Abstract
Methicillin-resistant Staphylococcus aureus is becoming increasingly prevalent as both a nosocomial and a community-acquired pathogen. Daptomycin, a lipopeptide antibiotic now in phase III clinical trials, is rapidly bactericidal in vitro against a range of gram-positive organisms, including methicillin-resistant S. aureus (MRSA). In this study, we compared the efficacy of daptomycin with that of vancomycin, each with or without rifampin, in a model of experimental aortic valve endocarditis due to MRSA. The infecting strain (MRSA strain 32) was susceptible to daptomycin (MIC = 1 μg/ml), vancomycin (MIC = 0.5 μg/ml), and rifampin (MIC = 0.5 μg/ml). Daptomycin was administered at 25 or 40 mg/kg q24h (q24h) by subcutaneous injection in an attempt to simulate human doses of 4 and 6 mg/kg q24h, respectively. Vancomycin was given at 150 mg/kg q24h by continuous intravenous infusion. Rifampin was given at 25 mg/kg by intramuscular injection q24h. Treatment was started 6 h postinoculation and continued for 4.5 days. Outcome was assessed by counting the residual viable bacteria in vegetations. The mean peak daptomycin levels in serum at 2 h after subcutaneous administration of 25 and 40 mg/kg were 64 and 91 μg/ml, respectively. Daptomycin was undetectable in serum at 24 h. The total exposure was comparable to that achieved clinically in humans receiving the drug. Bacterial counts (mean log10 number of CFU per gram ± the standard deviation) in untreated controls reached 10.6 ± 0.8. In treated rats, bacterial counts were as follows: vancomycin, 7.1 ± 2.5; daptomycin at 25 mg/kg, 5.5 ± 1.7; daptomycin at 40 mg/kg, 4.2 ± 1.5. The difference between daptomycin at 40 mg/kg and vancomycin at 150 mg/kg was statistically significant (P = 0.004). In the study of combination therapy, vegetation bacterial counts were as follows: daptomycin at 40 mg/kg, 4.6 ± 1.6; rifampin, 3.6 ± 1.3; vancomycin plus rifampin, 3.3 ± 1.1; daptomycin plus rifampin, 2.9 ± 0.8. The difference between daptomycin and daptomycin plus rifampin was statistically significant (P = 0.006). These results support the continued evaluation of daptomycin for serious MRSA infections, including infective endocarditis.
Daptomycin is a lipopeptide antibiotic derived from Streptomyces roseosporus with potent bactericidal activity in vitro against most clinically relevant gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA), coagulase-negative staphylococci, vancomycin-resistant enterococci, and penicillin-resistant Streptococcus pneumoniae (1, 2, 10, 11, 24, 28). The mechanism of action, while not yet fully elucidated, appears to involve the disruption of bacterial plasma membrane function (3, 6, 13, 16).
To date, daptomycin has been studied in complicated skin and soft-tissue infections (P. A. Matthews and M. F. DeBruin, Infect. Dis. Soc. Am., abstr. 112, 2001) and bacteremia due to gram-positive bacteria (29). A previous study was performed with daptomycin in bacterial endocarditis (data on file: study B8B-MC-AVAG, Cubist Pharmaceuticals, Inc.) (29). Given the lack of currently available antimicrobials with rapid bactericidal activity against MRSA, the continued evaluation of daptomycin in animal models of MRSA infection and in clinical MRSA infection in humans is warranted, particularly in infections such as infective endocarditis, where bactericidal therapy is crucial.
A previous animal study demonstrated that administration of daptomycin once daily (q24h) may reduce toxicity while maintaining good antibacterial activity (22). Therefore, we examined the activity of daptomycin in comparison with that of vancomycin in a rat model of MRSA aortic valve endocarditis with once-daily dosing to simulate clinical therapy. In addition, because rifampin is used clinically with vancomycin for the treatment of serious MRSA infections, we also examined the efficacy of regimens combining daptomycin or vancomycin with rifampin.
MATERIALS AND METHODS
Antimicrobial agents.
Daptomycin powder (lot 670113A) was supplied by Cubist Pharmaceuticals (Lexington, Mass.). A solution of 25 mg/ml was prepared for subcutaneous injection in sterile distilled water. Rifampin (Merrell Pharmaceuticals, Kansas City, Mo.) was reconstituted with sterile distilled water to prepare a 60-mg/ml solution for intramuscular (i.m.) injection. Vancomycin hydrochloride (American Pharmaceutical Partners, Inc., Los Angeles, Calif.) was infused at 7.2 ml/24 h intravenously (i.v.) in sterile saline to administer doses of 150 mg/kg/24 h. We have previously shown that this dose results in mean vancomycin concentrations in serum of approximately 15 μg/ml (31).
Microorganism.
MRSA strain 32 is a clinical isolate with the following MIC profile: daptomycin, 1 μg/ml; vancomycin, 0.5 μg/ml; oxacillin, >128 μg/ml; rifampin, 0.5 μg/ml. The MIC of vancomycin was determined by agar dilution testing in accordance with NCCLS recommendations (21). Daptomycin susceptibility testing was performed with Mueller-Hinton II agar (Becton Dickinson, Cockeysville, Md.) supplemented with calcium chloride at 50 μg/ml and confirmed by broth microdilution done separately at Cubist Pharmaceuticals (MIC, 0.8 μg/ml). Rifampin susceptibility testing was performed with a VITEK-2 AST-GP55 card (bioMérieux, St. Louis, Mo.).
Animal model.
Aortic valve endocarditis was created in male Sprague-Dawley rats (Charles River Laboratories, Wilmington, Mass.) by transvalvular placement of a polyethylene catheter and injection through the catheter of a 0.5-ml suspension of MRSA strain 32 in sterile saline (106 to 107 CFU/ml) as described previously (25), with modifications (30, 32). Animals were randomized 6 h later into different treatment groups. In the first phase of the study, groups included rats given daptomycin at 25 mg/kg subcutaneously (s.c.) q24h, daptomycin at 40 mg/kg s.c. q24h, or vancomycin at 150 mg/kg q24h as a continuous i.v. infusion and untreated controls sacrificed at day 5. The second phase of the study included administration of daptomycin at 40 mg/kg s.c. q24h, daptomycin at 40 mg/kg s.c. q24h plus rifampin at 25 mg/kg i.m. q24h, vancomycin at 150 mg/kg q24h plus rifampin at 25 mg/kg i.m. q24h, or rifampin at 25 mg/kg i.m. q24h; untreated controls sacrificed on day 5; and controls sacrificed at the time of therapy initiation (6 h). For animals receiving daptomycin with rifampin, the antibiotics were administered at the same time at two different sites. Animals that died prior to the 5-day study period were excluded if they had received less than 2 days of therapy. All control animals were included regardless of time of death.
Aortic valve vegetations were harvested aseptically on day 5 or, when dead animals were discovered, as soon as possible after death. Vegetations were weighed, homogenized in saline, serially diluted, and plated onto blood agar plates and mannitol salt agar plates. Vegetation bacterial density was expressed as the log10 number of CFU per gram of vegetation tissue. When vegetations were sterile, the value of the lowest limit of detection was assigned for analysis and statistical calculations.
Examination of surviving bacteria.
Individual bacterial colonies obtained from aortic valve tissue homogenates were grown and resuspended in 1 ml of Mueller-Hinton broth to a density equivalent to a 1.0 McFarland standard. Twenty-five microliters of this suspension and of dilutions of 101 to 107 were plated on Mueller-Hinton II agar with various concentrations of vancomycin or daptomycin (0 to 4 μg/ml). Mueller-Hinton agar was supplemented with CaCl2 at 50 μg/ml in daptomycin resistance studies. Colonies were counted at 48 h.
Pharmacokinetics.
Daptomycin (25 or 40 mg/kg) or rifampin was administered as a single s.c. or i.m. dose, respectively, to healthy male 200- to 225-g Sprague-Dawley rats (three per treatment group). Blood samples were collected in heparinized tubes via jugular vein catheters over a time range of 2 min through 24 h. Plasma was collected by centrifugation, and samples were stored at −20°C until analysis.
Daptomycin was detected with an internal standard of ethylparaben and protein precipitation, followed by high-performance liquid chromatography (HPLC) by using methodology developed by Eli Lilly and modified slightly by Cubist Pharmaceuticals (validation report LC 309.4, version 1.00, HPLC analysis of daptomycin in rat plasma, 2001 [report on file, Cubist Pharmaceuticals]). The method has been validated for use in clinical trials. In brief, drug concentrations in serum were determined by reversed-phase HPLC with a Metachem Hypersil C8 analytical column and a Waters Xterra RP18 guard column. Daptomycin and the internal standard, ethylparaben, were isolated from rat plasma by protein precipitation. The mobile phase was 32.6% acetonitrile, and 67.4% of it was a 0.5% ammonium phosphate solution. At a flow rate of 1.5 ml/min, daptomycin shows a retention time of 14 to 16 min. Samples were analyzed at 214 nm. Detection of daptomycin concentrations in rat plasma was linear across a range of 7.5 to 400 μg/ml. Rifampin was detected by HPLC analysis as described previously (7).
Statistical analysis.
The chi-square test was used to examine differences in discrete variables such as mortality. Differences in vegetation bacterial densities were evaluated by the Kruskal-Wallis test.
RESULTS
Pharmacokinetics.
The pharmacokinetic profiles of daptomycin and rifampin in our rat model are demonstrated in Table 1. Mean peak levels in serum were 64 μg/ml for the 25-mg/kg dose of daptomycin and 91 μg/ml for the 40-mg/kg dose. The drug was eliminated with a half-life of 1.6 to 2.9 h in rats, and daptomycin was undetectable in serum at 24 h. Protein binding of daptomycin is comparable (90 to 94%) across various species, including rodents (19) and humans (17); therefore, a comparison of total daptomycin concentrations is appropriate. Peak levels obtained in rats were comparable to the target values obtained in humans during treatment with a daily dose of 4 or 6 mg/kg, while the areas under the concentration-time curves of 278 and 605 μg × h/ml bracket the exposure obtained in humans at 4 mg/kg and are lower than the exposure of humans treated with the 6-mg/kg dose.
TABLE 1.
Pharmacokinetics of daptomycin and rifampin in the rat
| Antibiotic | Dose (mg/kg) route | Species | Cmaxc | Tmaxd (h) | AUC0-24e | t1/2f |
|---|---|---|---|---|---|---|
| Daptomycin | 25, s.c. | Rat | 63.6 | 2 | 278.4 | 1.6 |
| Daptomycin | 40, s.c. | Rat | 90.9 | 2 | 605.4 | 2.9 |
| Daptomycin | 4, i.v.a | Humanb | 57.8 | 0.5 | 493.5 | 8.2 |
| Daptomycin | 6, i.v.a | Human | 98.6 | 0.5 | 747.4 | 8.9 |
| Rifampin | 25, i.m. | Rat | 15.6 | 2 | 258.8 | 9.5 |
Administered as a 30-min-infusion.
The following data come from a single- and multiple-dose proportionality study with healthy human subjects. The values shown are the mean values at steady state (day 7 of q-24 h administration).
Cmax, maximum drug concentration in serum.
Tmax, time required to reach Cmax.
AUC0-24, area under the concentration-time curve from 0 to 24 h.
t1/2; half-life.
Control animals.
Vegetation bacterial density was determined for two groups of untreated animals at 5 days and for one group of rats at the time of initiation of therapy (6 h). The mean bacterial densities (mean log10 numbers of CFU per gram ± the standard deviation [SD]) on harvested aortic valve vegetations in the two groups of untreated controls at 5 days were 10.6 ± 0.8 (n = 12) and 10.3 ± 0.5 (n = 5). At the time of initiation of therapy, the mean bacterial density was 6.4 ± 1.8 (n = 8).
Monotherapy.
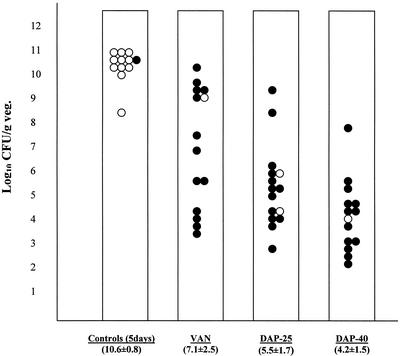
The first phase of this investigation compared daily daptomycin monotherapy at 25 or 40 mg/kg with vancomycin. Results are shown in Fig. 1. Mean bacterial densities (mean log10 numbers of CFU per gram ± the SD) on harvested aortic valve vegetations were 7.1 ± 2.5, 5.5 ± 1.7, and 4.2 ± 1.5 for animals treated with vancomycin, daptomycin at 25 mg/kg, and daptomycin at 40 mg/kg, respectively. Treatment with daptomycin resulted in a dose-dependent reduction in the number of viable bacteria, although the difference between the effects obtained with two doses of daptomycin was not statistically significant. The difference between daptomycin at 40 mg/kg and vancomycin at 150 mg/kg was statistically significant (P = 0.004). There was no significant difference in mortality at 5 days between the treatment groups, but all treatment arms showed significantly increased survival (86%) compared to that of untreated controls (8.3%).
FIG. 1.
Vegetation (veg.) bacterial densities after monotherapy for 5 days with vancomycin (VAN; n = 14), daptomycin at 25 mg/kg (DAP-25; n = 15), or daptomycin at 40 mg/kg (DAP-40; n = 14) and in control animals sacrificed at 5 days (n = 12). Each datum point represents one rat. The mean log10 number of CFU per gram ± the SD for each group is noted at the bottom of each column. P < 0.05 for controls versus all treatment groups and for vancomycin versus daptomycin at 40 mg/kg. Surviving animals at 5 days are shown as solid circles, while animals that were sacrificed or that died before 5 days are shown as open circles.
Combination therapy.
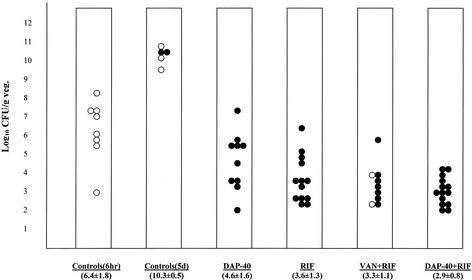
For combination therapy, we used daptomycin at 40 mg/kg, the more effective dose in the earlier study. The results of the combination therapy study are shown in Fig. 2. Mean bacterial densities (mean log10 numbers of CFU per gram ± the SD) on harvested aortic valve vegetations from animals treated with the different therapies were 4.6 ± 1.6 (daptomycin at 40 mg/kg), 3.6 ± 1.3 (rifampin at 25 mg/kg), 3.3 ± 1.1 (vancomycin plus rifampin), and 2.9 ± 0.8 (daptomycin at 40 mg/kg plus rifampin). The difference between daptomycin plus rifampin and daptomycin monotherapy was statistically significant (P = 0.006) but not different from vancomycin plus rifampin or rifampin alone. No significant survival difference between the therapeutic arms was detected.
FIG. 2.
Vegetation (veg.) bacterial densities after therapy with vancomycin (VAN) plus rifampin (RIF) (n = 9), daptomycin at 40 mg/kg plus rifampin (n = 14), daptomycin at 40 mg/kg (DAP-40; n = 10), or rifampin (n = 13) for 5 days and in control animals (n = 5) that received no therapy. The far left set of data (controls at 6 h, n = 8) demonstrates the vegetation bacterial density at the start of therapy. The mean log10 number of CFU per gram ± the SD for each group is noted at the bottom of each column. P < 0.05 for controls at 5 days versus all treatment groups and for monotherapy with daptomycin at 40 mg/kg versus daptomycin at 40 mg/kg plus rifampin. Surviving animals at 5 days are shown as solid circles, while animals that were sacrificed or that died before 5 days are shown as open circles.
Surviving bacteria.
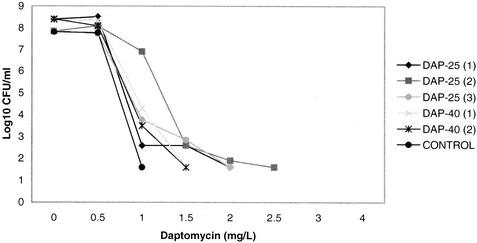
A subset of bacterial isolates obtained from aortic valve vegetations of treated rats was evaluated by population analysis to test for the development of resistance or heteroresistance to daptomycin or vancomycin. We used population analysis instead of routine susceptibility testing, given the recently characterized heterogeneous nature of daptomycin resistance in staphylococci (26). Representative population analyses of bacterial colonies harvested from three rats treated with daptomycin at 25 mg/kg q24h, two rats treated with daptomycin at 40 mg/kg q24h, and one untreated rat are displayed in Fig. 3. While isolates from daptomycin-treated animals demonstrated a consistent rightward shift of the population curve, the MIC increase was extrapolated to be less than twofold. With a 108-CFU/ml inoculum, no isolate showed growth at daptomycin concentrations of ≥3 μg/ml. Isolates treated with vancomycin did not demonstrate a shift in heteroresistance to vancomycin (data not shown). Of four isolates analyzed from four different rifampin-treated animals, one demonstrated intermediate resistance, with a fourfold increase in the MIC to 2 μg/ml.
FIG. 3.
Population analysis of surviving colonies from daptomycin-treated animals. Individual colonies were isolated and analyzed as described in Materials and Methods. Shown in the graph are three representative clones isolated from three individual animals treated with daptomycin at 25 mg/kg (DAP-25), two colonies from two individual animals treated with daptomycin at 40 mg/kg (DAP-40), and one colony from an untreated animal (CONTROL).
DISCUSSION
Over the past decade, the incidence of multidrug-resistant gram-positive organisms has been increasing at an alarming rate (5). A recent analysis showed that more than one-third of the S. aureus bloodstream isolates in the United States are methicillin resistant and that many of these isolates are resistant to multiple antibiotics (9). Even more troubling is a report that MRSA appears to be spreading into the community (9). Because of the current lack of available antimicrobials with consistent bactericidal activity against methicillin-resistant staphylococci and vancomycin-resistant enterococci, therapeutic options for patients with endovascular infections due to these organisms pose major therapeutic challenges for clinicians. Daptomycin is a cyclic lipopeptide antibiotic with a spectrum of activity similar to that of glycopeptides, except that it is active against vancomycin-resistant enterococci (29). Its mechanism of action is incompletely defined but differs from that of glycopeptides, involving disruption of the cell membrane potential (3, 4, 6, 13, 16).
The bactericidal activity of daptomycin in vitro is dependent on the pH and physiologic concentrations of ionized calcium (16). Furthermore, daptomycin is approximately 95% protein bound and its in vitro activity can be altered by the addition of serum or albumin (13, 17, 19, 23). The complex interactions of daptomycin with the surrounding environment and the influence of these interactions on its activity make it essential to demonstrate a correlation between in vitro activity and in vivo activity. Therefore, our in vivo model provides strong support for the activity of daptomycin against infection with MRSA.
Various animal studies evaluating the in vivo activity of daptomycin have demonstrated it to be comparable to that of glycopeptides (14, 15, 20). Some of these studies were performed in models in which the pharmacokinetic profile of daptomycin might not have been optimal for efficacy. Phase II clinical studies with daptomycin have demonstrated it to be comparable to conventional therapy for the treatment of gram-positive bacteremia and endocarditis (F. P. Tally, F. B. Oleson, C. L. Berman, and M. F. DeBruin, ECCMID, abstr. WeP233:8/1, 2000). A recent phase III study demonstrated a shorter duration of therapy with daptomycin compared to conventional therapy in complicated soft-tissue infections. (Matthews and DeBruin, Abstr. Infect. Dis. Soc. Am.)
In this investigation, we applied daptomycin to a well-established in vivo infection model of rat aortic valve endocarditis. It was determined that s.c. administration of daptomycin to rats at 25 and 40 mg/kg q24h resulted in maximum drug concentrations in serum that approximate those observed in humans given 4 and 6 mg/kg q24h i.v. The rat dosages resulted in exposures that bracket the 4-mg/kg clinical dose. These doses appear to be well tolerated by humans (Matthews and DeBruin, Abstr. Infect. Dis. Soc. Am.).
We demonstrated that daptomycin monotherapy administered to rats at 40 mg/kg was superior to vancomycin monotherapy, as determined by a statistically significant decrease in the bacterial density of aortic valve vegetations after 5 days of therapy. Daptomycin at 25 mg/kg showed a trend toward higher activity than vancomycin in this model, but the differences did not reach statistical significance. Although animals treated with daptomycin at 25 mg/kg or vancomycin had lower vegetation bacterial densities than untreated control animals, final counts in these treated animals were generally similar to those expected at the time treatment is begun. However, daptomycin at 40 mg/kg led to an approximately 2-log reduction and both combination regimens resulted in at least a 3-log reduction in bacterial densities over the treatment period compared with bacterial densities at the start of therapy.
Given that the pharmacokinetic profile of daptomycin in this model resulted in trough levels that were nondetectable, potential development of resistance was a concern. Evaluation of bacteria harvested from aortic valve vegetations of treated animals showed a small rightward shift in the population analysis curves, but the extrapolated MICs of daptomycin were less than twofold greater than those for the strains isolated from untreated animals. These findings are consistent with previous reports that found that the frequency of spontaneous daptomycin resistance is S. aureus is very low (14, 18).
This study provides evidence that the bactericidal activity of daptomycin is augmented by the addition of rifampin. Both daptomycin and vancomycin performed better in combination with rifampin. Bacterial endocarditis is a serious infection that could benefit from aggressive combination bactericidal therapy that maintains acceptable safety.
We chose rifampin for our combination therapy study primarily because it has been used concomitantly with β-lactams and glycopeptides to enhance the bactericidal activity of clinically applied antimicrobial regimens (12, 27). Rifampin has the ability to attain high intracellular concentrations in endothelial cells (8). It has been suggested that bacteria within endothelial cells may be important in the pathogenesis of bacterial endocarditis, particularly with the ability of bacteria to cause infection on normal valves and relapses despite long courses of treatment (8). Therefore, antimicrobials targeting bacteria in endothelial cells may provide advantages in endocarditis management. Rifampin maintains activity against most clinical MRSA isolates (9). Also, the target of action of rifampin lies within the bacterium and therefore might possibly complement daptomycin, which targets the cell membrane. Furthermore, rifampin avoids the ototoxicity and nephrotoxicity concerns that accompany the use of aminoglycosides. Nevertheless, the in vivo activity of daptomycin-aminoglycoside combination therapy probably warrants further study, particularly for enterococci.
In our infection model, rifampin monotherapy demonstrated unexpected efficacy. This may perhaps be explained by the fact that spontaneous resistance to rifampin in S. aureus occurs in approximately 1 in 107 organisms. Therefore, the inoculum size of infection at the time treatment was begun in the model was probably not sufficiently great to allow selection of resistance to rifampin.
In summary, we demonstrated with an in vivo experimental model of MRSA endocarditis that daptomycin administered at a dose corresponding to a human dose of 4 to 6 mg/kg q24h was comparable to or better than therapy with vancomycin and that the combination of rifampin with daptomycin was superior to daptomycin alone. No development of resistance to daptomycin was detected, and it was not possible to evaluate whether daptomycin would prevent the emergence of resistance to rifampin. The excellent bactericidal activity of daptomycin-rifampin combination therapy in vivo warrants further investigation of this regimen in humans with serious endovascular infections due to MRSA.
Acknowledgments
This work was supported by funding from Cubist Pharmaceuticals, Lexington, Mass.
REFERENCES
- 1.Akins, R. L., and M. J. Rybak. 2000. In vitro activities of daptomycin, arbekacin, vancomycin, and gentamicin alone and/or in combination against glycopeptide-intermediate Staphylococcus aureus in an infection model. Antimicrob. Agents Chemother. 44:1925-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, R. L., and M. J. Rybak. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alborn, W. E., Jr., N. E. Allen, and D. A. Preston. 1991. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob. Agents Chemother. 35:2282-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen, N. E., W. E. Alborn, Jr., and J. N. Hobbs, Jr. 1991. Inhibition of membrane potential-dependent amino acid transport by daptomycin. Antimicrob. Agents Chemother. 35:2639-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, J. M., and D. D. Turnidge. 2002. High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalized patients in Asia-Pacific and South African: results from the SENTRY antimicrobial surveillance program, 1998-1999. Antimicrob. Agents Chemother. 46:879-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canepari, P., M. Boaretti, M. del Mar Lleo, and G. Satta. 1990. Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin (LY146032). Antimicrob. Agents Chemother. 34:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte, J. E., Jr., E. Lin, and E. Zurlinden. 2000. Liquid chromatographic determination of rifampin in human plasma, bronchoalveolar lavage fluid, and alveolar cells. J. Chromatogr. Sci. 38:72-76. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche, R. O., and R. J. Hamill. 1994. Antibiotic penetration of and bacterial activity within endothelial cells. Antimicrob. Agents Chemother. 38:1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diekema, D. J., M. A. Pfaller, E. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, and The SENTRY Participants Group. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, G. M., C. Thauvin, B. Gerson, and R. C. Moellering. 1985. In vitro activity and mechanism of action of A21978C1, a novel cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 27:357-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliopoulos, G. M., S. Willey, E. Reiszner, P. G. Spitzer, G. Caputo, and R. C. Moellering, Jr. 1986. In vitro and in vivo activity of LY146032, a new cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 30:532-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faville, R. J., D. E. Zaske, E. L. Kaplan, et al. 1978. Staphylococcus aureus endocarditis: combined therapy with vancomycin and rifampin. JAMA 240:1963.. [DOI] [PubMed] [Google Scholar]
- 13.Hanberger, H. L., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaatz, G. W., S. M. Seo, V. N. Reddy, E. M. Bailey, and M. J. Rybak. 1990. Daptomycin compared with teicoplanin and vancomycin for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 34:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy, S., and H. F. Chambers. 1989. Daptomycin (LY146032) for prevention and treatment of experimental aortic valve endocarditis in rabbits. Antimicrob. Agents Chemother. 33:1522-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, B. L., M. Sachdeva, and H. F. Chambers. 1991. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob. Agents Chemother. 35:2505-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebowitz, L. D., J. Saunders, L. J. Chalkley, and H. J. Koornhof. 1988. In vitro selection of bacteria resistant to LY146032, a new cyclic lipopeptide. Antimicrob. Agents Chemother. 32:24-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie, A., P. Kaw, W. Liu, N. Jumbe, M. H. Miller, and G. L. Drusano. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mader, J. T., and K. Adams. 1989. Comparative evaluation of daptomycin (LY146032) and vancomycin in the treatment of experimental methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrob. Agents Chemother. 33:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Document M7-A5. NCCLS, Wayne, Pa.
- 22.Oleson, F. B., Jr., C. L. Bermen, J. B. Kirkpatrick, K. S. Regan, J. J. Lai, and F. P. Tally. 2000. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob. Agents Chemother. 44:2948-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rybak, M. J., E. M. Bailey, K. C. Lamp, and G. W. Kaatz. 1992. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob. Agents Chemother. 36:1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoro, J., and M. E. Levison. 1978. Rat model of experimental endocarditis. Infect. Immun. 19:915-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverman, J. A., N. Oliver, T. Andrew, and T. Li. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons, N. A. 1977. Synergy and rifampicin. J. Antimicrob. Chemother. 3:109.. [DOI] [PubMed] [Google Scholar]
- 28.Snydman, D. R., N. V. Jacobus, L. A. McDermott, J. R. Lonks, and J. M. Boyce. 2000. Comparative in vitro activities of daptomycin and vancomycin against resistant gram-positive pathogens. Antimicrob. Agents Chemother. 44:3447-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tally, F. P., M. Zeckel, M. M. Wasilewski, C. Carini, C. L. Berman, G. L. Drusano, and F. B. Olsen, Jr. 1999. Daptomycin: a novel agent for gram-positive infections. Exp. Opin. Investig. Drugs 8:1223-1238. [DOI] [PubMed] [Google Scholar]
- 30.Thauvin, C., G. M. Eliopoulos, S. Willey, C. Wennersten, and R. C. Moellering, Jr. 1987. Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob. Agents Chemother. 31:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao, J. C., C. Thauvin-Eliopoulos, G. M. Eliopoulos, and R. C. Moellering, Jr. 1990. Efficacy of teicoplanin in two dosage regimens for experimental endocarditis caused by a β-lactamase-producing strain of Enterococcus faecalis with high-level resistance to gentamicin. Antimicrob. Agents Chemother. 34:827-830. [DOI] [PMC free article] [PubMed] [Google Scholar]