Abstract
Wolbachia spp. are strict intracellular bacteria that infect a wide range of arthropods and filarial nematodes. Filarial nematodes are important causes of human diseases. There is increasing evidence that Wolbachia spp. influence important functions in the biology of the hosts, specifically, infertility. Preliminary experiments with humans and animals have suggested that antibiotics with activity against Wolbachia may help to treat filariasis. In this study, we determined using a real-time quantitative PCR assay the growth kinetics of a strain of Wolbachia pipientis from a mosquito grown in Aa23 cells. The doubling time was estimated to be 14 h. We then determined the susceptibilities of this strain to 13 antibiotics by two methods: an immunofluorescent-antibody test and a real-time quantitative PCR assay. Both techniques gave similar results. Doxycycline and rifampin were the most effective compounds, with MICs of 0.125 and 0.06 to 0.125 μg/ml, respectively. Fluoroquinolones were less effective, with MICs of 2 to 4 μg/ml for ciprofloxacin, 2 μg/ml for ofloxacin, and 1 μg/ml for levofloxacin. β-Lactams (penicillin G, amoxicillin, ceftriaxone) were not effective at concentrations up to 128 μg/ml. The MIC of erythromycin was >32 μg/ml, whereas that of telithromycin was 8 μg/ml. Other antibiotic compounds were bacteriostatic only at high concentrations, including gentamicin, co-trimoxazole, and thiamphenicol. The real-time PCR assay was a convenient and reliable technique for determination of the antibiotic susceptibilities of Wolbachia. It may help in the future to simplify antibiotic susceptibility testing of strict intracellular pathogens.
Filariasis affect approximately 150 million people in the world (30). It is an important cause of morbidity in the tropics (25, 30). The drugs used to treat filariasis, including diethylcarbamazine and ivermectin, are in general considered effective only against mature microfilariae, but they may be effective against adult worms. This is a major problem for the treatment of filariasis because adult worms live for up to 15 years, which explains the reappearance of microfilariae several months after treatment (25, 30). Thus, mass treatment should be applied for prolonged periods to interrupt the transmission of filarial nematods. Wolbachia spp. are endosymbiotic bacteria that infect a large number of arthropods and filarial worm species (32). All filarial parasites responsible for human diseases studied so far by PCR are infected with Wolbachia, with the possible exception of Loa loa worms (4, 32). Investigators have recently emphasized the association between Wolbachia and filarial nematods and have suggested that the Wolbachia bacteria of filarial parasites could provide a novel target for antibiotic-based therapy. Since 1993, several studies with animals or humans have suggested that antibiotics with activities against Wolbachia may help eradicate filariasis (2, 3, 11, 13, 14, 15, 18, 28, 32, 33, 35), due to induction of degeneration and sterility in adult worms. At present, no nematode-derived cell culture system for Wolbachia is available. However, Wolbachia pipientis, a species that infects arthropods, was cultured in a continuous cell line obtained from the mosquito Aedes albopictus (19). In initial work, Hermans et al. (12) studied the susceptibilities of this strain to five antibiotics by a Giemsa staining-based method. In the present study, we investigated the growth kinetics and the efficiencies of 13 antibiotics against the same strain of W. pipientis grown in an insect cell culture system. Bacterial growth was detected by two methods, an immunofluorescent-antibody (IFA) test and a real-time PCR assay.
MATERIALS AND METHODS
Strain cultivation.
W. pipientis was cultured in Aa23 cell monolayers grown in 150-cm2 cell culture flasks with 30 ml of a mixture (1:1 [vol/vol]) of Mitsuhashi-Maramorosh insect medium (Sigma, St. Louis, Mo.) and Schneider's insect medium (Sigma). The mixture was supplemented with 10% bovine fetal serum (Gibco, Cergy Pontoise, France). A cell line from an A. albopictus mosquito (Aa23 cells) infected with W. pipientis was kindly supplied by Mark Taylor (Liverpool School of Tropical Medicine, Liverpool, United Kingdom). The cells were cured of infection in our laboratory by adding 10 μg of doxycycline per ml in culture medium for three passages. All cell culture flasks were incubated at 28°C. Medium was replaced every week.
Growth kinetics.
W. pipientis was grown in a 150-cm2 tissue culture flask for 6 days, and then the cells were harvested. To quantify growth, 2.5 μl of 10-fold serial dilutions (10−1 to 10−6) of the suspension were deposited onto wells of 30-well microscope slides (Dynatech Laboratories Ltd., Billingshurt, United Kingdom) and stained with Diff-Quick*. The numbers of bacteria in five different fields were counted at ×1,000 magnification of the appropriate dilution. The number of fields per well was calculated on the basis of the surface area of a field at ×1,000 magnification and the surface area of a one-well microscope slide. The number of bacteria per milliliter was then evaluated on the basis of the average number of bacteria counted in the five different fields, the number of fields per well, the volume of the suspension of bacteria deposited per well, and the dilution used to count the bacteria.
Aa23 cells were cultured in 24-well microtiter plates (Costar, Cambridge, England). Each row was infected with a fresh suspension containing 103 bacteria, as determined by the method described above. The cells were incubated for 9 days at 28°C. The viability of the bacteria was demonstrated by the growth of the Wolbachia inoculum in subculture. Cell cultures were harvested each day by gently scraping the cells with a pipette, followed by verification with an inverted microscope that all the cells had been removed. The suspension was then stored at −20°C in sterile tubes for PCR assays to determine the growth kinetics by the real-time PCR assays.
Antibiotic susceptibility.
The antibiotics tested were penicillin G (Diamant, Paris, France), amoxicillin (Beecham-Sevigne, Paris, France), ceftriaxone (Roche, Paris, France), doxycycline (Pfizer, Neuilly, France), rifampin (Cassene, Puteaux, France), co-trimoxazole (Roche), ciprofloxacin (Bayer Pharma, Sens, France), ofloxacin (Diamant), telithromycin (Hoescht-Marion-Roussel, Romainville, France), levofloxacin (Hoescht-Marion-Roussel), erythromycin (Abbott Laboratories, Rungis, France), gentamicin (Dakota Pharm, Creteil, France), and thiamphenicol (Sanofi Winthrop, Gentilly, France). Stock solutions of all antibiotics except telithromycin were prepared by solubilization of antibiotic powders in sterile distilled water; telithromycin was first dissolved in methanol before being diluted in sterile distilled water. Stock solutions of the antibiotics to be tested were prepared and stored at −20°C. Final antibiotic solutions were made up fresh before use by dilution of concentrated stock solutions in culture medium.
For the antibiotic assays, Aa23 cells cultured in 24-well microtiter plates (Costar) were infected with a W. pipientis inoculum for 1 h at room temperature. The antibiotics were added at serial twofold concentrations in rows, with three different rows used for each concentration. Drug-free rows infected with W. pipientis served as growth (positive) controls. Uninfected rows served as negative controls. At day 0, six rows of infected and uninfected cells were harvested and stored at −20°C for the determination of the primary inoculum by quantitative PCR. The microplates were incubated for 6 days at 28°C. At the end of the experiments, the contents of the wells in the rows were harvested and were either stored at −20°C to perform the real-time PCR assay or centrifuged on a slide to perform the IFA test. The experiments were performed three different times to confirm the results.
Animal immunization.
Rabbit polyclonal antibodies were produced as follows. Rabbits were immunized by intradermal inoculation of a total of 1 mg of purified bacteria and Freund's complete adjuvant. Rabbits were given a booster immunization by intramuscular inoculation on day 28. Serum was sampled on day 45 and was frozen at −80°C for further studies.
IFA test.
Cells were harvested, centrifuged on a slide by using the Cytospin II system, dried, and revealed by immunofluorescence with homemade rabbit polyclonal serum. For immunofluorescence staining, the slides were fixed for 10 min with methanol. One hundred microliters of the primary antibody (rabbit polyclonal antibodies) was diluted 1:1,600 in phosphate-buffered saline (PBS) with 3% (wt/vol) nonfat dry milk, added to the slides, and incubated in a moist chamber at 37°C for 30 min. After three washes in PBS, the slides were incubated for 30 min at 37°C with 100 μl of a fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin (bioMérieux) diluted 1:200 in PBS containing 0.2% Evans blue. After three washes with PBS, the slides were mounted in buffered glycerol (Fluoprep; bioMérieux) and read with a Zeiss epifluorescence microscope at ×400 magnification to count the number of intracellular W. pipientis organisms at each antibiotic concentration. The minimal antibiotic concentration that allowed complete inhibition of the growth of bacteria compared to the growth of the drug-free control was recorded as the MIC.
Real-time PCR.
Real-time PCR was performed on a LightCycler instrument (Roche Biochemicals, Mannheim, Germany). The LightCycler instrument is a combined thermocycler and fluorimeter that offers rapid thermocycling (45 min). Samples and the PCR master mixture are contained in 30-μl glass cuvettes. Sample detection is based on the principle of fluorescence resonance energy transfer, with adjacent hybridization probes directed against the intended PCR product. With fluorescein serving as the donor fluorophore and LC-Red 640 (Roche Biochemicals) serving as the acceptor fluorophore, the presence of PCR amplicons is assessed by detection of LC-Red 640 fluorescence. Samples are assayed for the presence of this signal in real time during each PCR cycle, and the cycle number at which the signal is first detected is correlated to the original concentration of DNA. The specificity of amplification is confirmed by melting curve analysis. Single melting peaks are generated by depicting the negative derivative of the fluorescence versus the derivative of the temperature (−dF/dT) over the course of gradual melting of the PCR product. Extraction of DNA was performed with the High Pure 16 System viral nucleic acid kit (Roche Biochemicals), according to the instructions of the manufacturer. PCR master mixtures were prepared according to the instructions of the manufacturer by using primers WolbF (5′-GATCCTTTAAAAGCATCTTT-3′) and WolbR (5′-CACCAGCTTTTGCTTGAT-3′), which target the wsp (Wolbachia surface protein) gene. In each experiment the 20-μl sample volume in each capillary tube contained 2 μl of LightCycler DNA Master SYBR Green (Roche Biochemicals), 1.6 μl of MgCl2, 1 μl of each primer at 0.5 μM, 1.4 μl of sterile distilled water, and 2 μl of DNA, as proposed by the manufacturer. After one pulse of centrifugation to allow mixing and to drive the mix into the distal end of each tube, the glass capillaries were placed in the LightCycler instrument. The amplification program included an initial denaturation step for 1 cycle at 95°C for 8 min and 40 cycles of denaturation at 95°C for 10 s, annealing at 48°C for 10 s, and extension at 72°C for 20 s. Melting curve analysis was done at 45 to 90°C (temperature transition, 20°C/s) with stepwise fluorescence acquisition by real-time measurement of the fluorescence directly in the clear glass capillary tubes. Sequence-specific standard curves were generated by using 10-fold serial dilutions of a standard concentration of W. pipientis. The number of copies of each sample transcript was then calculated from the standard curve with the LightCycler software. The MIC was defined as the first antibiotic concentration that allowed the inhibition of bacterial growth compared to the growth (number of DNA copies) at day 0.
RESULTS
Figure 1 shows the growth kinetics of W. pipientis determined by quantitative PCR assay. The primary inoculum was found to contain approximately 103 DNA copies/ml. After latency of approximately 24 h, the growth of W. pipientis was exponential, with a nearly 2.5-log increase in the bacterial load after 6 days of incubation of the cultures. Thus, the doubling time during this period could be evaluated to be 14 h. Then, bacterial growth reached a plateau for the following 3 days. This growth curve allowed us to test the bacteriostatic activities of antibiotics against W. pipientis in Aa23 cells. MICs were determined by two methods: an IFA test and quantitative PCR assay. Figure 2 shows the data obtained by the quantitative PCR assay. The melting curve obtained with standard concentrations of W. pipientis confirms the specificity of the PCR product, with a single peak obtained for all PCR products. The antibiotic susceptibilities of W. pipientis are presented in Table 1. Doxycycline and rifampin were the most effective compounds, with MICs of 0.125 and 0.06 to 0.125 μg/ml, respectively. Fluoroquinolone compounds were bacteriostatic, with MICs of 1 μg/ml for levofloxacin, 2 μg/ml for ofloxacin, and 2 to 4 μg/ml for ciprofloxacin. The macrolide compound erythromycin did not inhibit W. pipientis growth at concentrations up to 32 μg/ml, whereas the ketolide compound telithromycin was effective at concentrations of 4 to 8 μg/ml. The MICs of gentamicin (32 μg/ml), thiamphenicol (32 μg/ml), and co-trimoxazole (32 μg/ml for sulfamethoxazole and 8 μg/ml for trimethoprim) for W. pipientis were high. Growth was not inhibited by β-lactam compounds (i.e., penicillin G, amoxicillin, or ceftriaxone) at concentrations up to 128 μg/ml.
FIG. 1.
Growth kinetics of W. pipientis in culture as determined by quantitative PCR assay.
FIG. 2.
Light Cycler PCR melting curve obtained with standard concentrations of W. pipientis showing the specificity of the PCR product as a single peak.
TABLE 1.
In vitro susceptibilities of W. pipientis to antibiotics determined by two methods
| Antibiotic | MIC (μg/ml)
|
|
|---|---|---|
| Immunofluorescence staining method | LightCycler real-time PCR | |
| Penicillin G | >128 | >128 |
| Amoxicillin | >128 | >128 |
| Ceftriaxone | >128 | >128 |
| Gentamicin | 32 | 32 |
| Thiamphenicol | 32 | 16 |
| Doxycycline | 0.125 | 0.125 |
| Erythromycin | >32 | >32 |
| Telithromycin | 8 | 4 |
| Rifampicin | 0.125 | 0.0625 |
| Co-trimoxazole | 32/8 | 32/8 |
| Ciprofloxacin | 2 | 4 |
| Ofloxacin | 2 | 2 |
| Levofloxacin | 1 | 1 |
DISCUSSION
The recent culture of W. pipientis and the new concept that antibiotics may help in the treatment of filariasis led us to develop and standardize techniques to test the antibiotic susceptibilities of this species. Since Wolbachia organisms are obligate intracellular bacteria, in vitro studies of their susceptibilities to antibiotics require the use of cell culture systems. To test the antibiotic susceptibilities of W. pipientis, we have used two different techniques: an IFA test and a new quantitative PCR assay with the LightCycler system. The former method, which is based on enumeration of bacteria after immunofluorescence antibody staining, has been known to be a valuable technique for testing of the antibiotic susceptibilities of intracellular bacteria (21). However, it is very fastidious and time-consuming; and the recent development of new quantitative PCR systems, such as the LightCycler system, which are more rapid and reproducible, has focused our attention on such systems. The LightCycler quantitative PCR assay has already been used for the diagnosis of tuberculosis (10) and the detection of antibiotic-resistant strains of Mycobacterium tuberculosis (34) and only recently has been used for the determination of MICs and the growth kinetics of Rickettsia in our laboratory (24). For the antibiotic susceptibility tests, the results obtained with the LightCycler system were comparable to those obtained by IFA. The LightCycler technique is specific, reproducible, easy to perform, and rapid. Moreover, this technique allowed us to evaluate the growth kinetics of W. pipientis for the first time. In culture, W. pipientis grows rapidly, with maximum numbers of infected cells achieved after 6 days, which corresponds to a doubling time of 14 h. This doubling time has not been evaluated before.
Our data from the antibiotic susceptibility testing of W. pipientis expand those of Hermans et al. (12). W. pipientis was found to be highly susceptible to doxycycline in vitro. Data for other antibiotics are limited. In vitro and in vivo studies with a worm (Brugia pahangi) model have previously shown that rifampin is active against W. pipientis (35). Rifampin was also found to be highly active in vitro against W. pipientis (12). Both techniques confirm the activity of rifampin. Ciprofloxacin has been reported to be inactive in vitro (MIC > 8 μg/ml) in worms (12, 15). Levofloxacin, the l isomer of ofloxacin was the most effective fluoroquinolone tested (MIC = 1 μg/ml), whereas the maximal concentration of this compound achievable in human serum is 6.5 μg/ml (29). Ciprofloxacin seems less promising, as its MICs range from 2 to 4 μg/ml and the maximal concentration achievable in serum is 2.5 μg/ml (26). The MIC of ofloxacin was 2 μg/ml, double that of levoflaxin. W. pipientis is resistant to erythromycin, co-trimoxazole, thiamphenicol, and β-lactams, as is the case for Ehrlichia spp. (5, 6, 7, 16, 17, 22).
The in vitro activity of doxycycline is in accordance with in vivo data on the activities of doxycycline against nematodes in animal and human filarial infections. If antibiotic therapy could be administered for filariasis, doxycycline seems to be the most promising compound. However, the antifilarial properties of rifampin merit further investigation in animal models or clinical trials. This drug could be a good alternative to doxycycline in young children, pregnant woman, or patients allergic to doxycycline. The other antibiotics tested are unlikely to be useful for the treatment of filariasis.
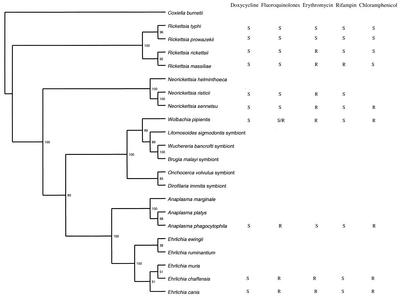
An important issue is the possibility of extrapolating the antibiotic susceptibilities of W. pipientis to those of Wolbachia spp. infecting nematodes. Initial phylogenetic analyses have shown that the Wolbachia endosymbionts of filarial nematodes are closely related to Wolbachia endosymbionts of arthropods (27). Additional phylogenetic analyses by comparison of ftsZ sequences showed that all filarial Wolbachia spp. are closely related and, in general, form a group separate from the Wolbachia spp. of arthropods (1, 31). Analysis of the ftsZ gene has shown that the Wolbachia spp. infecting filarial nematods segregate into two clusters (clusters C and D), which diverge from the clusters (clusters A and B) recognized for arthropod Wolbachia. A representative phylogenetic 16S rRNA tree constructed by the parsimony method with the TreeView program (20) shows the association of the intracellular bacteria Rickettsia spp., Ehrlichia spp., Anaplasma spp., Neorickettsia spp., W. pipientis, and several Wolbachia endosymbionts from nematodes and their susceptibilities to five antibiotics (Fig. 3). The phylogenetic studies based on 16S rRNA gene sequences have placed W. pipientis together with the genera Ehrlichia, Neorickettsia, and Anaplasma; and species belonging to these different genera have been placed into one of the four following phylogenetic groups (8, 9). The Neorickettsia genus contains Neorickettsia sennetsu, Neorickettsia risticii, and Neorickettsia helminthoeca. The Ehrlichia genus includes Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia ewingii, Ehrlichia muris, and Ehrlichia ruminatium. The Anaplasma genus includes Anaplasma phagocytophila (including Anaplasma platys and the agent of human granulocytic ehrlichiosis) and Anaplasma marginale. The fourth genus includes only Wolbachia spp. that are arthropod and nematode endosymbionts. It is noteworthy that all fluoroquinolone-resistant strains belonged to a single phylogenetic subgroup. Fluoroquinolone resistance may reflect a divergence during the evolution of this subgroup. Likewise, the high levels of susceptibility of the typhus group rickettsiae (Rickettsia typhi and Rickettsia prowazekii) to erythromycin compared to those of the spotted fever group rickettsiae (Rickettsia rickettsii and Rickettsia massiliae) and Ehrlichia spp. tested and W. pipientis may reflect a divergent strategic evolution involving susceptibility to macrolide antibiotics (23). We have also observed that all members of the Rickettsia group are susceptible to chloramphenicol, whereas all Ehrlichia spp., Anaplasma spp., Neorickettsia spp., and W. pipientis are resistant (23). Besides, it was recently described that all rifampin-resistant strains of Rickettsia (R. massiliae, Rickettsia rhipicephali, Rickettsia aeschlimanni, Rickettsia montana, Bar 29) belonged to a single phylogenetic group (23). In our phylogenetic tree, it is noteworthy that not only all fluoroquinolone-resistant strains but also all erythromycin-susceptible strains each belonged to a single phylogenetic subgroup. Based on these examples, we can speculate that the Wolbachia endosymbiont from nematodes, which clearly belongs to the genogroup W. pipientis, may have antibiotic susceptibilities comparable to those of W. pipientis.
FIG.3.
Phylogenetic tree based on 16S rRNA gene sequences showing the association of the intracellular bacteria of the Rickettsia spp., Ehrlichia spp., Anaplasma spp., Neorickettsia spp., W. pipientis, and several Wolbachia endosymbionts from nematods and correlation between doxycycline, fluoroquinolone, and erythromycin susceptibilities. R, resistant; S, susceptible. For doxycycline susceptibility was considered an MIC ≤4 μg/ml, for fluoroquinolones susceptibility was considered an MIC ≤2 μg/ml and resistance was considered an MIC >2 μg/ml, for erythromycin susceptibility was considered an MIC ≤1 μg/ml and resistance was considered an MIC >1 μg/ml, for rifampin susceptibility was considered an MIC ≤1 μg/ml and resistance was considered an MIC >1 μg/ml, and for chloramphenicol susceptibility was considered an MIC ≤8 μg/ml and resistance was considered an MIC >8 μg/ml. The numbers at the nodes represent the bootstrap confidence value after 100 replicates. Coxiella burnetii was defined as the outgroup. The sequences of the following species were used in the construction of the tree (GenBank accession numbers are given in parentheses): C. burnetii (D89799), R. typhi (L36221), R. prowazekii (M21789), R. massiliae (L36106), R. rickettsii (L36217), N. risticii (AF037211), N. sennetsu (M73225), N. helminthoeca (U12457), W. pipientis (X61768), A. marginale (AF311303), A. platys (AF287153), A. phagocytophila (M73224), Cowdria ruminantium (U03777), E. ewingii (U96436), E. canis (AF373613), E. muris (U15527), E. chaffeensis (U60476), Wuchereria bancroftii endosymbiont (AF093510), B. malayi endosymbiont (AF051145), Onchocerca volvulus endosymbiont (AF069069), L. sigmodontis endosymbiont (AF069068), and Dirofilaria immitis endosymbiont (Z49261).
In a model of infection with the worm Litomosoides sigmodontis, penicillin, gentamicin, the macrolides (erythromycin and azithromycin), ciprofloxacin, and chloramphenicol were shown to be ineffective against filarial Wolbachia (13, 15), whereas doxycycline was active. These in vivo data from experimental models are consistent with those from the model described here, but the possibility that different pharmacokinetics may result in different outcomes in other models of filariasis cannot be excluded. Clinical trials are necessary and are under way to determine whether antibiotic therapy could be effective in the control of filarial parasites. Besides, the possibility of establishing cell cultures for nematode-associated Wolbachia spp. is desirable for the development of better assays for the screening of antibiotics that can be used to treat filariasis.
In conclusion, our report describes the susceptibilities of a mosquito strain of Wolbachia, W. pipientis, to a wide range of antibiotics by a new quantitative PCR assay. Our results show that doxycycline and rifampin are highly effective against W. pipientis and that the fluoroquinolone compounds are poorly active, whereas co-trimoxazole, telithromycin, β-lactams, gentamicin, thiamphenicol, and erythromycin are not active. These results seems atypical for strict intracellular bacteria but are similar to those for Ehrlichia spp., Neorickettsia spp., and Anaplasma spp. Real-time quantitative PCR seems to be a good tool for evaluation of the antibiotic susceptibilities of strict intracellular bacteria.
Acknowledgments
We are indebted to Kelly Johnston and Mark Taylor for reviewing the manuscript.
REFERENCES
- 1.Bandi, C., T. J. C. Anderson, C. Genchi, and M. L. Blaxter. 1998. Phylogeny of Wolbachia in filarial nematodes. Proc. R. Soc. London 265:2407-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandi, C., J. W. McCall, C. Genchi, S. Corona, L. Venco, and L. Sacchi. 1999. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int. J. Parasitol. 29:357-364. [DOI] [PubMed] [Google Scholar]
- 3.Boohardt, S. C., J. W. McCall, S. U. Coleman, K. L. Jones, T. A. Petit, and T. R. Kiel. 1993. Prophylactic activity of tetracyclines against Brugia pahangi infection in jirds (Meriones unguiculatus). J. Parasitol. 79:775-777. [PubMed] [Google Scholar]
- 4.Brouqui, P., P. E. Fournier, and D. Raoult. 2001. Doxycycline and eradication of microfilaremia in patients with loiasis. Emerg. Infect. Dis. 7(Suppl. 3):604-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouqui, P., and D. Raoult. 1990. In vitro susceptibility of Ehrilichia sennetsu to antibiotics. Antimicrob. Agents Chemother. 34:1593-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouqui, P., and D. Raoult. 1992. In vitro antibiotic susceptibility of the newly recognized agent of ehrlichiosis in humans, Ehrlichia chaffeensis. Antimicrob. Agents Chemother. 36:2799-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouqui, P., and D. Raoult. 1993. Susceptibilities of Ehrlichiae to antibiotics, p. 181-199. In D. Raoult (ed.), Antimicrobial agents and intracellular pathogens. CRC Press, Inc., Boca Raton, Fla.
- 8.Dumler, J. S., and J. S. Bakken. 1996. Ehrlichial diseases of humans: emerging tick-borne infections. Clin. Infect. Dis 20:1102-1110. [DOI] [PubMed] [Google Scholar]
- 9.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. E vol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 10.Fenollar, F., P. E. Fournier, R. Gerolami, H. Lepidi, C. Poyart, and D. Raoult. 2002. Quantitative detection of Tropheryma whipplei DNA by real-time PCR. J. Clin. Microbiol. 40:1119-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genchi, C., L. Sacchi, and L. Venco. 1998. Preliminary results on the effects of tetracycline on the embryogenesis and symbiotic bacteria (Wolbachia) of Dirofilaria immitis. An update and discussion. Parasitologia 40:247-249. [PubMed] [Google Scholar]
- 12.Hermans, P. G., C. A. Hart, and A. J. Trees. 2001. In vitro activity of antimicrobial agents against the endosymbiont Wolbachia pipientis. J. Antimicrob. Chemother. 47:659-663. [DOI] [PubMed] [Google Scholar]
- 13.Hoerauf, A., K. Nissen-Paehle, C. Schmetz, K. Henkle-Dührsen, M. L. Blaxter, D. W. Büttner, M. Y. Gallin, K. M. Al-Qaoud, R. Lucius, and B. Fleischer. 1999. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litosmosoides sigmodontis and results in filarial infertility. J. Clin. Investig. 103:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoerauf, A., L. Volkmann, C. Hamelmann, O. Adjei, I. B. Autenrieth, B. Fleischer, and D. W. Büttner. 2000. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet 355:1242-1243. [DOI] [PubMed] [Google Scholar]
- 15.Hoerauf, A., L. Volkmann, K. Nissen-Paehle, C. Schmetz, I. B. Autenrieth, D. W. Büttner, and B. Fleischer. 2000. Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Trop. Med. Int. Health 5:275-279. [PubMed] [Google Scholar]
- 16.Horowitz, H., T. C. Hsieh, M. E. Aguero-Rosenfeld, F. Kalantarpour, I. Chowdhury, G. P. Wormser, and J. M. Wu. 2001. Antimicrobial susceptibility of Ehrlichia phagocytophila. Antimicrob. Agents Chemother. 45:786-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein, M. B., C. M. Nelson, and J. L. Goodman. 1997. Antibiotic susceptibility of the newly cultivated agent of human granulocytic ehrlichiosis: promising activity of quinolones and rifamycins. Antimicrob. Agents Chemother. 41:76-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langworthy, N. G., A. Renz, U. Mackenstedt, K. Henkle-Dührsen, M. B. de C. Bronsvoort, V. N. Tanya, M. J. Donnelly, and A. J. Trees. 2000. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc. R. Soc. London 267:1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neill, S. L., M. M. Pettigrew, S. P. Sinkins, H. R. Braig, T. G. Andreadis, and R. B. Tesh. 1997. In vitro cultivation of Wolbacha pipientis in an Aedes albopictus cell line. Insect Mol. Biol. 6:33-39. [DOI] [PubMed] [Google Scholar]
- 20.Page, R. D. M. 1996. TREEVIEW. An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 21.Raoult, D., H. Torres, and M. Drancourt. 1991. Shell-vial assay: evaluation of a new technique for determining antibiotic susceptibility, tested in 13 isolates of Coxiella burnetii. Antimicrob. Agents Chemother. 35:2020-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rikihisa, Y., and B. M. Jiang. 1988. In vitro susceptibilities of Ehrlichia risticii to eight antibiotics. Antimicrob. Agents Chemother. 32:986-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolain, J. M., M. Maurin, G. Vestris, and D. Raoult. 1998. In vitro susceptibility of 27 rickettsiae to 13 antimicrobials. Antimicrob. Agents Chemother. 42:1537-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolain, J. M., L. Sthul, M. Maurin, and D. Raoult. 2002. Evaluation of antibiotic susceptibilities of three rickettsial species including Rickettsia felis by a quantitative PCR DNA assay. Antimicrob. Agents Chemother. 46:2747-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saint André, A. V., N. M. Blackwell, L. R. Hall, A. Hoerauf, N. W. Brattig, L. Volkmann, M. J. Taylor, L. Ford, A. G. Hise, J. H. Lass, E. Diaconu, and E. Pearlman. 2002. The role of endosymbiontic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892-1895. [DOI] [PubMed] [Google Scholar]
- 26.Schentag, J. J. 2000. Clinical pharmacology of the fluoroquinolones: studies in human dynamic/kinetic model. Clin. Infect. Dis. 31(Suppl. 2):S40-S44. [DOI] [PubMed] [Google Scholar]
- 27.Sironi, M., C. Bandi, L. Sacchi, B. Di Sacco, G. Damiani, and C. Genchi. 1995. Molecular evidence for a close relative of the arthropod endosymbiont Wolbachia in filarial nematodes. Mol. Biochem. Parasitol. 75:223-227. [DOI] [PubMed] [Google Scholar]
- 28.Smith, H. L., and T. V. Rajan. 2000. Tetracyclines inhibit development of the infective-stage larvae of filarial nematodes in vitro. Exp. Parasitol. 95:265-270. [DOI] [PubMed] [Google Scholar]
- 29.Stein, G. E. 1996. Pharmacokinetics and pharmacodynamics of newer fluoroquinolones. Clin. Infect. Dis. 23(Suppl. 1):S19-S24. [DOI] [PubMed] [Google Scholar]
- 30.Taylor, M. J., C. Bandi, A. Hoerauf, and J. Lazdins. 2000. Wolbachia bacteria of filarial nematodes. A target for control. Parasitol. Today 16:179-180. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, M. J., K. Bilo, H. F. Cross, J. P. Archer, and A. P. Underwood. 1999. 16S rDNA phylogeny and ultrastructural characterization of Wolbachia intracellular bacteria of the filarial nematodes Brugia malayi, B. pahangi and Wuchereria bancrofti. Exp. Parasitol. 91:356-361. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, M. J., and A. Hoerauf. 1999. Wolbachia bacteria of filarial nematodes. Parasitol. Today 15:437-442. [DOI] [PubMed] [Google Scholar]
- 33.Taylor, M. J., and A. Hoerauf. 2001. A new approach to the treatment of filariasis. Curr. Opin. Infect. Dis. 14:727-731. [DOI] [PubMed] [Google Scholar]
- 34.Torres, M. J., A. Criado, J. C. Palomares, and J. C. Aznar. 2000. Use of real-time PCR and fluorimetry for rapid detection of rifampin and isoniazid resistance-associated mutations in Mycobacterium tuberculosis. J. Clin. Microbiol. 38:3194-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towson, S., D. Hutton, J. Siemienska, L. Hollick, T. Scanlon, and S. K. Tagboto. 2000. Antibiotics and Wolbachia in filarial nematodes: antifilarial activity of rifampicin, oxytetracycline and chloramphenicol against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Ann. Trop. Med. Parasitol. 94:801-816. [DOI] [PubMed] [Google Scholar]