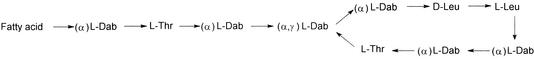Abstract
The pharmacokinetics of colistin was investigated using specific high-performance liquid chromatography (HPLC) to measure the concentrations of colistin and colistin A and B in plasma and urine in five rats after administration of an intravenous bolus of 1 mg of colistin sulfate/kg of body weight. There were differences in the pharmacokinetic behaviors of unbound colistin A and B. This is the first report of the use of HPLC to study the pharmacokinetics of colistin and its two major components.
Over the past several decades, Pseudomonas aeruginosa has become the most frequently detected pathogen in infections of the lower respiratory tract in patients with cystic fibrosis (11, 23). Given its notorious propensity for rapidly developing resistance against common antimicrobial agents (9, 20), P. aeruginosa is being recognized as a public health threat and presents an increasing clinical problem (17, 24). More recently, there has been an increasing awareness of the value of parenteral colistin for treating infections caused by this organism (3).
Colistin (polymyxin E) is most notable for its activity against the multidrug-resistant P. aeruginosa with slow development of resistance. It is a cationic polypeptide antibiotic produced by Bacillus polymyxa subsp. colistinus (13) and is bactericidal to gram-negative bacteria, with a detergent-like mechanism (10). Colistin (Fig. 1) is a complex mixture of at least 30 different components (18, 19). The two major components, colistin A and B, differ only in the fatty acid side chain (Fig. 1). Different pharmaceutical preparations of colistin may differ in the ratios of the major components (7). There are two forms of colistin available for clinical use: colistin sulfate (for topical use) and colistin methanesulfonate (for parenteral and aerosol therapy).
FIG. 1.
Chemical structures of colistin A and B. Fatty acid: 6-methyloctanoic acid for colistin A and 6-methylheptanoic acid for colistin B. Thr, threonine; Leu, leucine; Dab, α,γ-diaminobutyric acid. α and γ indicate the respective −NH2s involved in the peptide linkage.
The use of colistin is limited by its potential nephrotoxicity, neurotoxicity, and hypersensitivity (12, 25). While colistin has been available clinically for decades, its use diminished following the availability of potentially less toxic aminoglycosides and other antipseudomonal agents. However, recent reports of critical life-threatening infections caused by multidrug-resistant P. aeruginosa have shown that it may be of value to revisit this potentially useful antibiotic (4, 8, 14, 16).
There are only a few reports on the pharmacokinetics of colistin. These have arisen from studies with intravenous administration of colistin sulfate in the calf (22, 26, 27) and intramuscularly in the dog (2). All of these studies used microbiological methods to quantify colistin in plasma and urine. Unfortunately, these methods lack the ability to distinguish the two major components of colistin. To the best of our knowledge, the pharmacokinetics of colistin has not been studied using high-performance liquid chromatography (HPLC). The objective of this study was to investigate the pharmacokinetics of colistin in rats, after administration of an intravenous bolus of 1 mg of colistin sulfate/kg of body weight, with concentrations measured by a specific HPLC method developed previously in our laboratory (15). An improved understanding of the pharmacokinetics of colistin should allow it to be used more safely and efficaciously.
All experiments performed with rats were in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (sixth edition, 1997). The animal protocols were approved by the Animal Ethics Committee of the Institute of Medical and Veterinary Sciences (Adelaide, South Australia, Australia). Five male Sprague-Dawley rats (300 to 350 g) (Gilles Plains Animal House, Adelaide, Australia) were housed in an animal care facility and were acclimated for a minimum of 5 days prior to experimentation. At least 2 days prior to the administration of colistin sulfate, the jugular vein of the rat was cannulated with silicone rubber tubing. The cannula (filled with heparinized saline [10 U/ml]) was exteriorized in the dorsal neck region. After recovery from anesthesia, each rat was housed individually in a rat metabolic cage with free access to food and water.
On the day of the experiment, colistin sulfate (Sigma, St. Louis, Mo.) (1 mg/kg in 300 μl of sterile saline) was injected in less than 20 s into the jugular vein and the cannula was flushed with 1 ml of heparinized saline. There were no visual signs of toxicity. Studies conducted in vitro indicated that less than 0.02% of the dose of colistin sulfate remained in the cannula after the flush. Colistin used in the dosing solution was shown by HPLC analysis with UV detection (7) to comprise 86.5% colistin A and B. Blood (400 μl) was collected prior to administration of colistin and at 10, 20, 30, 60, 90, 120, 180, and 240 min thereafter. When collecting samples, the first 100 μl of blood was withdrawn and kept in the syringe. After collecting the actual sample with another syringe, the 100 μl of blood in the first syringe was returned to the rat together with 400 μl of heparinized saline to ensure that the cannula was flushed and a constant intravascular volume was maintained. Blood samples were placed into 1.5-ml centrifuge tubes containing 5 μl of heparin (1 U/μl) and centrifuged (1,000 × g, 10 min) immediately, and the plasma was stored at −20°C pending the assay. Urine was collected from the metabolic cage prior to administration of colistin and over intervals of 0 to 4, 4 to 8, and 8 to 12 h thereafter. Urine samples were stored at −20°C pending the assay.
Concentrations of colistin sulfate in plasma and urine were determined by HPLC (15), with minor modifications. These included changes to the volume of plasma and urine samples (200 μl), the amount of internal standard (10 μl of 2.5 mg of netilmicin sulfate/liter), the solid-phase-extraction C18 cartridges (Sep-Pak, Waters, Mass.), the injection volume for HPLC (30 μl), and the mobile phase. The latter, consisting of acetonitrile-tetrahydrofuran-water (50:30:20 [vol/vol/vol]), generated a shortened overall run time of 18 min with retention times for derivatized netilmicin, colistin B, and colistin A of approximately 10, 12, and 14 min, respectively. For the plasma assay, calibration curves were constructed with concentrations of colistin sulfate ranging from 0.1 to 4.0 mg/liter. The intraday accuracy and reproducibility were 0.25 ± 0.02 mg/liter and 3.74 ± 0.11 mg/liter (n = 6) for colistin sulfate at 0.25 mg/liter and 3.75 mg/liter, respectively, and the corresponding interday accuracy and reproducibility were 0.26 ± 0.02 mg/liter and 3.74 ± 0.12 mg/liter (n = 6). For urine, calibration curves were constructed with concentrations of colistin sulfate ranging from 0.1 to 0.8 mg/liter. The intraday accuracy and reproducibility were 0.20 ± 0.01 mg/liter and 0.64 ± 0.02 mg/liter (n = 6) for colistin sulfate at 0.20 mg/liter and 0.60 mg/liter, respectively, while the respective interday accuracy and reproducibility were 0.21 ± 0.01 mg/liter and 0.60 ± 0.03 mg/liter (n = 6).
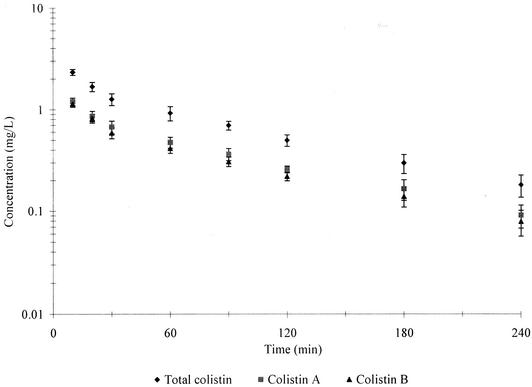
Colistin A and B were chosen as the quantitation markers of colistin. Because all of the components of colistin have analogous structures (18, 19), it was assumed that they have similar molecular UV absorbance. Therefore, the sum of colistin A and B in the dose of colistin was evaluated by HPLC with UV detection (7) and found to account for 86.5% of the total peak areas (data not shown). Hence, given that the sample of colistin sulfate administered was also used as the reference standard for quantitation, the concentrations of colistin A and B (as the sulfate salts) were calculated from the concentrations of colistin and the ratios of the areas of the respective chromatographic peaks and their summed areas. Figure 2 shows the mean (±standard deviation [SD]) concentrations of colistin and colistin A and B (as the sulfate) in plasma for five rats as a function of time. There was a short initial distribution phase followed by a slower log-linear decline in the concentrations over approximately 1 order of magnitude for colistin and colistin A and B. The concentrations of colistin in plasma were of the same magnitude as those measured in calves by a microbiological assay (26). However, a third disposition phase (as seen for calves) (26) was not observed in the present study with rats.
FIG. 2.
Mean (±SD) plasma concentration versus time profiles for colistin and colistin A and B (all as the sulfate) in rats (n = 5).
Noncompartmental analysis of colistin and colistin A and B (as the sulfate salts) in plasma was performed using WinNonlin (Version 3.0; Pharsight Corp., Cary, N.C.). The terminal rate constant (λz) was calculated by linear least-squares regression analysis using the last five log-transformed plasma concentration-versus-time points. The area under the concentration versus time curve to infinite time (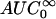 ) was calculated by the linear trapezoidal rule to the last quantifiable plasma concentration (Clast), and the area extrapolated beyond this point was calculated as Clast/λz. The mean extrapolated AUC for the five rats represented 10 ± 4% of the
) was calculated by the linear trapezoidal rule to the last quantifiable plasma concentration (Clast), and the area extrapolated beyond this point was calculated as Clast/λz. The mean extrapolated AUC for the five rats represented 10 ± 4% of the 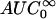 (n = 5). The other pharmacokinetic parameters were calculated with the following equations:
(n = 5). The other pharmacokinetic parameters were calculated with the following equations:
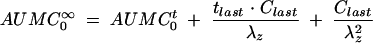 |
(1) |
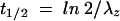 |
(2) |
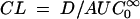 |
(3) |
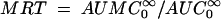 |
(4) |
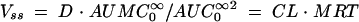 |
(5) |
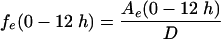 |
(6) |
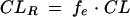 |
(7) |
where Ae (0-12 h) is the amount of unchanged drug recovered in urine in 12 h, 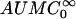 is the area under the first moment curve to infinite time,
is the area under the first moment curve to infinite time, 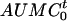 is the area under the first moment curve to tlast, fe (0-12 h) is the fraction of the dose recovered in unchanged form in urine in 12 h, t1/2 is the terminal half-life, CL is the total body clearance, CLR is the renal clearance, D is the dose, MRT is the mean residence time, tlast is the time of the last quantifiable concentration, and Vss is the volume of distribution at steady state.
is the area under the first moment curve to tlast, fe (0-12 h) is the fraction of the dose recovered in unchanged form in urine in 12 h, t1/2 is the terminal half-life, CL is the total body clearance, CLR is the renal clearance, D is the dose, MRT is the mean residence time, tlast is the time of the last quantifiable concentration, and Vss is the volume of distribution at steady state.
The pharmacokinetic parameters for colistin and colistin A and B are presented in Table 1. There were no significant differences in the CL (P > 0.25), Vss (P > 0.29), and t1/2 (P > 0.89) between colistin A and B. Overall, the coefficients of variation for these parameters were less than 19% (Table 1). During the first 12 h after dosing, 0.18 ± 0.14% (n = 5) of the dose was recovered in urine as unchanged colistin, the majority (97.3%) of which was recovered during the first 4 h. There was no colistin detected in urine collected in the 8 to 12 h interval. The CLR of colistin was 0.010 ± 0.008 ml/min/kg of body weight. No significant difference (P > 0.13) was observed in the 12-h urinary recovery of colistin A (0.13 ± 0.08%) and colistin B (0.23 ± 0.19%). The low urinary recovery of unchanged colistin indicates a very low renal clearance (0.010 ± 0.008 ml/min/kg). This value is far lower than the anticipated clearance of colistin by glomerular filtration (2.3 ml/min/kg), which can be calculated as the product of the unbound fraction of colistin (fu = 0.44) and a glomerular filtration rate of approximately 5.2 ml/min/kg in rats (6). There must, therefore, be very extensive net reabsorption of colistin from tubular urine back into blood. The lack of difference between colistin A and B in urinary recovery is not surprising, given the variability about the mean values. The cumulative urinary recovery was very similar to the results reported for dogs at a dosage of 1.1 mg of colistin sulfate/kg of body weight, in which 0.13 ± 0.09% of the dose was recovered in urine in 12 h (2).
TABLE 1.
Mean pharmacokinetic parameters ± SD for colistin and colistin A and B in rats (n = 5)
| Drug | CL (ml/min/kg) | Vss (ml/kg) | MRT (min) | t1/2 (min) |
|---|---|---|---|---|
| Colistin | 5.2 ± 0.4 | 496 ± 60 | 94.9 ± 12.3 | 74.6 ± 13.2 |
| Colistin A | 5.1 ± 0.5 | 498 ± 70 | 98.1 ± 15.9 | 77.1 ± 12.8 |
| Colistin B | 5.3 ± 0.5 | 509 ± 64 | 96.1 ± 17.2 | 77.4 ± 14.8 |
The total body clearance of colistin ranged from 4.5 to 6.1 ml/min/kg and, as discussed above, renal clearance (0.010 ± 0.008 ml/min/kg) was only a very small component. Therefore, the majority of the total body clearance must be via nonrenal pathways. Comparison of the magnitude of the nonrenal clearance (essentially the same as the total body clearance) and normal hepatic blood flow in the rat (72 to 95 ml/min/kg [5]) indicates that colistin must have a very low hepatic extraction ratio. Abe et al. (1) administered colistin methanesulfonate intravenously to rabbits and used thin-layer chromatography and a reference standard to tentatively identify a metabolite of colistin, colistin-N-glucuronide, in the urine (1.7% of the dose) and the bile (6.7% of the dose). However, there has not been any report on biliary excretion following a dose of colistin sulfate. Further studies will be needed to determine the routes for the elimination and metabolism of colistin.
Protein binding of colistin was studied by equilibrium dialysis at concentrations spanning the range of values observed in vivo in rat plasma. The perspex dialysis cell unit contained two reservoirs (750-μl volume in each) separated by a Spectra/Por-2 membrane (Spectrum Laboratories, Inc., Rancho Dominguez, Calif.). Fresh plasma pooled from four drug-free rats was adjusted to pH 7.4 with 0.5 M hydrochloric acid and spiked with colistin sulfate at concentrations of 4.0, 8.0, and 12.0 mg/liter. Two replicates at each concentration were incubated at 37°C for 30 min. A portion (600 μl) of the plasma (donor) was dialyzed against the same volume of isotonic phosphate buffer (pH 7.4, 0.067 M) (acceptor) at 37°C for 12 h. Preliminary experiments showed that 12 h was sufficient to achieve equilibrium of colistin between the two chambers. Samples of plasma and buffer were removed from each reservoir and stored at −20°C until analysis. The fraction of drug unbound in plasma (fu) was calculated as follows: (acceptor colistin concentration)/(donor colistin concentration). Values of fu were 0.44, 0.45, and 0.43 at equilibrium concentrations of 1.5, 3.4, and 6.0 mg/liter, respectively. The fu values for colistin A (0.35, 0.36, and 0.36 at equilibrium concentrations for colistin sulfate of 1.5, 3.4, and 6.0 mg/liter, respectively) were significantly (P < 0.006) less than those for colistin B (0.53, 0.52, and 0.51, respectively).
Extensive nonspecific binding (>99% at 10 mg/liter) to a commonly used membrane (Amicon regenerated cellulose YM-10) precluded the use of ultrafiltration. For colistin, a value of about 55 to 57% bound in rat plasma is similar to values determined in plasma from dogs (2) and calves (27), both by equilibrium dialysis at 4°C for 24 h (2) and 48 h (27). The higher binding for colistin A is most likely the result of its longer chain fatty acid (6-methyloctanoic acid) compared with colistin B (6-methylheptanoic acid), since this is the only difference in the structures between the two molecules (Fig. 1).
While the substantial difference in the unbound fractions between colistin A and B is interesting and somewhat surprising, it conceals other apparent differences in the pharmacokinetic parameters for these very closely related compounds. For both total body clearance and volume of distribution, it is possible to calculate the corresponding parameters with reference to the unbound drug in plasma by dividing each parameter by the respective fu value (0.36 for colistin A and 0.52 for colistin B). Thus, while the total body clearance levels of colistin A and B were comparable (mean values of 5.1 and 5.3 ml/min/kg; Table 1), corresponding clearances with reference to unbound concentration in plasma were approximately 14.2 and 10.2 ml/min/kg. The respective values for clearance of unbound colistin A and B suggest marked differences in the removal of the two unbound components from plasma by processes which presumably distinguish them on the basis of their differences in the hydrophobicity of their fatty acids (Fig. 1). Similarly, the volumes of distribution with reference to unbound drug in plasma were 1,383 and 979 ml/kg for colistin A and B, respectively, although the corresponding values referenced to total concentration were very similar (498 and 509 ml/kg; Table 1). From the respective values for the volume of distribution of unbound drug in plasma, it is evident that colistin A (containing the longer chain fatty acid) must be more extensively bound in tissues than colistin B. It is interesting that the modest difference in the structures of colistin A and B (arising from the presence or absence of one CH2 in the fatty acid) also leads to a substantial difference in retention time on a reversed-phase HPLC column, as observed by us (data not shown) and others (7, 18, 19); colistin A is more strongly retained in such a chromatographic system. Clearly, the subtle difference in the structures of these two molecules (molecular weight difference of only 14 in a total of approximately 1,163) leads to substantial differences in not only chromatographic behavior but also disposition in the body.
Recently, a pharmacokinetic study was conducted in patients with cystic fibrosis following the intravenous administration of colistin methanesulfonate (21). Concentrations of drug in plasma, urine, and sputum were measured by HPLC after derivatization with dansyl chloride. However, colistin methanesulfonate has the potential to hydrolyze to colistin in aqueous medium. Results from our laboratory have shown that 31.2% of colistin methanesulfonate in human plasma was hydrolyzed to colistin in 4 h at 37°C in vitro (17a). The derivatizing method used by Reed et al. (21) included heating of plasma samples at 54°C for 2 h, which is most likely to have increased the hydrolysis of colistin methanesulfonate. Therefore, the concentrations of colistin might have been elevated spuriously by hydrolysis of colistin methanesulfonate present in plasma at the time of collection from the patients.
In conclusion, this is the first report using an HPLC method specific for colistin (and colistin A and B) in plasma and urine to examine the pharmacokinetics of colistin following intravenous administration of colistin sulfate. While the total body clearance and volume of distribution with reference to total drug in plasma were not significantly different between colistin A and B, there were differences in these parameters calculated with reference to unbound drug in plasma which may be attributable to a difference in hydrophobicity between the two molecules. Only a very small fraction of the dose was recovered in urine, and the value for renal clearance suggests extensive tubular reabsorption of colistin by the kidney.15a
REFERENCES
- 1.Abe, M., K. Shimizu, M. Ouchi, and T. Matsumoto. 1976. Studies on sodium colistin methane sulfonate (CL-M) on metabolism in the urine and bile of rabbits. Chemotherapy 24:1592-1596. [Google Scholar]
- 2.Al-Khayyat, A. A., and A. L. Aronson. 1973. Pharmacologic and toxicologic studies with the polymyxins. II. Comparative pharmacologic studies of the sulfate and methanesulfonate salts of polymyxin B and colistin in dogs. Chemotherapy 19:82-97. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, D., and D. Stableforth. 2000. The treatment of respiratory pseudomonas infection in cystic fibrosis: what drug and which way? Drugs 60:1053-1064. [DOI] [PubMed] [Google Scholar]
- 4.Beringer, P. 2001. The clinical use of colistin in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 7:434-440. [DOI] [PubMed] [Google Scholar]
- 5.Birnie, J. H., and J. Grayson. 1952. Observations on temperature distribution and liver blood flow in the rat. J. Physiol. 116:189-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharmacol. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 7.Decolin, D., P. Leroy, A. Nicolas, and P. Archimbault. 1997. Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J. Chromatogr. Sci. 35:557-564. [DOI] [PubMed] [Google Scholar]
- 8.Evans, M. E., D. J. Feola, and R. P. Rapp. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960-967. [DOI] [PubMed] [Google Scholar]
- 9.Hanberger, H., D. Diekema, A. Fluit, R. Jones, M. Struelens, R. Spencer, and M. Wolff. 2001. Surveillance of antibiotic resistance in European ICUs. J. Hosp. Infect. 48:161-176. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch, C., and N. Hoiby. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065-1069. [DOI] [PubMed] [Google Scholar]
- 12.Koch-Weser, J., V. W. Sidel, E. B. Federman, P. Kanarek, D. C. Finer, and A. E. Eaton. 1970. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann. Intern. Med. 72:857-868. [DOI] [PubMed] [Google Scholar]
- 13.Koyama, Y., A. Kurosasa, A. Tsuchiya, and K. Takakuta. 1950. A new antibiotic ‘colistin’ produced by spore-forming soil bacteria. J. Antibiot. 3:457-458. [Google Scholar]
- 14.Levin, A. S., A. A. Barone, J. Penco, M. V. Santos, I. S. Marinho, E. A. Arruda, E. I. Manrique, and S. F. Costa. 1999. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Infect. Dis. 28:1008-1011. [DOI] [PubMed] [Google Scholar]
- 15.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, K. Coulthard, and D. W. Johnson. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B 761:167-175. [DOI] [PubMed] [Google Scholar]
- 15a.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, and K. Coulthard. 2003. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob. Agents Chemother. 47:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Littlewood, J. M., C. Koch, P. A. Lambert, N. Hoiby, J. S. Elborn, S. P. Conway, R. Dinwiddie, and F. Duncan-Skingle. 2000. A ten year review of colomycin. Respir. Med. 94:632-640. [DOI] [PubMed] [Google Scholar]
- 17.National Nosocomial Infections Surveillance System. 1998. National Nosocomial Infections Surveillance (NNIS) System report; data summary from October 1986-April 1998. Am. J. Infect. Control 26:522-533. [DOI] [PubMed] [Google Scholar]
- 18.Orwa, J. A., C. Govaerts, R. Busson, E. Roets, A. Van Schepdael, and J. Hoogmartens. 2001. Isolation and structural characterization of colistin components. J. Antibiot. 54:595-599. [DOI] [PubMed] [Google Scholar]
- 19.Orwa, J. A., A. Van Gerven, E. Roets, and J. Hoogmartens. 2000. Development and validation of a liquid chromatography method for analysis of colistin sulphate. Chromatographia 51:433-436. [Google Scholar]
- 20.Poole, K. 2001. Multidrug resistance in Gram-negative bacteria. Curr. Opin. Microbiol. 4:500-508. [DOI] [PubMed] [Google Scholar]
- 21.Reed, M. D., R. C. Stern, M. A. O'Riordan, and J. L. Blumer. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645-654. [DOI] [PubMed] [Google Scholar]
- 22.Renard, L., P. Sanders, and M. Laurentie. 1991. Pharmacokinetics of colistin sulfate administered by intravenous and intramuscular routes in the calf. Ann. Rech. Vet. 22:387-394. [PubMed] [Google Scholar]
- 23.Stern, R. C. 1996. Denmark to the rescue. Pediatr. Pulmonol. 21:151-152. [DOI] [PubMed] [Google Scholar]
- 24.Troillet, N., M. H. Samore, and Y. Carmeli. 1997. Imipenem-resistant Pseudomonas aeruginosa: risk factors and antibiotic susceptibility patterns. Clin. Infect. Dis. 25:1094-1098. [DOI] [PubMed] [Google Scholar]
- 25.Wolinsky, E., and J. D. Hines. 1962. Neurotoxic and nephrotoxic effects of colistin in patients with renal disease. N. Engl. J. Med. 266:759-768. [DOI] [PubMed] [Google Scholar]
- 26.Ziv, G., J. F. Nouws, and C. A. van Ginneken. 1982. The pharmacokinetics and tissue levels of polymyxin B, colistin and gentamicin in calves. J. Vet. Pharmacol. Ther. 5:45-58. [DOI] [PubMed] [Google Scholar]
- 27.Ziv, G., M. Wanner, and J. Nicolet. 1980. Clinical pharmacology of polymyxin B, colistin, and colistimethate in young dairy calves. J. Vet. Pharmacol. Ther. 3:87-94. [Google Scholar]