Abstract
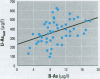
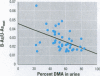
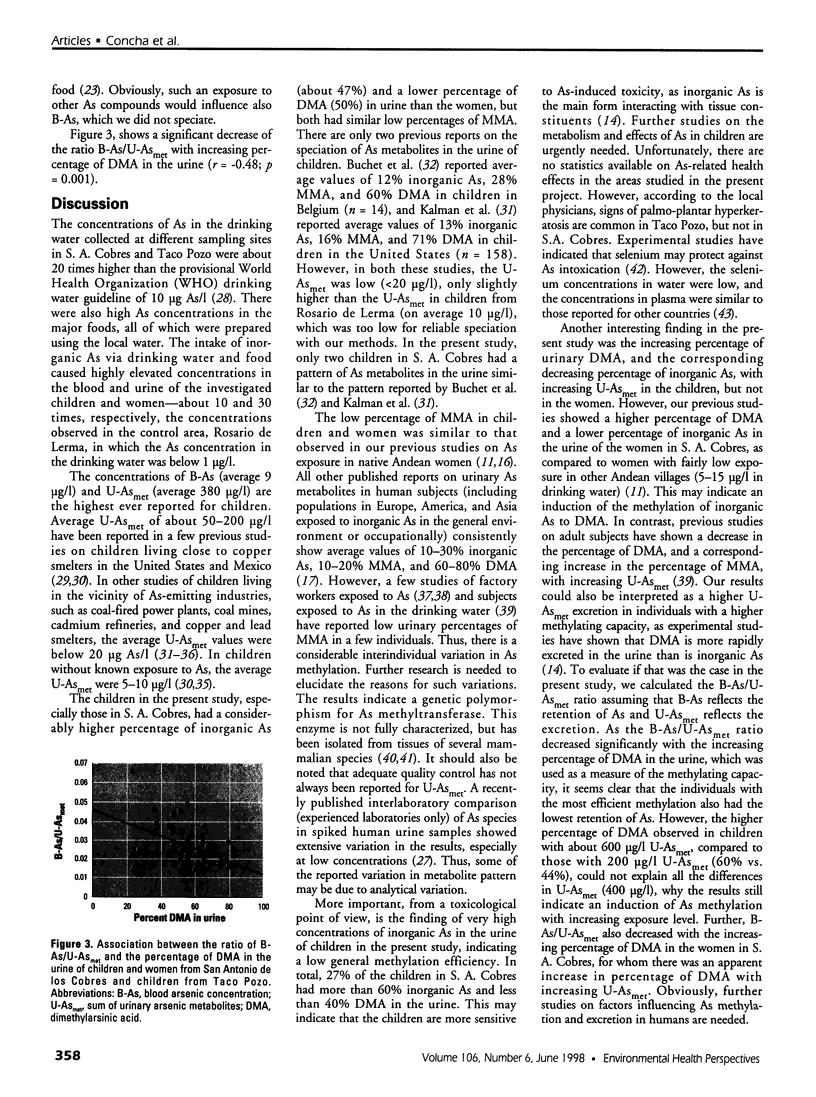
This study concerns the metabolism of inorganic arsenic (As) in children in three villages in northern Argentina: San Antonio de los Cobres and Taco Pozo, each with about 200 microg As/l in the drinking water, and Rosario de Lerma, with 0.65 microg As/l. Findings show that the concentrations of As in the blood and urine of the children in the two As-rich villages were on average 9 and 380 microg/l, respectively, the highest ever recorded for children. The concentrations were about 10 and 30 times higher for blood and urine, respectively, than in Rosario de Lerma. Total As in urine was only slightly higher than the sum of metabolites of inorganic As (U-Asmet), i.e., inorganic As, methylarsonic acid (MMA), and dimethylarsinic acid (DMA); this shows that inorganic As was the main form of As ingested. In contrast to previous studies on urinary metabolites of inorganic As in various population groups, the children and women in the present study excreted very little MMA. Thus, there seems to be a polymorphism for the enzymes (methyltransferases) involved in the methylation of As. Interestingly, the children had a significantly higher percentage of inorganic As in urine than the women, about 50% versus 32%. Also, the percentage of inorganic As in the children is considerably higher than in previous studies on children (about 13% in the two studies available) and adults (about 15-25%) in other population groups. This may indicate that children are more sensitive to As-induced toxicity than adults, as the methylated metabolites bind less to tissue constituents than inorganic As. In the children, the percentage inorganic arsenic in urine decreased, and the percentage of DMA increased with increasing U-Asmet, indicating an induction of As methylation with increasing exposure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder S., Forney D., Kaye W., Paschal D. Arsenic exposure in children living near a former copper smelter. Bull Environ Contam Toxicol. 1987 Jul;39(1):114–121. doi: 10.1007/BF01691798. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Lauwerys R. Role of thiols in the in-vitro methylation of inorganic arsenic by rat liver cytosol. Biochem Pharmacol. 1988 Aug 15;37(16):3149–3153. doi: 10.1016/0006-2952(88)90313-9. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Roels H., Lauwerys R., Bruaux P., Claeys-Thoreau F., Lafontaine A., Verduyn G. Repeated surveillance of exposure to cadmium, manganese, and arsenic in school-age children living in rural, urban, and nonferrous smelter areas in Belgium. Environ Res. 1980 Jun;22(1):95–108. doi: 10.1016/0013-9351(80)90122-x. [DOI] [PubMed] [Google Scholar]
- Cebrián M. E., Albores A., Aguilar M., Blakely E. Chronic arsenic poisoning in the north of Mexico. Hum Toxicol. 1983 Jan;2(1):121–133. doi: 10.1177/096032718300200110. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chen C. W., Wu M. M., Kuo T. L. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992 Nov;66(5):888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha G., Vogler G., Nermell B., Vahter M. Low-level arsenic excretion in breast milk of native Andean women exposed to high levels of arsenic in the drinking water. Int Arch Occup Environ Health. 1998 Feb;71(1):42–46. doi: 10.1007/s004200050248. [DOI] [PubMed] [Google Scholar]
- Crecelius E., Yager J. Intercomparison of analytical methods for arsenic speciation in human urine. Environ Health Perspect. 1997 Jun;105(6):650–653. doi: 10.1289/ehp.97105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulout F. N., Grillo C. A., Seoane A. I., Maderna C. R., Nilsson R., Vahter M., Darroudi F., Natarajan A. T. Chromosomal aberrations in peripheral blood lymphocytes from native Andean women and children from northwestern Argentina exposed to arsenic in drinking water. Mutat Res. 1996 Oct 1;370(3-4):151–158. doi: 10.1016/s0165-1218(96)00060-2. [DOI] [PubMed] [Google Scholar]
- Díaz-Barriga F., Santos M. A., Mejía J. J., Batres L., Yáez L., Carrizales L., Vera E., del Razo L. M., Cebrián M. E. Arsenic and cadmium exposure in children living near a smelter complex in San Luis Potosí, Mexico. Environ Res. 1993 Aug;62(2):242–250. doi: 10.1006/enrs.1993.1109. [DOI] [PubMed] [Google Scholar]
- Foy H. M., Tarmapai S., Eamchan P., Metdilogkul O. Chronic arsenic poisoning from well water in a mining area in Thailand. Asia Pac J Public Health. 1992;6(3):150–152. doi: 10.1177/101053959200600306. [DOI] [PubMed] [Google Scholar]
- Foà V., Colombi A., Maroni M., Buratti M., Calzaferri G. The speciation of the chemical forms of arsenic in the biological monitoring of exposure to inorganic arsenic. Sci Total Environ. 1984 Mar 15;34(3):241–259. doi: 10.1016/0048-9697(84)90066-4. [DOI] [PubMed] [Google Scholar]
- Gottlieb K., Koehler J. R., Tessari J. Non-analytic problems in detecting arsenic and cadmium in children living near a cadmium refinery in Denver, Colorado. J Expo Anal Environ Epidemiol. 1993 Apr-Jun;3(2):139–153. [PubMed] [Google Scholar]
- Hopenhayn-Rich C., Biggs M. L., Smith A. H., Kalman D. A., Moore L. E. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect. 1996 Jun;104(6):620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopenhayn-Rich C., Smith A. H., Goeden H. M. Human studies do not support the methylation threshold hypothesis for the toxicity of inorganic arsenic. Environ Res. 1993 Feb;60(2):161–177. doi: 10.1006/enrs.1993.1024. [DOI] [PubMed] [Google Scholar]
- Hwang Y. H., Bornschein R. L., Grote J., Menrath W., Roda S. Environmental arsenic exposure of children around a former copper smelter site. Environ Res. 1997 Jan;72(1):72–81. doi: 10.1006/enrs.1996.3691. [DOI] [PubMed] [Google Scholar]
- Jensen G. E., Christensen J. M., Poulsen O. M. Occupational and environmental exposure to arsenic--increased urinary arsenic level in children. Sci Total Environ. 1991 Sep;107:169–177. doi: 10.1016/0048-9697(91)90258-g. [DOI] [PubMed] [Google Scholar]
- Levander O. A. Metabolic interrelationships between arsenic and selenium. Environ Health Perspect. 1977 Aug;19:159–164. doi: 10.1289/ehp.7719159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockitch G. Selenium: clinical significance and analytical concepts. Crit Rev Clin Lab Sci. 1989;27(6):483–541. doi: 10.3109/10408368909114596. [DOI] [PubMed] [Google Scholar]
- Norin H., Vahter M. A rapid method for the selective analysis of total urinary metabolites of inorganic arsenic. Scand J Work Environ Health. 1981 Mar;7(1):38–44. doi: 10.5271/sjweh.2568. [DOI] [PubMed] [Google Scholar]
- Tam G. K., Lacroix G. Dry ashing, hydride generation atomic absorption spectrometric determination of arsenic and selenium in foods. J Assoc Off Anal Chem. 1982 May;65(3):647–650. [PubMed] [Google Scholar]
- Tam K. H., Charbonneau S. M., Bryce F., Lacroix G. Separation of arsenic metabolites in dog plasma and urine following intravenous injection of 74As. Anal Biochem. 1978 Jun 1;86(2):505–511. doi: 10.1016/0003-2697(78)90775-3. [DOI] [PubMed] [Google Scholar]
- Tam K. H., Conacher H. B. The suitability of the dry ashing procedure for determination of arsenic in marine samples. J Environ Sci Health B. 1977;12(3):213–227. doi: 10.1080/03601237709372065. [DOI] [PubMed] [Google Scholar]
- Trepka M. J., Heinrich J., Schulz C., Krause C., Popescu M., Wjst M., Wichmann H. E. Arsenic burden among children in industrial areas of eastern Germany. Sci Total Environ. 1996 Feb 9;180(2):95–105. doi: 10.1016/0048-9697(95)04945-2. [DOI] [PubMed] [Google Scholar]
- Vahter M., Concha G., Nermell B., Nilsson R., Dulout F., Natarajan A. T. A unique metabolism of inorganic arsenic in native Andean women. Eur J Pharmacol. 1995 Dec 7;293(4):455–462. doi: 10.1016/0926-6917(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Vahter M., Friberg L., Rahnster B., Nygren A., Nolinder P. Airborne arsenic and urinary excretion of metabolites of inorganic arsenic among smelter workers. Int Arch Occup Environ Health. 1986;57(2):79–91. doi: 10.1007/BF00381375. [DOI] [PubMed] [Google Scholar]
- Vahter M., Lind B. Concentrations of arsenic in urine of the general population in Sweden. Sci Total Environ. 1986 Oct;54:1–12. doi: 10.1016/0048-9697(86)90252-4. [DOI] [PubMed] [Google Scholar]
- Vahter M., Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett. 1987 Jun;37(1):41–46. doi: 10.1016/0378-4274(87)90165-2. [DOI] [PubMed] [Google Scholar]
- Yamamura Y., Yamauchi H. Arsenic metabolites in hair, blood and urine in workers exposed to arsenic trioxide. Ind Health. 1980;18(4):203–210. doi: 10.2486/indhealth.18.203. [DOI] [PubMed] [Google Scholar]
- Zakharyan R. A., Wildfang E., Aposhian H. V. Enzymatic methylation of arsenic compounds. III. The marmoset and tamarin, but not the rhesus, monkeys are deficient in methyltransferases that methylate inorganic arsenic. Toxicol Appl Pharmacol. 1996 Sep;140(1):77–84. doi: 10.1006/taap.1996.0199. [DOI] [PubMed] [Google Scholar]
- Zakharyan R., Wu Y., Bogdan G. M., Aposhian H. V. Enzymatic methylation of arsenic compounds: assay, partial purification, and properties of arsenite methyltransferase and monomethylarsonic acid methyltransferase of rabbit liver. Chem Res Toxicol. 1995 Dec;8(8):1029–1038. doi: 10.1021/tx00050a006. [DOI] [PubMed] [Google Scholar]
- Zaldívar R., Guillier A. Environmental and clinical investigations on endemic chronic arsenic poisoning in infants and children. Zentralbl Bakteriol Orig B. 1977 Oct;165(2):226–234. [PubMed] [Google Scholar]