Abstract
An in vitro model was used to compare the effects of linezolid, clindamycin, and penicillin, alone and in combination, on streptococcal pyrogenic exotoxin A (SPE A) release against virulent group A streptococci (GAS). All regimens exhibited lower (P < 0.05) SPE A release at 1 h than those with penicillin alone. Linezolid and clindamycin, alone or in combination with penicillin, may optimize the treatment of GAS infections by reducing bacterial burden and exotoxin release.
Management of severe infections due to Streptococcus pyogenes requires aggressive antibiotic treatment and supportive measures. S. pyogenes continues to be susceptible to penicillin and other β-lactam antibiotics. Although penicillin remains the drug of choice for uncomplicated S. pyogenes infections, the overall response to treatment with penicillin has decreased and can be associated with high morbidity and mortality (4, 5, 17, 18). Clindamycin, whether used in monotherapy or in combination with antimicrobial agents, has been shown to be beneficial in in vitro evaluations and in the clinical treatment of invasive S. pyogenes infections (5, 11, 18). Linezolid is active against S. pyogenes and is similar to clindamycin in that it inhibits protein synthesis by binding to the 50S ribosomal subunit and exhibits a relatively long postantibiotic effect (3, 22, 23). In an effort to characterize the effects of antimicrobial agents on bacterial killing and the exotoxin release of S. pyogenes, we simulated regimens of linezolid, penicillin, and clindamycin, alone and in combination, by using an in vitro model.
A known streptococcal pyrogenic exotoxin A (SPE A)-producing S. pyogenes isolate (MGAS166; hereafter referred to as 166) and its hypervirulent mutant (MGAS2616; hereafter referred to as 2616) were acquired from Cary Engleberg at the University of Michigan (Ann Arbor, Mich.) (8, 13). Susceptibility testing was determined by broth microdilution according to NCCLS guidelines (14).
Todd-Hewitt broth supplemented with 0.5% yeast extract (Difco Laboratories, Detroit, Mich.) was utilized for implementation of the previously described in vitro pharmacodynamic models (2). Simulated regimens included linezolid (Pharmacia, Kalamazoo, Mich.) at 600 mg every 12 h, clindamycin (Sigma Chemical Co., St. Louis, Mo.) at 900 mg every 8 h, and penicillin G (Sigma Chemical Co.) at 4,000,000 U every 4 h. In addition, combinations of penicillin and clindamycin, penicillin and linezolid, and clindamycin and linezolid were evaluated. A peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Ill.) was used to simulate the half-lives of linezolid (6 h), clindamycin (3 h), and penicillin G (1 h). For the combination regimens, an additional supplemental compartment was used to compensate for different half-lives (1). Growth control simulations were performed at the shortest half-life of the tested antimicrobial agents (1 h). Each model was placed in a water bath and maintained at 37°C for the entire 24-h study period. Model experiments were performed in duplicate to ensure reproducibility.
Samples were obtained at 0, 0.5, 2, 4, 8, and 24 h for bacterial quantification and antibiotic concentrations. Bacteria were quantified by plating serial saline dilutions of samples onto tryptic soy agar with 5% sheep blood (Difco Laboratories), followed by overnight incubation. Antimicrobial carryover was minimized with serial dilutions. Concentrations of linezolid were determined by a validated high-performance liquid chromatography assay. The standards ranged from 0.5 to 30 mg/ml, and the intraday coefficient of variation percentages across all standards were 1.04 to 4.39%. Concentrations of penicillin G and clindamycin were determined by microbioassay with Bacillus subtilis ATCC 6633 and Micrococcus luteus ATCC 9341, respectively (2). Correlation coefficients for both assays were >0.95, and the intraday coefficient of variation percentage was less than 6.25% across all standards. The lower limits of detection for the microbioassays were 0.0625 μg/ml. Additional samples were drawn from the model at 0, 1, and 6 h for SPE A quantification. SPE A was measured by an enzyme-linked immunosorbent assay technique previously described by K. Miwa of Pioneering Research Laboratories, Toray Industries, Inc. (Otsu, Shiga, Japan) (12). Optical density demonstrated a linear relationship (r > 0.999), with standard concentrations of 0 to 800 pg/ml and a lower limit of detection of 1.40 pg/ml. Changes in log10 CFU per milliliter and in SPE A concentrations (picograms per milliliter) for each simulation were compared by using one-way analysis of variance with Tukey's posthoc test. A P value of ≤0.05 was considered significant.
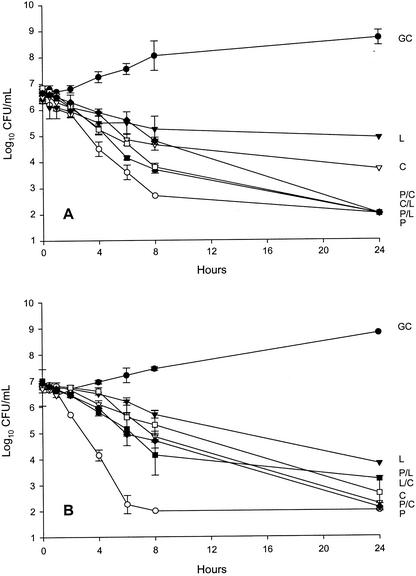
Linezolid, clindamycin, and penicillin MICs for strains 166 and 2616 were 2.0 and 1.0, 0.125 and 0.125, and 0.008 and 0.008 μg/ml, respectively. Regimen simulations were within 10% of target concentrations and half-lives. Figure 1 portrays the activity of all regimens against strains 166 and 2616, respectively. Against strain 166, the time to 99.9% kill (3-log reduction in CFU per milliliter) was achieved within 6 h for penicillin alone, 8 h for penicillin-containing combinations, and 24 h for the linezolid-clindamycin combination. A 2- to 2.5-log reduction in CFU per milliliter was achieved within 24 h by linezolid and clindamycin alone. Against strain 2616, penicillin alone achieved 99.9% kill within 4 h and reached the limit of detection by 8 h. All other regimens achieved 99.9% kill by 24 h. No regrowth was observed.
FIG. 1.
In vitro pharmacodynamic activity of study antimicrobial regimens against S. pyogenes 166 (A) and 2616 (B). Since no statistically significant differences were observed between growth controls that were run at the three drug elimination rates, the growth curve for the fastest elimination rate (1 h) is depicted for each respective study isolate. GC, growth control (•); P, penicillin (○); C, clindamycin (▿); L, linezolid (▾); P/C, penicillin plus clindamycin (♦); P/L, penicillin plus linezolid (▪); L/C or C/L, linezolid plus clindamycin (□).
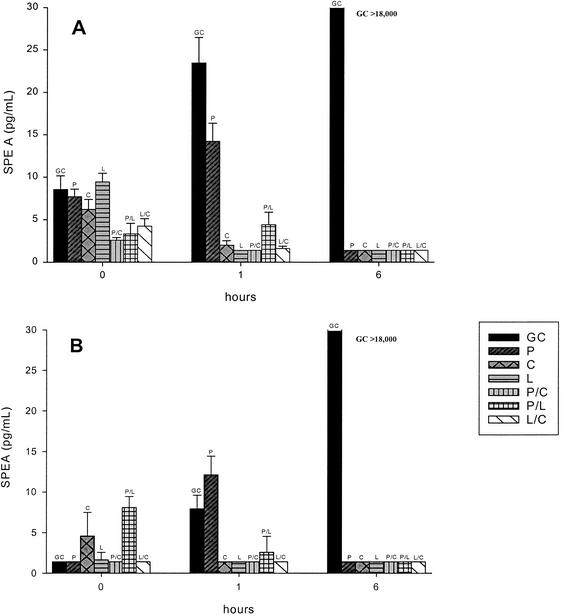
Figure 2 portrays the SPE A quantification results for S. pyogenes 166 and 2616, respectively. Clindamycin (2.00 and 1.40 pg/ml), linezolid (1.40 and 1.40 pg/ml), penicillin-clindamycin (1.40 and 1.40 pg/ml), penicillin-linezolid (4.41 and 2.57), and linezolid-clindamycin (1.61 and 1.40 pg/ml) each displayed significantly (P < 0.05) lower SPE A levels at 1 h than penicillin (14.23 and 12.11 pg/ml) against both study isolates (166 and 2616, respectively). All antibiotics, alone and in combination, displayed significantly lower (P < 0.05) SPE A levels at 6 h (1.40 pg/ml) than the growth control (>18,000 pg/ml). Standard deviations for all quantified samples were <2.3 pg/ml.
FIG. 2.
SPE A quantification (pg/ml) with S. pyogenes 166 (A) and 2616 (B). GC, growth control; P, penicillin; C, clindamycin; L, linezolid; P/C, penicillin plus clindamycin; P/L, penicillin plus linezolid; L/C, linezolid plus clindamycin. The lower limit of this assay was 1.4 pg/ml.
Numerous in vitro and in vivo studies have investigated the efficacy of clindamycin in the treatment of group A streptococcus infections (5, 15, 18, 20, 21). Stevens et al. investigated the relative efficacies of penicillin, clindamycin, and erythromycin in a mouse model of myositis due to S. pyogenes (19). They suggested that the enhanced efficacy of clindamycin could be related to several inherent properties, including activity unaffected by inoculum size or growth stage, a long postantibiotic effect, and the inhibition of S. pyogenes virulence factors, particularly M protein (19). M proteins contribute to exotoxin release and resistance to opsonization and subsequent phagocytosis (7, 10, 16, 18). Furthermore, Gemmell et al. have determined that, in the presence of varying concentrations of clindamycin and linezolid, enhanced opsonization and phagocytosis of S. pyogenes are seen (5, 6).
In our experiments, clindamycin and linezolid displayed less antibacterial activity than penicillin; however, these experiments were conducted in an in vitro model and the effect of polymorphonuclear lymphocytes and macrophages could not be evaluated. In vivo, the bacterial susceptibility of protein synthesis inhibitors such as clindamycin and linezolid is improved due to the increased phagocytosis (5, 6). Combination regimens exhibited similar or enhanced bacterial density reductions compared to the most active respective monoregimens. The slow bactericidal activity seen with the penicillin combination regimens compared to that seen with penicillin alone may be due to the inhibition of synthesis of penicillin binding proteins by the protein synthesis inhibitors linezolid and clindamycin (20, 24). No antagonism was observed with these regimens. The effect of staggered dosing administration times with combination regimens was not tested in these experiments. This might have an impact on the release of SPE A and should be evaluated in future studies.
This study was unique in that we utilized in vitro models to quantify SPE A levels in relation to antimicrobial therapy and included linezolid simulations (2, 9). As opposed to the utilization of fixed concentrations in traditional time-kill experiments, these models permit us to characterize SPE A release in response to changing antimicrobial concentrations according to population pharmacokinetics. We were able to demonstrate significant decreases in SPE A production at 1 h for all regimens containing clindamycin or linezolid compared to that for penicillin alone, despite their respective contrast in the bacterial kill. In conclusion, our study demonstrated that clindamycin and linezolid, alone and in combination with penicillin, significantly reduce the early release of SPE A. The treatment of necrotizing fasciitis and streptococcal toxic shock due to group A streptococci with linezolid and clindamycin, alone or in combination with penicillin, may optimize the treatment of these severe and acute infections.
Acknowledgments
This project was supported by grants from Pharmacia Pharmaceuticals.
We are grateful for C. Engleberg's contribution of organisms and to K. Miwa for performance of exotoxin assays. We are grateful to Charles Peloquin from the Division of Infectious Diseases at the National Jewish Medical & Research Center (Denver, Colo.) for the performance of linezolid assays.
REFERENCES
- 1.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 2.Cappelletty, D. M., and M. J. Rybak. 1996. Bactericidal activities of cefprozil, penicillin, cefaclor, cefixime, and loracarbef against penicillin-susceptible and -resistant Streptococcus pneumoniae in an in vitro pharmacodynamic infection model. Antimicrob. Agents Chemother. 40:1148-1152. [DOI] [PMC free article] [PubMed]
- 3.Dresser, L. D., and M. J. Rybak. 1998. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 18:456-462. [PubMed] [Google Scholar]
- 4.Gemmell, C. G., and M. McLeod. 1992. The effect of roxithromycin on the virulence of gram-positive cocci. Diagn. Microbiol. Infect. Dis. 15:67S-70S. [DOI] [PubMed]
- 5.Gemmell, C. G., P. K. Peterson, D. Schmeling, Y. Kim, J. Mathews, L. Wannamaker, and P. G. Quie. 1981. Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. J. Clin. Investig. 67:1249-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gemmell, C. G., and C. W. Ford. 2002. Virulence factor expression by gram-positive cocci exposed to subinhibitory concentrations of linezolid. J. Antimicrob. Chemother. 50:665-672. [DOI] [PubMed] [Google Scholar]
- 7.Hauser, A. R., D. L. Stevens, E. L. Kaplan, and P. M. Schlievert. 1991. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J. Clin. Microbiol. 29:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heath, A., V. J. DiRita, N. L. Barg, and N. C. Engleberg. 1999. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang, S. L., and M. J. Rybak. 1997. In vitro bactericidal activity of quinupristin/dalfopristin alone and in combination against resistant strains of Enterococcus species and Staphylococcus aureus. J. Antimicrob. Chemother. 39(Suppl. A):33-39. [DOI] [PubMed] [Google Scholar]
- 10.Kline, J. B., and C. M. Collins. 1996. Analysis of the superantigenic activity of mutant and allelic forms of streptococcal pyrogenic exotoxin A. Infect. Immun. 64:861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, P. K., and P. M. Schlievert. 1989. Quantification and toxicity of group A streptococcal pyrogenic exotoxins in an animal model of toxic shock syndrome-like illness. J. Clin. Microbiol. 27:1890-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miwa, K., M. Fukuyama, R. Sakai, S. Shimizu, N. Ida, M. Endo, and H. Igarashi. 2000. Sensitive enzyme-linked immunosorbent assays for the detection of bacterial superantigens and antibodies against them in human plasma. Microbiol. Immunol. 44:519-523. [DOI] [PubMed] [Google Scholar]
- 13.Musser, J. M., V. Kapur, S. Kanjilal, U. Shah, D. M. Musher, N. L. Barg, K. H. Johnston, P. M. Schlievert, J. Henrichsen, D. Gerlach, R. M. Rakita, A. Tanna, B. D. Cookson, and J. C. Huang. 1993. Geographic and temporal distribution and molecular characterization of two highly pathogenic clones of Streptococcus pyogenes expressing allelic variants of pyrogenic exotoxin A (Scarlet fever toxin). J. Infect. Dis. 167:337-346. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1993. Methods for dilution antimicrobial susceptibility test for bacteria to grow aerobically, 3rd ed. Approved standard M7-A3. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 15.Roggiani, M., J. A. Stoehr, B. A. B. Leonard, and P. M. Schlievert. 1997. Analysis of toxicity of streptococcal pyrogenic exotoxin A mutants. Infect. Immun. 65:2868-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sriskandan, S., D. Moyes, L. K. Buttery, T. Krausz, T. J. Evans, J. Polak, and J. Cohen. 1996. Streptococcal pyrogenic exotoxin release, distribution, and role in a murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes. J. Infect. Dis. 173:1399-1407. [DOI] [PubMed] [Google Scholar]
- 17.Sriskandan, S., D. Moyes, K. Buttery, J. Wilkinson, T. J. Evans, J. Polak, and J. Cohen. 1997. The role of nitric oxide in experimental murine sepsis due to pyrogenic exotoxin A-producing Streptococcus pyogenes. Infect. Immun. 65:1767-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens, D. L., A. E. Gibbons, R. Bergstrom, and V. Winn. 1988. The eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 158:23-28. [DOI] [PubMed] [Google Scholar]
- 19.Stevens, D. L., K. A. Maier, and J. E. Mitten. 1987. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob. Agents Chemother. 31:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens, D. L., K. J. Madaras-Kelly, and D. M. Richards. 1998. In vitro antimicrobial effects of various combinations of penicillin and clindamycin against four strains of Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:1266-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens, D. L., S. Yan, and A. E. Bryant. 1993. Penicillin-binding protein expression at different growth stages determine penicillin efficacy in vitro and in vivo: an explanation for the inoculum effect. J. Infect. Dis. 167:1401-1405. [DOI] [PubMed] [Google Scholar]
- 22.Swaney, S. M., H. A. Aoki, M. C. Ganoza, and D. L. Shinabarger. 1998. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabau, F., R. Fernandez-Robles, J. Linares, R. Martin, and F. Soriano. 2001. In vitro activity of linezolid and 11 other antimicrobials against 566 clinical isolates and comparison between NCCLS microdilution and Etest methods. J. Antimicrob. Chemother. 47:675-680. [DOI] [PubMed] [Google Scholar]
- 24.Yan, S., G. A. Bohach, and D. L. Stevens. 1994. Persistent acylation of high-molecular-weight penicillin-binding proteins by penicillin induces the postantibiotic effect in Streptococcus pyogenes. J. Infect. Dis. 170:609-614. [DOI] [PubMed] [Google Scholar]