Abstract
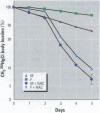
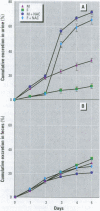
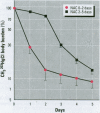
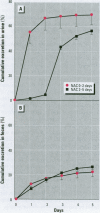
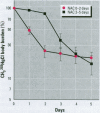
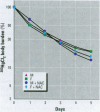
Methylmercury is a ubiquitous environmental pollutant and potent neurotoxin. Treatment of methylmercury poisoning relies almost exclusively on the use of chelating agents to accelerate excretion of the metal. The present study demonstrates that oral administration of N-acetylcysteine (NAC), a widely available and largely nontoxic amino acid derivative, produces a profound acceleration of urinary methylmercury excretion in mice. Mice that received NAC in the drinking water (10 mg/ml) starting at 48 hr after methylmercury administration excreted from 47 to 54% of the 203Hg in urine over the subsequent 48 hr, as compared to 4-10% excretion in control animals. When NAC-containing water was given from the time of methylmercury administration, it was even more effective at enhancing urinary methylmercury excretion and at lowering tissue mercury levels. In contrast, excretion of inorganic mercury was not affected by oral NAC administration. The ability of NAC to enhance methylmercury excretion when given orally, its relatively low toxicity, and is wide availability in the clinical setting indicate that it may be an ideal therapeutic agent for use in methylmercury poisoning.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaseth J., Frieheim E. A. Treatment of methyl mercury poisoning in mice with 2,3-dimercaptosuccinic acid and other complexing thiols. Acta Pharmacol Toxicol (Copenh) 1978 Apr;42(4):248–252. doi: 10.1111/j.1600-0773.1978.tb02196.x. [DOI] [PubMed] [Google Scholar]
- Aaseth J. Mobilization of methyl mercury in vivo and vitro using N-acetyl-DL-penicillamine and other complexing agents. Acta Pharmacol Toxicol (Copenh) 1976 Sep;39(3):289–301. doi: 10.1111/j.1600-0773.1976.tb03180.x. [DOI] [PubMed] [Google Scholar]
- Al-Abbasi A. H., Kostyniak P. J., Clarkson T. W. An extracorporeal complexing hemodialysis system for the treatment of methylmercury poisoning. III. Clinical applications. J Pharmacol Exp Ther. 1978 Oct;207(1):249–254. [PubMed] [Google Scholar]
- Aposhian H. V., Maiorino R. M., Gonzalez-Ramirez D., Zuniga-Charles M., Xu Z., Hurlbut K. M., Junco-Munoz P., Dart R. C., Aposhian M. M. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology. 1995 Mar 31;97(1-3):23–38. doi: 10.1016/0300-483x(95)02965-b. [DOI] [PubMed] [Google Scholar]
- Bakir F., Damluji S. F., Amin-Zaki L., Murtadha M., Khalidi A., al-Rawi N. Y., Tikriti S., Dahahir H. I., Clarkson T. W., Smith J. C. Methylmercury poisoning in Iraq. Science. 1973 Jul 20;181(4096):230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Banner W., Jr, Koch M., Capin D. M., Hopf S. B., Chang S., Tong T. G. Experimental chelation therapy in chromium, lead, and boron intoxication with N-acetylcysteine and other compounds. Toxicol Appl Pharmacol. 1986 Mar 30;83(1):142–147. doi: 10.1016/0041-008x(86)90331-5. [DOI] [PubMed] [Google Scholar]
- Bonanomi L., Gazzaniga A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur J Respir Dis Suppl. 1980;111:45–51. [PubMed] [Google Scholar]
- Borgström L., Kågedal B., Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31(2):217–222. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- Cantilena L. R., Jr, Klaassen C. D. The effect of chelating agents on the excretion of endogenous metals. Toxicol Appl Pharmacol. 1982 May;63(3):344–350. doi: 10.1016/0041-008x(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Clarkson T. W., Magos L., Cox C., Greenwood M. R., Amin-Zaki L., Majeed M. A., Al-Damluji S. F. Tests of efficacy of antidotes for removal of methylmercury in human poisoning during the Iraq outbreak. J Pharmacol Exp Ther. 1981 Jul;218(1):74–83. [PubMed] [Google Scholar]
- Domingo J. L. Prevention by chelating agents of metal-induced developmental toxicity. Reprod Toxicol. 1995 Mar-Apr;9(2):105–113. doi: 10.1016/0890-6238(94)00060-3. [DOI] [PubMed] [Google Scholar]
- Elhassani S. B. The many faces of methylmercury poisoning. J Toxicol Clin Toxicol. 1982 Oct;19(8):875–906. doi: 10.3109/15563658208992523. [DOI] [PubMed] [Google Scholar]
- Endo A., Watanabe T. Analysis of protective activity of N-acetylcysteine against teratogenicity of heavy metals. Reprod Toxicol. 1988;2(2):141–144. doi: 10.1016/0890-6238(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Ewan K. B., Pamphlett R. Increased inorganic mercury in spinal motor neurons following chelating agents. Neurotoxicology. 1996 Summer;17(2):343–349. [PubMed] [Google Scholar]
- Friedheim E., Corvi C. Meso-dimercaptosuccinic acid, a chelating agent for the treatment of mercury poisoning. J Pharm Pharmacol. 1975 Aug;27(8):624–626. doi: 10.1111/j.2042-7158.1975.tb09522.x. [DOI] [PubMed] [Google Scholar]
- Gabard B. Improvement of oral chelation treatment of methyl mercury poisoning in rats. Acta Pharmacol Toxicol (Copenh) 1976 Aug;39(2):250–255. doi: 10.1111/j.1600-0773.1976.tb03176.x. [DOI] [PubMed] [Google Scholar]
- Girardi G., Elias M. M. Effectiveness of N-acetylcysteine in protecting against mercuric chloride-induced nephrotoxicity. Toxicology. 1991 Apr 8;67(2):155–164. doi: 10.1016/0300-483x(91)90139-r. [DOI] [PubMed] [Google Scholar]
- Godfrey N. F., Peter A., Simon T. M., Lorber A. IV N-acetylcysteine treatment of hematologic reactions to chrysotherapy. J Rheumatol. 1982 Jul-Aug;9(4):519–526. [PubMed] [Google Scholar]
- Hannestad U., Sörbo B. Determination of 3-mercaptolactate, mercaptoacetate and N-acetylcysteine in urine by gas chromatography. Clin Chim Acta. 1979 Jul 16;95(2):189–200. doi: 10.1016/0009-8981(79)90359-0. [DOI] [PubMed] [Google Scholar]
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Henderson P., Hale T. W., Shum S., Habersang R. W. N-Acetylcysteine therapy of acute heavy metal poisoning in mice. Vet Hum Toxicol. 1985 Dec;27(6):522–525. [PubMed] [Google Scholar]
- Hinchman C. A., Matsumoto H., Simmons T. W., Ballatori N. Intrahepatic conversion of a glutathione conjugate to its mercapturic acid. Metabolism of 1-chloro-2,4-dinitrobenzene in isolated perfused rat and guinea pig livers. J Biol Chem. 1991 Nov 25;266(33):22179–22185. [PubMed] [Google Scholar]
- Hirayama K. Effects of combined administration of thiol compounds and methylmercury chloride on mercury distribution in rats. Biochem Pharmacol. 1985 Jun 1;34(11):2030–2032. doi: 10.1016/0006-2952(85)90328-4. [DOI] [PubMed] [Google Scholar]
- Hjortsø E., Fomsgaard J. S., Fogh-Andersen N. Does N-acetylcysteine increase the excretion of trace metals (calcium, magnesium, iron, zinc and copper) when given orally? Eur J Clin Pharmacol. 1990;39(1):29–31. doi: 10.1007/BF02657052. [DOI] [PubMed] [Google Scholar]
- Ishihara N., Shiojima S., Suzuki T. Selective enhancement of urinary organic mercury excretion by D-penicillamine. Br J Ind Med. 1974 Jul;31(3):245–249. doi: 10.1136/oem.31.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerper L. E., Ballatori N., Clarkson T. W. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am J Physiol. 1992 May;262(5 Pt 2):R761–R765. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- Kostyniak P. J., Clarkson T. W., Cestero R. V., Freeman R. B., Abbasi A. H. An extracorporeal complexing hemodialysis system for the treatment of methylmercury poisoning. I. In vitro studies of the effects of four complexing agents on the distribution and dialyzability of methylmercury in human blood. J Pharmacol Exp Ther. 1975 Feb;192(2):260–269. [PubMed] [Google Scholar]
- Kostyniak P. J., Soiefer A. I. A methylmercury toxicity model to test for possible adverse effects resulting from chelating agent therapy. J Appl Toxicol. 1984 Aug;4(4):206–210. doi: 10.1002/jat.2550040409. [DOI] [PubMed] [Google Scholar]
- Lewis P. A., Woodward A. J., Maddock J. High-performance liquid chromatographic assay for N-acetylcysteine in plasma and urine. J Pharm Sci. 1984 Jul;73(7):996–998. doi: 10.1002/jps.2600730736. [DOI] [PubMed] [Google Scholar]
- Lorber A., Baumgartner W. A., Bovy R. A., Chang C. C., Hollcraft R. Clinical application for heavy metal-complexing potential of N-acetylcysteine. J Clin Pharmacol. 1973 Aug-Sep;13(8):332–336. doi: 10.1002/j.1552-4604.1973.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Magos L., Clarkson T. W. Atomic absorption determination of total, inorganic, and organic mercury in blood. J Assoc Off Anal Chem. 1972 Sep;55(5):966–971. [PubMed] [Google Scholar]
- Magos L., Clarkson T. W. The effect of oral doses of a polythiol resin on the excretion of methylmercury in mice treated with cysteine, D-penicillamine or phenobarbitone. Chem Biol Interact. 1976 Aug;14(3-4):325–335. doi: 10.1016/0009-2797(76)90111-3. [DOI] [PubMed] [Google Scholar]
- Magos L. The effects of dimercaptosuccinic acid on the excretion and distribution of mercury in rats and mice treated with mercuric chloride and methylmercury chloride. Br J Pharmacol. 1976 Apr;56(4):479–484. doi: 10.1111/j.1476-5381.1976.tb07460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L. R., Holdiness M. R., Gillen L. E. N-acetylcysteine: its bioavailability and interaction with ifosfamide metabolites. Semin Oncol. 1983 Mar;10(1 Suppl 1):56–61. [PubMed] [Google Scholar]
- Ornaghi F., Ferrini S., Prati M., Giavini E. The protective effects of N-acetyl-L-cysteine against methyl mercury embryotoxicity in mice. Fundam Appl Toxicol. 1993 May;20(4):437–445. doi: 10.1006/faat.1993.1054. [DOI] [PubMed] [Google Scholar]
- Ottenwälder H., Simon P. Differential effect of N-acetylcysteine on excretion of the metals Hg, Cd, Pb and Au. Arch Toxicol. 1987 Jul;60(5):401–402. doi: 10.1007/BF00295763. [DOI] [PubMed] [Google Scholar]
- Planas-Bohne F. The influence of chelating agents on the distribution and biotransformation of methylmercuric chloride in rats. J Pharmacol Exp Ther. 1981 May;217(2):500–504. [PubMed] [Google Scholar]
- Prescott L. F., Illingworth R. N., Critchley J. A., Stewart M. J., Adam R. D., Proudfoot A. T. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J. 1979 Nov 3;2(6198):1097–1100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenstein D., DeCoster A., Gazzaniga A. Pharmacokinetics of oral acetylcysteine: absorption, binding and metabolism in patients with respiratory disorders. Clin Pharmacokinet. 1978 May-Jun;3(3):247–254. doi: 10.2165/00003088-197803030-00005. [DOI] [PubMed] [Google Scholar]
- SHEFFNER A. L. The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-L-cysteine. Ann N Y Acad Sci. 1963 Mar 30;106:298–310. doi: 10.1111/j.1749-6632.1963.tb16647.x. [DOI] [PubMed] [Google Scholar]
- Sheffner A. L., Medler E. M., Bailey K. R., Gallo D. G., Mueller A. J., Sarett H. P. Metabolic studies with acetylcysteine. Biochem Pharmacol. 1966 Oct;15(10):1523–1535. doi: 10.1016/0006-2952(66)90197-3. [DOI] [PubMed] [Google Scholar]
- Shih V. E., Schulman J. D. N-acetylcysteine-cysteine disulfide excretion in the urine following N-acetylcysteine administration. J Pediatr. 1969 Jan;74(1):129–131. doi: 10.1016/s0022-3476(69)80022-3. [DOI] [PubMed] [Google Scholar]
- Shum S., Skarbovig J., Habersang R. Acute lethal arsenite poisoning in mice: effect of treatment with N-acetylcysteine, D-penicillamine and dimercaprol on survival time. Vet Hum Toxicol. 1981;23(Suppl 1):39–42. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Toribara T. Y. Preparation of CH3 203HgCl of high specific activity. Int J Appl Radiat Isot. 1985 Nov;36(11):903–904. doi: 10.1016/0020-708x(85)90025-0. [DOI] [PubMed] [Google Scholar]
- Zimmer L. J., Carter D. E. The efficacy of 2,3-dimercaptopropanol and D-penicillamine on methyl mercury induced neurological signs and weight loss. Life Sci. 1978 Sep 11;23(10):1025–1034. doi: 10.1016/0024-3205(78)90662-8. [DOI] [PubMed] [Google Scholar]