IS were extracted from human plasma by liquid-liquid extraction with an ethyl acetate mixture. Sample extracts were analyzed by HPLC with UV detection. The retention times of the primary analytes were ∼9.1 min (ritonavir) and ∼13.1 min (IS). The specificity of the method in the presence of delavirdine and desalkyl-delavirdine had been established prior to specimen analysis. Calibration standard responses were linear over the curve range (0.01 to 15.0 μg/ml) by a weighted (1/concentration2) least-squares linear regression based on peak height ratios. Correlation coefficients were 0.9982 throughout the analyses. The lower limit of quantitation was 0.01 μg/ml. Clinical specimens with concentrations above the calibration standard range were diluted with blank human plasma prior to sample preparation. Acceptable performance of the method with similar dilutions was monitored with QC standards during specimen analysis. Interassay CV for back calculated concentrations of ritonavir calibration standards ranged from 1.6 to 8.5%, with mean accuracies within 96.1 to 104.6% of the nominal concentrations. Assay accuracies, expressed as the ratios of the estimated QC standard concentrations to the nominal QC standard concentrations, were 96.4, 99.5, 105.8, 100.1, and 95.6%, respectively, for the low (0.250 μg/ml), medium (0.350 μg/ml), high (10.0 μg/ml), and diluted (10.0 μg/ml; 5× and 10× dilutions) QC standards. Assay precision levels, expressed as the CV of the estimated concentrations of QC standards, were 12.5, 2.2, 3.3, 2.8, and 2.8%, respectively, for the low, medium, and high concentrations and the two diluted concentrations.
Pharmacokinetic and statistical analysis.
Pharmacokinetic parameters were calculated by using noncompartmental methods. For delavirdine, desalkyl-delavirdine, and ritonavir, the area under the plasma concentration-time curve for the steady-state dosing interval (AUC0-8 for delavirdine and AUC0-12 for ritonavir) was calculated by using the trapezoidal rule. Peak concentration in plasma (Cmax), time to peak concentration (Tmax), and minimum concentration (Cmin) during the steady-state dosing interval were tabulated. The ratio of desalkyl-delavirdine formation clearance to elimination clearance was calculated as the ratio of the desalkyl-delavirdine AUC0-8 to the delavirdine AUC0-8. Following log transformation, the geometric mean ratio (90% confidence interval) of day 28 values to day 7 values was determined for each ritonavir pharmacokinetic parameter.
For a patient to be considered able to be evaluated for the pharmacokinetic interaction between ritonavir and delavirdine, pharmacokinetic data were required from each of the two steady-state evaluations (treatment with ritonavir alone and in combination). Paired t tests were used to determine differences in pharmacokinetic parameters for ritonavir used in treatment with and without concomitant delavirdine. The delavirdine pharmacokinetic parameters were compared to those from a previous study by using Wilcoxon rank sum tests. The comparator database was a study by Morse et al. assessing the steady-state pharmacokinetics of delavirdine (400 mg three times daily) taken with a high-fat meal (14). Statistical significance in these tests was defined as a P value of <0.05. All statistical evaluations were conducted by using the Statistical Analysis System (version 6.08; SAS Institute, Cary, N.C.).
RESULTS
Fourteen patients (13 males and 1 female) were enrolled in this study, although only 12 completed the study. One patient (male) dropped out of the study because of personal reasons unrelated to the study, and another patient (female) was inappropriately enrolled due to elevated serum aminotransferase levels at screening but never received delavirdine. The demographics of the remaining 12 patients are given in Table 1. All patients were male (nine white, two black, one Hispanic), the mean age was 41 years (range, 32 to 54 years), and the mean weight was 79 kg (range, 61 to 108 kg). The mean duration of ritonavir use prior to starting the study was 7 ± 4 months (ranging from 1 to 12 months), and the mean number of concurrent medications taken by these patients was 4.9 ± 2.5. The majority (8 of 12) of these patients had undetectable HIV viral loads at baseline (<400 copies of HIV RNA/ml; data not shown).
Overall, the ritonavir-delavirdine regimen was well tolerated during the 21 days of concurrent administration. No subject discontinued the study because of an adverse event, and no serious events occurred. One grade-3 lab test abnormality was reported, a case of hypertriglyceridemia ascribed to HIV and ritonavir, but the case was not treated during the study time period. Conditions that emerged during treatment and required medication included bronchitis and cough on day 6; presumptive, mild to moderate Pneumocystis carinii pneumonia on day 6; and tendonitis. The most common adverse events emerging during treatment, without regard to causality, were upper respiratory infection (three cases), diarrhea (two cases), and nausea (two cases). All other events had only a single occurrence. Several nonserious adverse events occurred: hypertriglyceridemia (n = 1; grade 3) and tests showing elevated liver function (AST, ALT, GGT; n = 1; grade 3). Other changes noted in hematology and chemistry safety lab results were regarded to be the effects of preexisting conditions or to be related to disease or to nonstudy medications. By day 28, CD4 cell counts increased by an average of 71 cells/mm3, the levels of CD4 increased by an average of 3.1%, and HIV RNA was undetectable in 10 of 12 patients.
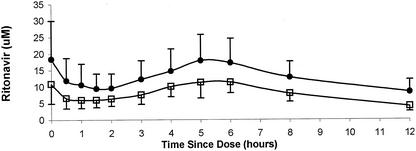
Mean plasma ritonavir concentrations before and after concomitant administration of delavirdine are shown in Fig. 1. Mean ritonavir pharmacokinetic parameters for the two treatments are listed in Table 2. Treatment with delavirdine resulted in statistically significant (P < 0.01) increases in the AUC0-12, Cmax, and Cmin of ritonavir. Delavirdine increased the ritonavir AUC0-12 in 11 of the 12 patients evaluated, with changes in the AUC0-12 ranging from a decrease of 29% to an increase of 214%. Ritonavir appeared to be slowly and variably absorbed in this study, with little absorption during the first hour after drug administration (Fig. 1). The Tmax values of ritonavir ranged from 0 to 6 h for both treatments, with four patients having ritonavir Tmax values of 0 h for both treatments. Delavirdine had no effect on the Tmax of ritonavir, with mean values (± standard deviations [SD]) of 3.0 ± 2.5 h and 2.6 ± 2.6 h for treatment with ritonavir alone and in combination with delavirdine, respectively. Although the intersubject variability in the Tmax of ritonavir was relatively large, intrasubject variability in this parameter was relatively low and Tmax values for the two treatments were significantly correlated (data not shown).
FIG. 1.
Mean (± SD) ritonavir concentrations in plasma from 12 patients with HIV-1 infection after treatment with ritonavir alone (600 mg every 12 h; open squares) and after treatment with ritonavir in combination with delavirdine (400 mg every 8 h; closed circles) for 21 days.
TABLE 2.
Ritonavir pharmacokinetic parameters (means ± SD) from treatment with ritonavir (600 mg every 12 h) alone and in combination with delavirdine (400 mg every 8 h)
| Drug(s) (day of assessment) | AUC0-12 (μM · h) | Cmax (μM) | Cmin (μM) | Tmax (h) |
|---|---|---|---|---|
| Ritonavir (day 7) | 94 ± 36 | 14.8 ± 6.7 | 3.6 ± 2.1 | 3.0 ± 2.5 |
| Ritonavir plus delavirdine (day 28) | 154 ± 83 | 24.6 ± 13.9 | 6.52 ± 4.85 | 2.6 ± 2.6 |
| Geometric mean ratioa (90% confidence interval) | 1.51 (1.24-1.83) | 1.54 (1.24-1.91) | 1.76 (1.5-2.05) | NDb |
Ratio of day 28 parameter to day 7 parameter.
ND, not determined.
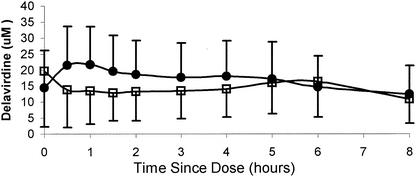
When the steady-state delavirdine concentrations from this study were compared to those from the reference database for delavirdine taken with food, there were no statistically significant differences (Table 3). Figure 2 illustrates the concentrations in plasma versus time profiles for delavirdine and desalkyl-delavirdine over an 8-h interval.
TABLE 3.
Delavirdine pharmacokinetic parameters (means ± SD) following administration of delavirdine mesylate (400 mg every 8 h, with meals) to HIV-1 infected patients
| Drug(s) (no. of patients) | AUC0-8 (μM · h) | Cmax (μM) | Tmax (h) | Cmin (μM) | CLF/CLMb |
|---|---|---|---|---|---|
| Delavirdine plus ritonavir (12) | 114 ± 75 | 23 ± 16 | 2.4 ± 2.7 | 9.1 ± 7.5 | 0.29 ± 0.15 |
| Delavirdine without ritonavir (13)a | 132 ± 87 | 23 ± 13 | 1.0 ± 0.9 | 11 ± 9 | 0.36 ± 0.58 |
A previous study (14) with 13 patients was used as a control.
CLF, formation clearance; CLM, elimination clearance.
FIG. 2.
Mean (± SD) concentrations of delavirdine (closed circles) and desalkyl-delavirdine (N-DLV; open squares) in plasma from 12 HIV-1-infected patients receiving ritonavir (600 mg every 12 h) and delavirdine (400 mg every 8 h) for 21 days.
DISCUSSION
Treatment with delavirdine has been shown in vitro to result in a noncompetitive inhibition of cytochrome P450 3A (CYP3A4) (5; Voorman et al., PhRMA 1997 Drug Metab. Fall Workshop: Metab.-Based Drug-Drug Interact.). Delavirdine mesylate may therefore have clinically important pharmacokinetic drug interactions with other drugs whose clearance is mediated by CYP3A. The results of pharmacokinetic studies suggest that delavirdine inhibits the metabolism of clarithromycin (2; S. R. Cox, M. T. Borin, M. R. Driver, B. Levy, and W. W. Freimuth, 2nd Natl. Conf. Hum. Retrovir. Related Infect., abstr. 487, 1995), rifabutin (S. R. Cox, D. W. Schneck, B. D. Herman, B. J. Carel, B. R. Gullotti, B. M. Kerr, and W. W. Freimuth, 5th Conf. Retrovir. Opportunistic Infect., abstr. 345, 1998), indinavir (7), saquinavir (S. R. Cox, J. J. Ferry, D. H. Batts, G. F. Carlson, D. W. Schneck, B. D. Herman, A. A. Della-Coletta, J. H. Chambers, N. K. Carel, F. Stewart, N. Buss, and A. Brown, 4th Conf. Retrovir. Opportunistic Infect., 1997), and nelfinavir (Cox et al., 5th Conf. Retrovir. Opportunistic Infect.). The primary route of delavirdine clearance is N dealkylation, which is mediated by CYP3A and possibly CYP2D6 (Rescriptor package insert). Inducers of CYP3A (such as rifabutin and rifampin) were shown to produce a marked increase in delavirdine clearance (1, 2). However, inhibitors of CYP3A (such as ketoconazole, itraconazole, and clarithromycin) were found to produce only modest reductions in delavirdine clearance.
Ritonavir is cleared primarily through oxidative metabolism, which is mediated by CYP3A and CYP2D6 (6). Rifampin, a potent inducer of CYP3A, reduced the systemic exposure to ritonavir by 35%, but inhibitors of CYP3A and/or CYP2D6 (clarithromycin and fluoxetine) produced modest increases (<20%) in ritonavir systemic exposure. In microsomal studies, ritonavir was found to inhibit several P450 isoforms, including CYP3A and CYP2D6 (6, 11). Consistent with these findings, ritonavir was shown to markedly reduce the clearance of rifabutin, saquinavir, and indinavir (13; N. Buss and the Fortovase Study Group, 5th Conf. Retrovir. Opportunistic Infect., abstr. 354, 1998; Hsu et al., 37th ICAAC). However, in recent studies, ritonavir induced the metabolism of alprazolam, mepiridine, and methadone (19; R. Frye, R. Bertz, G. R. Granneman, J. Qian, J. Lamm, and S. Dennis, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-59, 1997; A. Hsu, G. R. Granneman, L. Carothers, S. Dennis, Y.-L. Chiu, and J. Valdes, 5th Conf. Retrovir. Opportunistic Infect., abstr. 342, 1998). Therefore, predictions of the effects of ritonavir on the pharmacokinetics of other drugs based on findings of in vitro studies may not be reliable.
The ritonavir pharmacokinetic parameters in the present study are consistent with those in previous reports (Norvir package insert, Abbott Laboratories, Inc., Chicago, Ill.). In this study, delavirdine increased the mean systemic exposure to ritonavir by 51% and increased the Cmin of ritonavir by 76%. Ritonavir undergoes extensive oxidative metabolism into five metabolites. Microsomal studies showed that the primary P450 isoform involved in the metabolism of ritonavir is CYP3A but that CYP2D6 is also involved (6). Because of the lesser affinity of delavirdine for CYP2D6 (Voorman et al., PhRMA 1997 Drug Metab. Fall Workshop: Metab.-Based Drug-Drug Interact.), the most likely explanation for the reduction in ritonavir clearance by delavirdine is therefore the inhibition of CYP3A.
In previous studies, two inhibitors of CYP3A and CYP2D6, clarithromycin and fluoxetine, were shown to produce smaller increases (<20%) in plasma ritonavir concentrations than those observed in the present study, although these data were unlikely to reflect steady-state conditions for ritonavir. The ritonavir dose in the clarithromycin study (200 mg three times daily) was much less than the approved dose, and the duration of ritonavir treatment (4 days) in that study may have been insufficient to provide steady-state conditions (17). In the fluoxetine study, ritonavir was administered as a single 600-mg dose (18). It may be difficult to extrapolate the findings from the clarithromycin and fluoxetine interaction studies to steady-state conditions with ritonavir taken in 600-mg doses twice daily, since ritonavir induces its own pharmacokinetics and exhibits nonlinear steady-state pharmacokinetics (10).
The interaction between ritonavir and delavirdine has also been evaluated in healthy volunteers by using lower doses of both drugs: delavirdine at 400 mg twice daily and ritonavir at 300 mg twice daily (J. J. Ferry, D. W. Schneck, G. F. Carlson, P. A. Carberry, A. A. Della-Coletta, B. R. Gulotti, and S. R. Cox, 4th Conf. Retrovir. Opportunistic Infect., 1997). In that study, neither drug had any substantial effect on the clearance of the other. The differing results between that study and the present study may be related to differences between doses and/or subject populations. It is possible that the inhibitory effect of delavirdine on ritonavir clearance is more pronounced at the higher ritonavir concentrations, since ritonavir exhibits nonlinear pharmacokinetics (10). Subject differences (healthy volunteers versus HIV-1-infected patients) may also have contributed to the difference between study results regarding the effect of delavirdine on ritonavir clearance; altered patterns of drug metabolism have been observed in HIV-1-infected patients relative to those in normal volunteers (3, 4, 12, 15, 16). The concentration-time profiles for delavirdine in the present study generally differed from those in previous steady-state studies with either fasting or fed HIV-1-infected patients, and this difference is consistent with a more delayed and/or prolonged drug absorption (1, 2, 7). Although the possibility that ritonavir reduces the rate of delavirdine absorption cannot be ruled out, it is likely that flattened concentration-time profiles from the present study are the result of the effects of high-fat meals, which delay gastric emptying. In a previous steady-state study, meals had no effect on the AUC0-8, Cmin, or Tmax of delavirdine but did significantly reduce the Cmax of delavirdine by about 30% to 23 μM, a value in excellent agreement with the Cmax from the present study (14). The patients in the present study generally consumed higher-fat meals than those in the previous steady-state food effect study, and the longer mean Tmax of delavirdine from the present study is consistent with the more profoundly delayed rates of gastric emptying and delavirdine absorption resulting from higher-fat meals.
The present study suggests that delavirdine inhibits the metabolism of ritonavir, presumably through the CYP3A4 pathway, when both drugs are used at full dosages. At present, delavirdine appears to be the only antiretroviral agent that inhibits the metabolism of ritonavir to a clinically significant extent. The role of protein binding displacement or effects on P-glycoprotein also cannot be ruled out as potential explanatory mechanisms for the interaction. Based upon the results of this study, ritonavir doses should probably be reduced for patients taking both full-dosage ritonavir and delavirdine, given the poor tolerance for higher ritonavir doses and presumably for higher exposure. Extrapolation of these results to patients receiving delavirdine with lower doses (e.g., 100 to 200 mg every 12 h) of ritonavir as a pharmacologic enhancer of a second protease inhibitor will require further investigation to determine the net pharmacokinetic outcome of these three- or four-way interactions in HIV-infected patients during salvage therapy.
Acknowledgments
The assistance of the participating patients and the entire staff of the Erie County Medical Center, Immunodeficiency Services Clinic, is greatly appreciated.
This project was supported by a grant from Agouron Inc. (a Pfizer company).
REFERENCES
- 1.Borin, M. T., J. T. Chambers, B. J. Carel, W. W. Freimuth, S. Aksentijevich, and A. A. Piergies. 1997. Pharmacokinetic study of the interaction between rifabutin and delavirdine mesylate in HIV-1 infected patients. Antivir. Res. 35:53-63. [DOI] [PubMed] [Google Scholar]
- 2.Borin, M. T., J. T. Chambers, B. J. Carel, S. Gagnon, and W. W. Freimuth. 1997. Pharmacokinetic study of the interaction between rifampin and delavirdine mesylate. Clin. Pharmacol. Ther. 61:544-553. [DOI] [PubMed] [Google Scholar]
- 3.Brockmeyer, N. H., L. Mertins, D. Spatz, I. Tillmann, and M. Goos. 1992. Endogenous interferon plasma levels and antipyrine pharmacokinetics in patients with viral infections. Int. J. Clin. Pharmacol. Ther. Toxicol. 30:530-533. [PubMed] [Google Scholar]
- 4.Chen, Y. L., V. LeVraux, A. Leneveu, F. Dreyfus, A. Stheneur, I. Florentin, M. De Sousa, J. P. Giroud, B. Flouvat, and L. Chauvelot-Moachon. 1994. Acute-phase response, interleukin-6, and alteration of cyclosporine pharmacokinetics. Clin. Pharmacol. Ther. 55:649-660. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, C.-L., D. E. Smith, P. L. Carver, S. R. Cox, P. B. Watkins, D. S. Blake, C. A. Kauffman, K. M. Meyer, G. L. Amidon, and P. L. Stetson. 1997. Steady-state pharmacokinetics of delavirdine in HIV-positive patients: effect on erythromycin breath test. Clin. Pharmacol. Ther. 61:531-543. [DOI] [PubMed] [Google Scholar]
- 6.Eagling, V. A., D. J. Back, and M. G. Barry. 1997. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br. J. Clin. Pharmacol. 44(2):190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferry, J. J., B. D. Herman, B. J. Carel, G. F. Carlson, and D. H. Batts. 1998. Pharmacokinetic drug-drug interaction study of delavirdine and indinavir in healthy volunteers. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:252-259. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher, C. V., E. P. Acosta, H. Cheng, R. Haubrich, M. Fischl, R. Raasch, C. Mills, X. J. Hu, D. Katzenstein, R. P. Remmel, R. M. Gulick, and the ACTG Protocol Team. 2000. Competing drug-drug interactions among multidrug antiretroviral regimens used in the treatment of HIV-infected subjects: ACTG 884. AIDS 14:2495-2501. [DOI] [PubMed] [Google Scholar]
- 9.Friedland, G. H., R. Pollard, B. Griffith, M. Hughes, G. Morse, R. Bassett, W. Freimuth, L. Demeter, E. Connick, T. Nevin, M. Hirsch, and M. Fischl. 1999. Efficacy and safety of delavirdine mesylate with zidovudine and didanosine compared with two-drug combinations of these agents in persons with HIV disease with CD4 counts of 100 to 500 cells/mm3 (ACTG 261). J. Acquir. Immune Defic. Syndr. 21:281-292. [DOI] [PubMed] [Google Scholar]
- 9a.Hoetelmans, R. M., M. van Essenberg, M. Profijt, P. L. Meenhorst, J. W. Mulder, and J. H. Beijnen. 1998. High-performance liquid chromatographic determination of ritonavir in human plasma, cerebrospinal fluid and saliva. J. Chromatogr. B 705:119-126. [DOI] [PubMed] [Google Scholar]
- 10.Hsu, A., G. R. Granneman, G. Witt, C. Locke, J. Denissen, and A. Molla. 1997. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 41:898-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar, G. N., A. D. Rodrigues, A. M. Buko, and J. F. Denissen. 1996. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J. Pharmacol. Exp. Ther. 277(1):423-431. [PubMed] [Google Scholar]
- 12.Lee, B. L., D. Wong, N. L. Benowitz, and P. M. Sullam. 1993. Altered patterns of drug metabolism in patients with acquired immunodeficiency syndrome. Clin. Pharmacol. Ther. 53:529-535. [DOI] [PubMed] [Google Scholar]
- 13.Merry, C., M. G. Barry, F. Mulcahy, M. Ryan, J. Heavey, J. F. Tijia, K. L. Halifax, J. Heavey, C. Kelly, and D. J. Back. 1997. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS 11:F29-F33. [DOI] [PubMed] [Google Scholar]
- 14.Morse, G. D., M. A. Fischl, S. R. Cox, L. Thompson, A. A. Della-Coletta, and W. W. Freimuth. 2003. Effect of food on the steady-state pharmacokinetics of delavirdine mesylate in HIV+ patients. Clin. Drug Investig. 23:255-261. [DOI] [PubMed]
- 15.Nokta, M., J. P. Loh, S. M. Douidar, A. E. Ahmed, and R. B. Pollard. 1991. Metabolic interaction of recombinant interferon-β and zidovudine in AIDS patients. J. Interferon Res. 11:159-164. [DOI] [PubMed] [Google Scholar]
- 16.Okumo, H., Y. Kitao, M. Takasu, H. Kano, K. Kunieda, and T. Seki. 1990. Depression of drug metabolizing activity in the human liver by interferon-α. Eur. J. Clin. Pharmacol. 39:365-367. [DOI] [PubMed] [Google Scholar]
- 17.Ouellet, D., A. Hsu, G. R. Granneman, G. Carlson, J. Cavanaugh, H. Guenther, and J. M. Leonard. 1998. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin. Pharmacol. Ther. 64:355-362. [DOI] [PubMed] [Google Scholar]
- 18.Ouellet, D., A. Hsu, J. Qian, J. E. Lamm, J. H. Cavanaugh, J. M. Leonard, and G. R. Granneman. 1998. Effect of fluoxetine on pharmacokinetics of ritonavir. Antimicrob. Agents Chemother. 42:3107-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piscitelli, S., D. Rock-Kress, R. Bertz, A. Pau, and R. Davey. 2000. The effect of ritonavir on the pharmacokinetics of meperidine and normeperidine. Pharmacotherapy 20:549-553. [DOI] [PubMed] [Google Scholar]
- 20.Voorman, R. L., S. M. Maio, N. A. Payne, Z. Zhao, K. A. Koeplinger, and X. Wang. 1998. Microsomal metabolism of delavirdine: evidence for mechanism-based inactivation of human cytochrome P450 3A. J. Pharmacol. Exp. Ther. 287:381-388. [PubMed]