Abstract
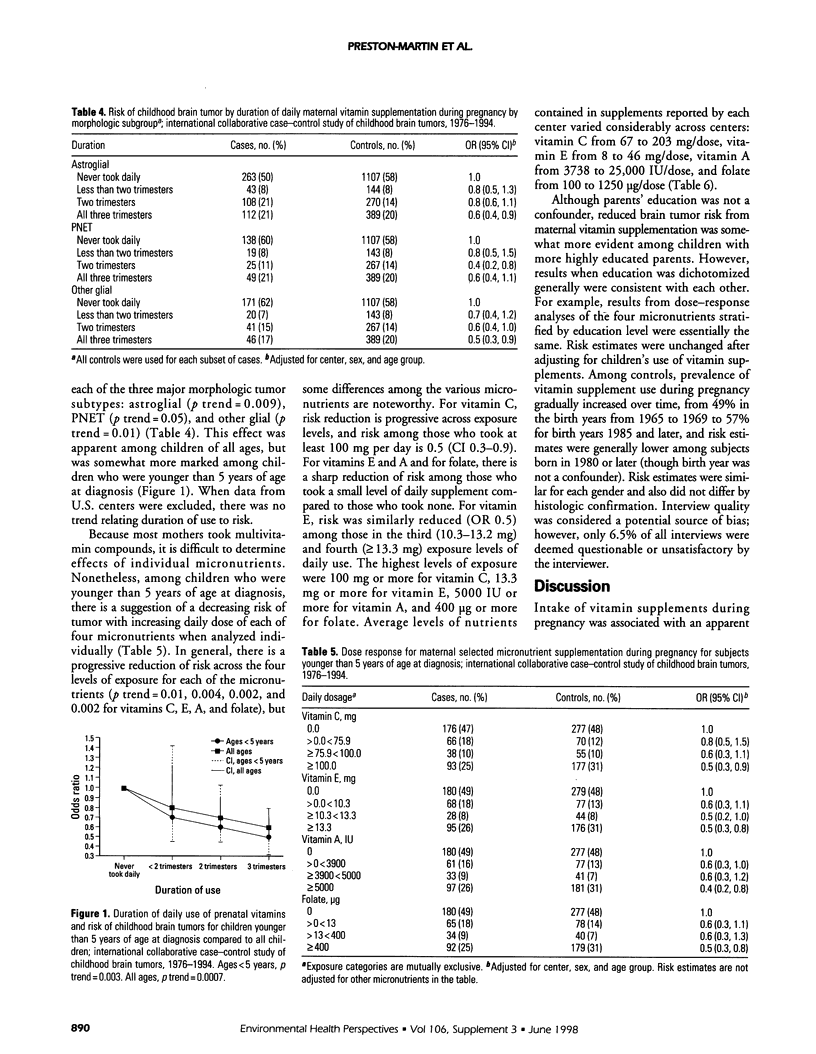
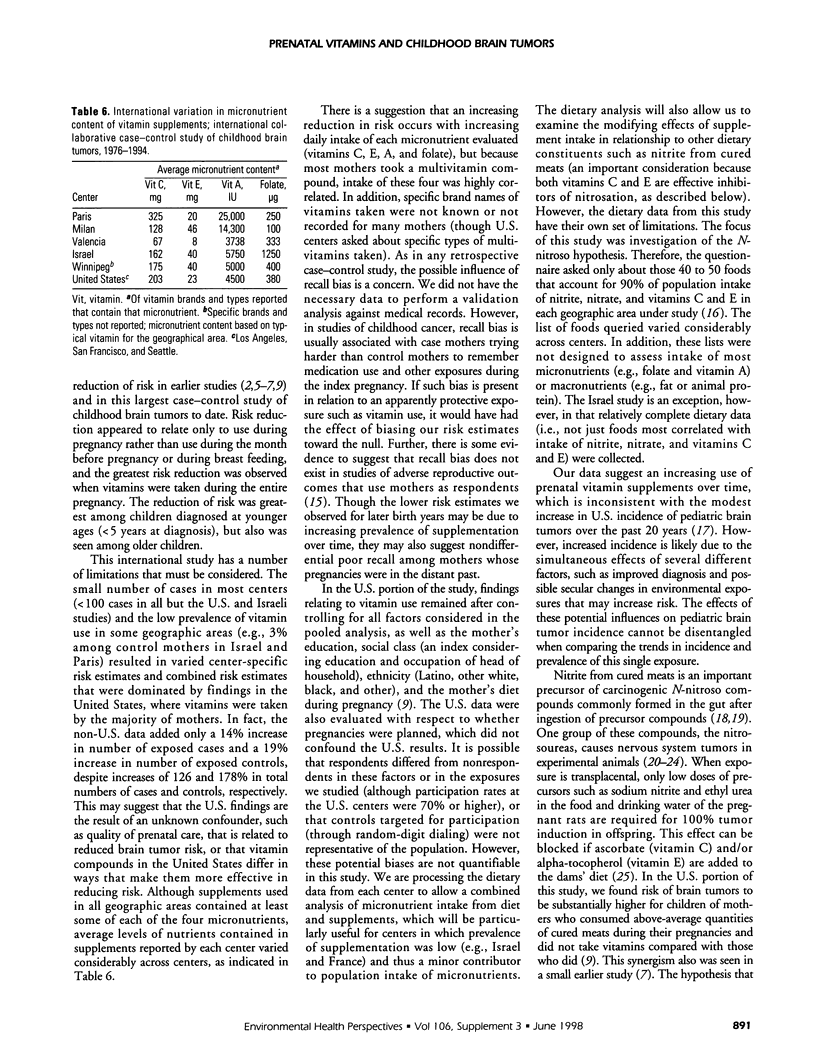
An international case-control study of primary pediatric brain tumors included interviews with mothers of cases diagnosed from 1976 to 1994 and mothers of population controls. Data are available on maternal vitamin use during pregnancy for 1051 cases and 1919 controls from eight geographic areas in North America, Europe, and Israel. Although risk estimates varied by study center, combined results suggest that maternal supplementation for two trimesters may decrease risk of brain tumor (odds ratio [OR] 0.7, 95% confidence interval [CI] 0.5-0.9), with a trend of less risk with longer duration of use (p trend = 0.0007). The greatest risk reduction was among children diagnosed under 5 years of age whose mothers used supplements during all three trimesters (OR 0.5, CI 0.3-0.8). This effect did not vary by histology and was seen for supplementation during pregnancy rather than during the month before pregnancy or while breast feeding. These findings are largely driven by data from the United States, where most mothers took vitamins. The proportion of control mothers who took vitamins during pregnancy varied tremendously: from 3% in Israel and France, 21% in Italy, 33% in Canada, 52% in Spain and 86 to 92% at the three U.S. centers. The composition of the various multivitamin compounds taken also varied: the daily dose of vitamin C ranged from 0 to 600 mg, vitamin E ranged from 0 to 70 mg, vitamin A ranged from 0 to 30,000 IU, and folate ranged from 0 to 2000 micrograms. Mothers also took individual micronutrient supplements (e.g., vitamin C tablets), but most mothers who took these also took multivitamins, making it impossible to determine potential independent effects of these micronutrients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bondy M. L., Lustbader E. D., Buffler P. A., Schull W. J., Hardy R. J., Strong L. C. Genetic epidemiology of childhood brain tumors. Genet Epidemiol. 1991;8(4):253–267. doi: 10.1002/gepi.1370080406. [DOI] [PubMed] [Google Scholar]
- Bostick R. M., Fosdick L., Wood J. R., Grambsch P., Grandits G. A., Lillemoe T. J., Louis T. A., Potter J. D. Calcium and colorectal epithelial cell proliferation in sporadic adenoma patients: a randomized, double-blinded, placebo-controlled clinical trial. J Natl Cancer Inst. 1995 Sep 6;87(17):1307–1315. doi: 10.1093/jnci/87.17.1307. [DOI] [PubMed] [Google Scholar]
- Bunin G. R., Kuijten R. R., Boesel C. P., Buckley J. D., Meadows A. T. Maternal diet and risk of astrocytic glioma in children: a report from the Childrens Cancer Group (United States and Canada) Cancer Causes Control. 1994 Mar;5(2):177–187. doi: 10.1007/BF01830264. [DOI] [PubMed] [Google Scholar]
- Bunin G. R., Kuijten R. R., Buckley J. D., Rorke L. B., Meadows A. T. Relation between maternal diet and subsequent primitive neuroectodermal brain tumors in young children. N Engl J Med. 1993 Aug 19;329(8):536–541. doi: 10.1056/NEJM199308193290804. [DOI] [PubMed] [Google Scholar]
- Cordier S., Iglesias M. J., Le Goaster C., Guyot M. M., Mandereau L., Hemon D. Incidence and risk factors for childhood brain tumors in the Ile de France. Int J Cancer. 1994 Dec 15;59(6):776–782. doi: 10.1002/ijc.2910590612. [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Filippini G., Farinotti M., Lovicu G., Maisonneuve P., Boyle P. Mothers' active and passive smoking during pregnancy and risk of brain tumours in children. Int J Cancer. 1994 Jun 15;57(6):769–774. doi: 10.1002/ijc.2910570602. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney J. G., Davis S., Severson R. K., Fang J. Y., Ross J. A., Robison L. L. Trends in cancer incidence among children in the U.S. Cancer. 1996 Aug 1;78(3):532–541. doi: 10.1002/(SICI)1097-0142(19960801)78:3<532::AID-CNCR22>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Howe G. R., Harrison L., Jain M. A short diet history for assessing dietary exposure to N-nitrosamines in epidemiologic studies. Am J Epidemiol. 1986 Oct;124(4):595–602. doi: 10.1093/oxfordjournals.aje.a114432. [DOI] [PubMed] [Google Scholar]
- Ivankovic S., Druckrey H. Transplacentare Erzeugung maligner Tumoren des Nervensystems. I. Athyl-nitroso-harnstoff (ANH) an BD IX-Ratten. Z Krebsforsch. 1968;71(4):320–360. [PubMed] [Google Scholar]
- Ivankovic S., Preussmann R. Transplazentale Erzeugung maligner Tumoren nach oraler Gabe von Athylharnstoff. Naturwissenschaften. 1970 Sep;57(9):460–460. doi: 10.1007/BF00607750. [DOI] [PubMed] [Google Scholar]
- Ivankovic S., Zeller W. J., Schmähl D., Preussmann R. Verhinderung der pränatal carcinogenen Wirkung von Athylharnstoff und Nitrit durch Ascorbinsäure. Naturwissenschaften. 1973 Nov;60(11):525–525. doi: 10.1007/BF00603269. [DOI] [PubMed] [Google Scholar]
- Mackenzie S. G., Lippman A. An investigation of report bias in a case-control study of pregnancy outcome. Am J Epidemiol. 1989 Jan;129(1):65–75. doi: 10.1093/oxfordjournals.aje.a115125. [DOI] [PubMed] [Google Scholar]
- Patterson R. E., White E., Kristal A. R., Neuhouser M. L., Potter J. D. Vitamin supplements and cancer risk: the epidemiologic evidence. Cancer Causes Control. 1997 Sep;8(5):786–802. doi: 10.1023/a:1018443724293. [DOI] [PubMed] [Google Scholar]
- Preston-Martin S., Pogoda J. M., Mueller B. A., Holly E. A., Lijinsky W., Davis R. L. Maternal consumption of cured meats and vitamins in relation to pediatric brain tumors. Cancer Epidemiol Biomarkers Prev. 1996 Aug;5(8):599–605. [PubMed] [Google Scholar]
- Preston-Martin S., Yu M. C., Benton B., Henderson B. E. N-Nitroso compounds and childhood brain tumors: a case-control study. Cancer Res. 1982 Dec;42(12):5240–5245. [PubMed] [Google Scholar]
- Rice J. M., Rehm S., Donovan P. J., Perantoni A. O. Comparative transplacental carcinogenesis by directly acting and metabolism-dependent alkylating agents in rodents and nonhuman primates. IARC Sci Publ. 1989;(96):17–34. [PubMed] [Google Scholar]
- Sarasua S., Savitz D. A. Cured and broiled meat consumption in relation to childhood cancer: Denver, Colorado (United States) Cancer Causes Control. 1994 Mar;5(2):141–148. doi: 10.1007/BF01830260. [DOI] [PubMed] [Google Scholar]


