Abstract
AM-112 [(1′R,5R,6R)-3-(4-amino-1,1-dimethyl-butyl)-6-(1′-hydroxyethyl)oxapenem-3-carboxylate] is a novel oxapenem compound which possesses potent β-lactamase-inhibitory properties. Fifty-percent inhibitory concentrations (IC50s) of AM-112 for class A enzymes were between 0.16 and 2.24 μM for three enzymes, compared to IC50s of 0.008 to 0.12 μM for clavulanic acid. Against class C and class D enzymes, however, the activity of AM-112 was between 1,000- and 100,000-fold greater than that of clavulanic acid. AM-112 had affinity for the penicillin-binding proteins (PBPs) of Escherichia coli DC0, with PBP2 being inhibited by the lowest concentration of AM-112 tested, 0.1 μg/ml. Ceftazidime was combined with AM-112 at 1:1 and 2:1 ratios in MIC determination studies against a panel of β-lactamase-producing organisms. These studies demonstrated that AM-112 was effective at protecting ceftazidime against extended-spectrum β-lactamase-producing strains and derepressed class C enzyme producers, reducing ceftazidime MICs by 16- and 2,048-fold. Similar results were obtained when AM-112 was combined with ceftriaxone, cefoperazone, or cefepime in a 1:2 ratio. Protection of ceftazidime with AM-112 was maintained against Enterobacter cloacae P99 and Klebsiella pneumoniae SHV-5 in a murine intraperitoneal sepsis model. The 50% effective dose of ceftazidime against E. cloacae P99 and K. pneumoniae SHV-5 was reduced from >100 and 160 mg/kg of body weight to 2 and 33.6 mg/kg, respectively, when it was combined with AM-112 at a 1:1 ratio. AM-112 demonstrates potential as a new β-lactamase inhibitor.
β-Lactamase-mediated resistance is one of the most important mechanisms of antibiotic resistance for bacteria (14). β-Lactamase inhibitors offer the means of overcoming this resistance, and the currently used inhibitors clavulanic acid, tazobactam, and sulbactam have found widespread clinical use. These inhibitors are highly active against class A and extended-spectrum β-lactamases (ESBLs) but lack significant activity against class C and class D enzymes (4, 18). There are currently no marketed β-lactamase inhibitors with good activity against class C and class D enzymes.
Extended-spectrum cephalosporins are generally less susceptible to class A β-lactamases but are readily hydrolyzed by ESBLs and class C enzymes (20). ESBLs are becoming increasingly widespread and represent the fastest-growing subgroup of β-lactamase enzymes (5). The chromosomal class C enzymes can become plasmid-borne and thus readily spread between bacterial species (6, 13, 22). Both of these factors threaten the future clinical usefulness of expanded spectrum cephalosporins, and there is an urgent requirement for new β-lactamase inhibitors which combine activity against class A, class C, and class D enzymes as well as ESBLs.
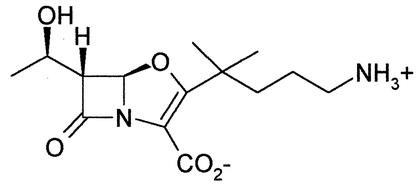
Oxapenems, containing a five-membered, oxygen-containing ring fused to the β-lactam ring with a double bond between C2 and C3, were first described in the late 1970s (9). They were found to have potent β-lactamase-inhibitory effects but poor stability. Pfaendler et al. (19) synthesized a novel series of more-stable oxapenems with potent β-lactamase-inhibitory properties. AM-112 [(1′R,5R,6R)-3-(4-amino-1,1-dimethyl-butyl)-6-(1′-hydroxyethyl)oxapenem-3-carboxylate] is one such novel oxapenem (Fig. 1). We have reported preliminary findings on the activity of AM-112 (C. E. Jamieson, P. A. Lambert, and I. N. Simpson, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F381 and F383, 2001). Here we report on the in vivo and in vitro activities of AM-112 in combination with various cephalosporins.
FIG. 1.
Chemical structure of AM-112.
MATERIALS AND METHODS
Antibacterial agents.
AM-112 was obtained from Amura Ltd. (Cambridge, United Kingdom). All other antibiotics were obtained from commercial sources.
Organisms.
Escherichia coli J53 transconjugants (TEM-1, TEM-3, TEM-6, TEM-9, TEM-10, SHV-1, SHV-2, SHV-3, SHV-4, SHV-5, OXA-1, OXA-2, OXA-3, OXA-5, and PSE-4) (see Tables 1 to 3) and the stably derepressed, constitutive β-lactamase-producing (con) strains Enterobacter cloacae 84-con, Citrobacter freundii C2-con, Serratia marcescens S2-con, Moraxella morganii M1-con, and Pseudomonas aeruginosa 1405-con and 2297-con (see Tables 2 and 3) were kindly supplied by D. M. Livermore, Central Public Health Laboratory, Colindale, London, United Kingdom. E. cloacae 1051E P99, E. cloacae 1194E Hennessey, and E. coli DC0 (10) were obtained from the GlaxoSmithKline culture collection. The mouse-virulent strains of Staphylococcus aureus (3816) and Klebsiella pneumoniae SHV-5 used in the in vivo infection models were supplied by Biosearch Italia and D. M. Livermore, respectively.
TABLE 1.
IC50 and Ki (preincubation) values (μM) of clavulanic acid and AM-112 against isolated β-lactamase enzymes
| Enzyme (group/classa) and inhibitor | IC50 | Ki (pre-inc.b) |
|---|---|---|
| E. coli | ||
| TEM-1 (2b/A) | ||
| Clavulanic acid | 0.12 | 7.1 × 10−4 |
| AM-112 | 2.26 | 0.014 |
| TEM-10 (2be/A) | ||
| Clavulanic acid | 0.03 | 2.5 × 10−5 |
| AM-112 | 0.224 | 5.6 × 10−5 |
| SHV-5 (2be/A) | ||
| Clavulanic acid | 0.008 | 1 × 10−5 |
| AM-112 | 0.16 | 2 × 10−4 |
| E. cloacae | ||
| P99 (1/C) | ||
| Clavulanic acid | 11 | 0.009 |
| AM-112 | 0.002 | 1.6 × 10−6 |
| S. marcescens | ||
| S2 (1/C) | ||
| Clavulanic acid | 327 | 0.15 |
| AM-112 | 0.07 | 3 × 10−5 |
| P. aeruginosa | ||
| S + A (1/C) | ||
| Clavulanic acid | 449 | 0.086 |
| AM-112 | 0.002 | 3.6 × 10−7 |
| E. coli | ||
| OXA-1 (2d/D) | ||
| Clavulanic acid | 99 | 2.3 |
| AM-112 | 0.005 | 1.1 × 10−4 |
| OXA-5 (2d/D) | ||
| Clavulanic acid | 202 | 0.322 |
| AM-112 | 0.0007 | 1 × 10−6 |
TABLE 3.
MICs of ceftriaxone, ceftazidime, cefoperazone, and cefepime alone and in combination with a 2:1 ratio of AM-112 against a panel of β-lactamase-producing bacteria
| Organism | MIC (μg/ml)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CRO | CRO + AM-112 | CAZ | CAZ + AM-112 | CFP | CFP + AM-112 | FEP | FEP + AM-112 | |
| E. coli J53 TEM-1 | 1.06 | <0.03 | 0.5 | 0.5 | 32 | 8 | 0.125 | 0.06 |
| E. coli J53 TEM-3 | 4 | 2 | 16 | 0.5 | 16 | <0.03 | 2 | <0.03 |
| E. coli J53 TEM-6 | 0.5 | <0.03 | >64 | 0.25 | 8 | <0.06 | <0.03 | <0.03 |
| E. coli J53 TEM-9 | 8 | 2 | >64 | 32 | 64 | 8 | 4 | 2 |
| E. coli J53 TEM-10 | 1 | 1 | >64 | 16 | 8 | 2 | 2 | 1 |
| E. coli J53 SHV-1 | <0.03 | <0.03 | 0.25 | 0.25 | 0.125 | 0.06 | <0.03 | <0.03 |
| E. coli J53 SHV-2 | 0.06 | <0.03 | 0.25 | 0.125 | 0.25 | 0.06 | <0.03 | <0.03 |
| E. coli J53 SHV-3 | 0.06 | <0.03 | 0.25 | <0.03 | 0.5 | <0.03 | <0.03 | <0.03 |
| E. coli J53 SHV-4 | 16 | 2 | >64 | 4 | 32 | 2 | 2 | 1 |
| E. coli J53 SHV-5 | 1 | <0.03 | 32 | 8 | 4 | 0.06 | 0.25 | <0.03 |
| E. coli J53 OXA-1 | 0.06 | <0.03 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.125 |
| E. coli J53 OXA-2 | <0.03 | <0.03 | 0.25 | 0.25 | 64 | 2 | <0.03 | <0.03 |
| E. coli J53 OXA-3 | 0.06 | <0.03 | 0.5 | <0.03 | 1 | 1 | <0.03 | <0.03 |
| E. coli J53 OXA-5 | 0.06 | <0.03 | 0.25 | <0.03 | 0.25 | <0.03 | <0.03 | <0.03 |
| E. cloacae P99 | >64 | 16 | >64 | 8 | >64 | 8 | 2 | 0.5 |
| E. cloacae Hennessey | >64 | 16 | >64 | 16 | >64 | 16 | 4 | 1 |
| E. cloacae 84-con | >64 | 16 | >64 | 32 | >64 | 32 | 4 | 2 |
| S. marcescens S2-con | 4 | 2 | 0.5 | 0.5 | 16 | 4 | 0.25 | 0.125 |
| M. morganii M1-con | 8 | 8 | 8 | 1 | >64 | 32 | 1 | 1 |
| C. freundii C2-con | >64 | 8 | >64 | 8 | 64 | 16 | 1 | 0.5 |
| P. aeruginosa ATCC 27853 | 8 | 4 | 2 | 0.5 | 4 | 2 | 0.5 | 0.5 |
| P. aeruginosa NCTC 10662 | >64 | 8 | 1 | <0.03 | 4 | 4 | 1 | <0.03 |
| P. aeruginosa 1407-con | >64 | >64 | 64 | 64 | >64 | >64 | 16 | 8 |
| P. aeruginosa 2297-con | >64 | >64 | 64 | 64 | >64 | >64 | 4 | 4 |
MICs quoted for antibiotic-AM-112 combinations refer to antibiotic component. AM-112 concentration is half that quoted for each ceftazidime MIC. CRO, ceftriaxone; CAZ, ceftazidime; CFP, cefoperazone; FEP, cefepime.
TABLE 2.
MICs of ceftazidime alone or in combination with AM-112 against β-lactamase-producing bacteria
| Organism | Group/classa | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|
| AM-112 | CAZb | CAZ + AM-112 (1:1)c | CAZ + AM-112 (2:1)b | ||
| E. coli J53 SHV-1 | 2b/A | 32 | 2 | 0.25 | 0.125 |
| E. coli J53 SHV-2 | 2be/A | 16 | 0.25 | 0.125 | 0.125 |
| E. coli J53 SHV-3 | 2be/A | 8 | 0.125 | 0.03 | 0.125 |
| E. coli J53 SHV-4 | 2be/A | 32 | >64 | 4 | 8 |
| E. coli J53 SHV-5 | 2be/A | 32 | 16 | 8 | 16 |
| E. coli J53 TEM-1 | 2b/A | 32 | 0.25 | 0.25 | 0.5 |
| E. coli J53 TEM-3 | 2be/A | 16 | 16 | 4 | 2 |
| E. coli J53 TEM-6 | 2be/A | 32 | >64 | 8 | 4 |
| E. coli J53 TEM-9 | 2be/A | 32 | >64 | 8 | 8 |
| E. coli J53 TEM-10 | 2be/A | 32 | >64 | 8 | 16 |
| P. aeruginosa 1405-con | 1/C | >128 | >64 | 32 | 32 |
| P. aeruginosa 2297-con | 1/C | >128 | >64 | 64 | 64 |
| P. aeruginosa ATCC 27853 | 1/C | >128 | 2 | 4 | 2 |
| E. cloacae P99 | 1/C | 16 | 32 | 4 | 4 |
| E. cloacae Hennessey | 1/C | 32 | >64 | 4 | 4 |
| E. cloacae 84-con | 1/C | 32 | >64 | 4 | 4 |
| C. freundii C2-con | 1/C | 32 | 64 | 0.03 | 2 |
| S. marcescens S2-con | 1/C | 64 | 1 | 0.03 | 0.5 |
| M. morganii M1-con | 1/C | 64 | 8 | 1 | 1 |
| E. coli J53 OXA-1 | 2d/D | 16 | 0.25 | 0.5 | 0.25 |
| E. coli J53 OXA-2 | 2d/D | 16 | 0.25 | 0.25 | 0.25 |
| E. coli J53 OXA-3 | —/D | 32 | 0.5 | 0.5 | 0.5 |
| E. coli J53 OXA-5 | 2d/D | 32 | 0.5 | 0.25 | 0.25 |
Isolation of β-lactamase enzymes.
Isolated enzyme extracts were prepared from the following organisms: E. coli J53 TEM-10, E. coli J53 SHV-5, E. coli J53 OXA-1, E. coli J53 OXA-5, E. cloacae 1051E P99, S. marcescens S2-con, and P. aeruginosa S+A (2). Overnight cultures of each organism were grown to log phase in Mueller-Hinton broth (Oxoid, Basingstoke, United Kingdom) at 37°C. Cells were harvested by centrifugation at 12,100 × g, washed, and resuspended in 10 mM sodium phosphate buffer (pH 7.0). Cells were disrupted by six 10-s cycles of sonication using an MSE Soniprep 150 (MSE Ltd., Crawley, United Kingdom) with constant cooling in an ice bath. Sonicated cells were then centrifuged for 30 min at 15,300 × g at 4°C to remove cell debris. The supernatant was retained and stored at −70°C until required. The crude enzyme extracts were further purified by preparative isoelectric focusing in Sephadex G-75 (Sigma, Poole, United Kingdom) over a pH range of 3.5 to 10 U for 16 to 18 h. The focused plasmidic enzyme (identified by nitrocefin staining) was excised from the gel in the region of its published pI (4) and eluted with sodium phosphate buffer. The eluted enzyme was stored at −70°C until required.
Cell-free β-lactamase inhibition studies.
β-Lactamase activity was determined by a spectrophotometric method using nitrocefin (Oxoid) as the substrate, according to the method of O'Callaghan (17). The assays were carried out in flat-bottomed microtiter plates, in triplicate, with a final reaction volume of 150 μl in each well. For the inhibition studies, the enzyme was preincubated with the inhibitor for 15 min at 37°C prior to the addition of the substrate. The absorbance at 492 nm was measured for a 10-min period after addition of nitrocefin using an Anthos 2001 plate reader (Anthos Labtech, Salzburg, Austria). The temperature was maintained at 37°C for the course of the assay. The initial rates of hydrolysis at all inhibitor concentrations were calculated. The percentage of inhibition for each concentration of inhibitor was determined by comparison to the control enzyme. The 50% inhibitory concentration (IC50) was calculated by nonlinear regression using the enzyme kinetics template of GraphPad Prism 3.02 for Windows (GraphPad Software, San Diego, Calif.), and Ki values (after a 15-min preincubation of enzyme and inhibitor) were calculated using the Cheng and Prusoff equation (8).
In vitro susceptibility tests.
MICs were determined by agar dilution carried out in accordance with NCCLS guidelines. Mueller-Hinton agar was used for MIC determination. The initial inoculum was 105 CFU/ml. The activities of ceftazidime, cefepime, ceftriaxone, and cefoperazone against a panel of β-lactamase-producing strains were determined alone and in combination at either a 1:1 (ceftazidime) or 2:1 (all agents) ratio with AM-112. The MIC was defined as the lowest cephalosporin concentration that prevented visible growth of the bacteria after overnight incubation at 37°C.
PBPs of Escherichia coli DC0.
Cell membranes of E. coli DC0 were prepared according to the method of Spratt (21) and stored at −70°C until required. The penicillin-binding proteins (PBPs) of E. coli DC0 were labeled with 3H-benzylpenicillin (10 to 30 Ci/mmol; Amersham, Little Chalfont, United Kingdom) in competition with AM-112, as described by Bryan and Godfrey (3). Labeled proteins were separated by sodium dodecyl sulfate gel electrophoresis, followed by immersion in Amplify (Amersham) for 30 min. Dried gels were placed in contact with X-ray film (Hyperfilm MP; Amersham) and stored at −70°C for 2 weeks prior to visualization.
In vivo efficacy of AM-112 alone and in combination with ceftazidime in a murine intraperitoneal infection model.
The activity of AM-112 alone and in combination with ceftazidime was determined in a murine intraperitoneal infection model. Three pathogenic strains were used, each of which expressed a β-lactamase enzyme: S. aureus 3816 (class A), K. pneumoniae SHV-5 (class A, ESBL), and E. cloacae P99 (class C). A volume of 0.1 ml of the bacterial suspension (106 to 107 CFU/ml) in 6% hog gastric mucin (Sigma) was inoculated intraperitoneally into either male ICR mice (20 to 22 g; Harlan Sprague Dawley, Indianapolis, Ind.) (10 mice per antibiotic dose; S. aureus 3816), male CD1 mice (20 to 22 g; Harlan Sprague Dawley) (five mice per antibiotic dose; E. cloacae P99), or female ICR mice (20 to 22 g; Harlan Sprague Dawley) (five mice per antibiotic dose; K. pneumoniae SHV-5). Ceftazidime and AM-112, alone and in either a 4:1 and 7:1 combination (S. aureus, 15 min postinfection), a 1:1, 2:1, and 4:1 combination (E. cloacae, 1 and 5 h postinfection), or a 1:1 and 2:1 combination (K. pneumoniae, 1 h postinfection), were administered by subcutaneous injection. The 50% effective dose (ED50) was calculated by the Spearman Kärber method from the survival rate at 4 days after infection. Untreated mice infected with S. aureus 3816, E. cloacae P99, or K. pneumoniae SHV-5 died within 2 days of infection.
RESULTS
β-Lactamase-inhibitory activity.
The inhibitory activity of AM-112 was compared to that of clavulanic acid against a panel of class A, class C, and class D enzymes (Table 1). Nitrocefin was a suitable substrate for the enzymes, with maximum rate (Vmax) values for the native enzymes ranging from 0.4 to 52 nmol/s. Clavulanic acid was an effective inhibitor of the class A enzymes, and in particular the ESBL enzymes TEM-10 and SHV-5. The activity of AM-112 against the class A enzymes was between 10- and 20-fold poorer. AM-112 was a strong inhibitor of the class C enzymes produced by E. cloacae, S. marcescens, and P. aeruginosa. The IC50s ranged from 0.002 to 0.07 μM. Clavulanic acid was poorly active against these enzymes and had IC50s between 103- and 105-fold higher than those of AM-112. AM-112 was also much more active against the two class D enzymes than clavulanic acid. IC50s for AM-112 were between 103- and 104-fold lower than those for clavulanic acid. IC50s for clavulanic acid against class A enzymes were similar to published values, while values for the class C enzymes were approximately 10-fold lower than published values (7, 18). Ki (preincubation) values for both inhibitors showed a pattern similar to that of the IC50s, with clavulanic acid having lower values against the class A enzymes while AM-112 was more potent against the class C and D enzymes (Table 1).
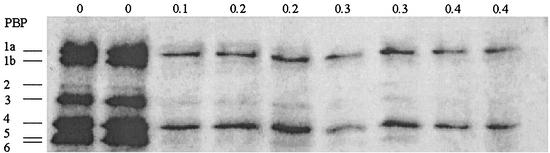
PBPs of E. coli DC0.
The binding affinity of AM-112 for the PBPs of E. coli DC0 is shown in Fig. 2. At an AM-112 concentration of 0.1 μg/ml, the binding of the radiolabel to PBP2 was completely inhibited while binding to PBP1b, PBP3, PBP5, and PBP6 was reduced. PBP1b was inhibited by AM-112 at a concentration of 0.3 μg/ml. At 0.4 μg/ml, the radiolabel binding to PBP3, 5, and 6 was inhibited, while binding to PBP1a and PBP4 was slightly reduced. While the visualization of PBP2 by the radiolabel in the control lanes was poor, studies on the morphology of E. coli DC0 in the presence of AM-112 indicated that at low concentrations of AM-112 (0.25 μg/ml) the cells assumed a round shape, indicating inhibition of PBP2 (data not shown), in agreement with the results of the radiolabeling study.
FIG. 2.
PBPs of E. coli DC0 labeled with 3H-benzylpenicillin, in competition with AM-112. Concentrations of AM-112 (μg/ml) are shown along the top of the gel. The PBP pattern of E. coli DC0 was determined by labeling with 3H-benzylpenicillin (10 to 30 Ci/mmol) after preincubation for 45 min at 37°C with AM-112 at the concentrations shown. Separation of the labeled proteins was achieved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 7.5% (wt/vol) acrylamide gels following the method of Laemmli (12). After electrophoresis, the gel was soaked in Amplify (Amersham) for 30 min. The gel was dried on a gel drier (Bio-Rad, Hemel Hempstead, United Kingdom) for 2 h. Hyperfilm MP (Amersham) was placed in contact with the dried gel and was exposed to the gel for 2 weeks at −70°C prior to visualization.
In vitro antibacterial activity of ceftazidime combined with AM-112 at 1:1 or 2:1 against β-lactamase-producing strains (Table 2).
Alone, AM-112 showed poor activity against the β-lactamase-producing strains, with MICs between 8 and >128 μg/ml. Ceftazidime exhibited good activity against non-ESBL class A enzyme-producing strains and class D enzyme-producing strains (MICs, 0.125 to 2 μg/ml) but lacked good activity against ESBL and class C enzyme-producing strains. The higher concentration of AM-112 (1:1) was effective in protecting ceftazidime against the extended-spectrum class A enzyme producers (group 2be). The protection was most pronounced against the TEM β-lactamase producers, where MICs were reduced 4- to 16-fold in comparison with those of ceftazidime alone. The combination of ceftazidime with AM-112 at either ratio did not confer additional activity over that of ceftazidime alone against P. aeruginosa strains. AM-112 at both concentrations lowered the MIC of ceftazidime against the hyperproducing class C β-lactamase producers between 8- and 2,048-fold compared to that of ceftazidime alone. AM-112 did not enhance the activity of ceftazidime against the class D enzyme producers (group 2d).
Activity in combination with ceftazidime, cefepime, ceftriaxone, or cefoperazone (Table 3).
Alone, ceftriaxone was not active against the class C enzyme-producing strains like E. cloacae and P. aeruginosa. MICs were raised against the ESBL TEM producers (MICs of 0.5 to 8 μg/ml) compared to those for the SHV and OXA enzyme producers. When ceftriaxone was combined with AM-112 in a 2:1 combination, the MICs against the ESBL TEM producers were lowered between 2- and 16-fold and lowered 2-fold for most of the SHV and OXA producers. The activity of ceftriaxone was enhanced fourfold against E. cloacae strains. The activity of ceftriaxone against the hyperproducing Pseudomonas strains was not enhanced.
The combination of ceftazidime and AM-112 was more active than ceftazidime alone against the TEM ESBLs, with reductions in the ceftazidime MIC of up to 512-fold, and was also more active against some organisms producing enzymes from the SHV and OXA groups. Similarly, the activity of ceftazidime against class C enzyme producers was enhanced by the combination with AM-112 compared to results with ceftazidime alone. Reductions of up to 16-fold in the ceftazidime MIC were observed against AmpC-hyperproducing organisms, such as E. cloacae P99. As with ceftriaxone, however, there was no augmentation of activity against the derepressed Pseudomonas strains.
The combination of AM-112 and cefoperazone was more active than cefoperazone alone against most class A and D enzyme producers. MICs were reduced against most strains, compared to results with cefoperazone alone, except OXA-1 and OXA-3. Reductions of between 2- and 512-fold were observed against other class A and D β-lactamase producers. The activity against the class C enzyme producers was also enhanced by the addition of AM-112, with activity being enhanced between two- and eightfold. As with other cephalosporins, there was no enhancement of activity against the Pseudomonas strains.
Cefepime had good activity against all the strains except P. aeruginosa 1405-con, a stable derepressed AmpC-producing strain. Activity was maintained against the ESBL-producing strains, which would be expected. Addition of AM-112 enhanced the activity against some strains, including some of the P. aeruginosa strains, between 2- and 64-fold.
In vivo efficacy of AM-112 alone and in combination with ceftazidime in a murine intraperitoneal infection model (Table 4).
TABLE 4.
ED50 values for combinations of ceftazidime and AM-112 against various pathogens in a murine intraperitoneal sepsis model
| Organism or inoculum (CFU/ml) | Compound (ratio) | MIC (μg/ml)a | ED50 (mg/kg)b | 95% CIc |
|---|---|---|---|---|
| S. aureus 3816 | ||||
| 8 × 108 | CAZd | 16 | 22.6 | 19.4-26.4 |
| 8 × 106 | AM-112 | 1 | 2.6 | 2.2-3.1 |
| 8 × 106 | CAZ-AM-112 (4:1) | NDe | 4.8 + 1.2 | 4.0-5.8 |
| 8 × 106 | CAZ-AM-112 (7:1) | ND | 7 + 1 | 6.6-9.7 |
| E. cloacae P99 | ||||
| 2.2 × 107 | CAZ | 128 | >100 | ND |
| 2.2 × 107 | AM-112 | 32 | 19 | 11.6-33.4 |
| 2.2 × 107 | CAZ-AM-112 (1:1) | 2 + 2 | 2 + 2 | 1.0-5.5 |
| 2.2 × 107 | CAZ-AM-112 (2:1) | 4 + 2 | 3.8 + 1.9 | 2.3-5.9 |
| 2.2 × 107 | CAZ-AM-112 (4:1) | 4 + 1 | 11.6 + 2.9 | 7.2-17.4 |
| K. pneumoniae SHV-5 | ||||
| 6 × 108 | CAZ | 128 | >160 | ND |
| 6 × 108 | AM-112 | 16 | >40 | ND |
| 6 × 108 | CAZ-AM-112 (1:1) | 16 + 16 | 33.6 + 33.6 | 25.1-45.1 |
| 6 × 108 | CAZ-AM-112 (2:1) | 32 + 16 | 23.8 + 11.9 | 18.8-30.1 |
MICs quoted for CAZ-AM-112 combinations are expressed as CAZ + AM-112 MIC.
ED50 values for CAZ-AM-112 combinations are expressed as CAZ + AM-112 ED50 values.
95% CI, confidence intervals for ceftazidime or AM-112; for combinations, 95% CI refers to ceftazidime component.
CAZ, ceftazidime.
ND, not determined.
When administered alone, ceftazidime had moderate activity against S. aureus 3816 (ED50, 18.2 mg/kg of body weight). AM-112 was very active against S. aureus 3816 (ED50, <3 mg/kg). When the two agents were combined in a 4:1 ratio of ceftazidime to AM-112, the ED50 was lowered fourfold compared to that of ceftazidime alone and halved compared to that of AM-112 alone (P < 0.05). The combination of ceftazidime and AM-112 in a 4:1 ratio was more potent than that in a 7:1 ratio.
Ceftazidime was not active against E. cloacae P99 when administered alone (ED50, >100 mg/kg). AM-112 had moderate activity (19 mg/kg) against E. cloacae after subcutaneous administration. Incorporation of AM-112 as the minor component in 1:1, 2:1, or 4:1 combinations with ceftazidime resulted in the lowering of the ED50 of ceftazidime at least 32-fold (P < 0.05) and that of AM-112 eightfold, indicative of synergy.
Ceftazidime was poorly active (MIC, 128 μg/ml) against K. pneumoniae SHV-5, a recent clinical isolate. AM-112 was more active, with a MIC of 16 μg/ml. Alone, ceftazidime and AM-112 were ineffective in vivo, with ED50s of >160 and >40 mg/kg, respectively. However, 1:1 and 2:1 combinations of ceftazidime and AM-112 were effective, with the ceftazidime ED50 reduced to 23.8 and 33.6 mg/kg. Statistical analysis was not possible as the values for single agents could not be determined.
DISCUSSION
Class C enzymes are cephalosporinases (4) and pose a significant threat to currently used cephalosporin antibiotics. AmpC enzymes can be plasmid borne and readily disseminated to strains not normally producing a class C enzyme (13), and chromosomally encoded AmpC can be hyperproduced by reversible induction or stable derepression (22). Between 15 and 25% of E. cloacae strains hyperproduce class C enzymes (15). None of the currently marked β-lactamase inhibitors has significant activity against class C β-lactamases.
The β-lactamase-inhibitory properties of AM-112 have been evaluated in comparison with those of clavulanic acid against a panel of class A, class C, and class D isolated enzymes. Neither clavulanic acid or AM-112 had any inhibitory activity against the class B metallo-β-lactamases (data not shown). AM-112 was between 10- and 20-fold less active than clavulanic acid against the class A enzymes, of which TEM-10 and SHV-5 were also ESBLs. AM-112 was much more active against the class C and class D enzymes than clavulanic acid.
The activity of AM-112 against isolated class C β-lactamases prompted its evaluation as a potential partner for cephalosporins, labile to such class C enzymes. The results presented in Tables 2 and 3 demonstrate that the activity of AM-112 against isolated β-lactamase enzymes is maintained in whole-cell tests. MICs of ceftazidime against class C enzyme hyperproducers were lowered between 8- and 2,046-fold by addition of AM-112 at either 1:1 or 2:1 in comparison with results with ceftazidime alone. For other cephalosporins, activities against these strains were enhanced by up to 8-fold. The activity of the combination of ceftazidime and AM-112 against ESBL-producing E. coli strains was similar to that of piperacillin and tazobactam (11). The MICs of the ceftazidime-AM-112 combination against class C enzyme-producing strains were between 2- and 64-fold lower than comparative MICs of piperacillin combined with tazobactam against the same strains (2). Interestingly, AM-112 enhanced the activities of all the cephalosporins against the TEM-derived ESBL strains, despite being less active than clavulanic acid against these enzymes in the inhibitor assays. AM-112 also enhanced the activities of some cephalosporins against SHV and OXA enzyme producers.
AM-112 failed to enhance the activities of the cephalosporins against P. aeruginosa strains, including derepressed strains which were resistant to the cephalosporins tested. Such cephalosporin resistance may be attributable to outer membrane permeability, which serves to decrease penetration of the antibiotic and the hyperproduction of β-lactamase, which overwhelms and destroys the antibiotic. The lack of activity of AM-112 against such resistant P. aeruginosa strains is disappointing. A recently described novel inhibitor, Syn2190, was designed to utilize the tonB-dependent iron transport system to enhance the penetration of the inhibitor into derepressed P. aeruginosa strains and showed synergy with ceftazidime (16). Syn2190 showed good inhibitory activity against class C enzymes but was much less active against class A enzymes.
The in vitro activity of AM-112 and cephalosporins was confirmed in in vivo models of infection using AM-112 and ceftazidime. AM-112 enhanced the activity of ceftazidime against S. aureus, E. cloacae P99, and E. coli SHV-5, organisms producing class A, class C, and ESBL enzymes, respectively. This extension of the activity of ceftazidime to include gram-positive organisms, cephalosporinase, and ESBL producers is very promising and offers scope for treatment of drug-resistant infections caused by these organisms.
In conclusion, AM-112 is a novel oxapenem which is an effective inhibitor of class A, ESBL, class C, and class D enzymes. AM-112 protects cephalosporins against TEM ESBL producers and hyperproducers of class C enzymes, such as E. cloacae. In common with other β-lactams, it has affinity for the PBPs of E. coli and appears to bind to PBP2 as an initial target. In vitro activity is mirrored by in vivo activity in animal models of infection. AM-112 offers scope for further investigation as a potential new β-lactamase inhibitor.
Acknowledgments
We acknowledge the help and technical support from Glaxo Wellcome, Verona, Italy (now GlaxoSmithKline) and Biosearch Italia, Gerenzano, Italy, in work with the animal infection models.
We thank Amusa for financial support.
REFERENCES
- 1.Ambler, R. P. 1980. The structure of β-lactamases. Phil. Trans. R. Soc. Lond. Biol. Sci. 289:321-331. [DOI] [PubMed] [Google Scholar]
- 2.Babini, G. S., and D. M. Livermore. 2000. Effect of conalbumin on the activity of Syn 2190, a 1,5 dihydroxy-4-pyridon monobactam inhibitor of AmpC β-lactamases. J. Antimicrob. Chemother. 45:105-109. [DOI] [PubMed] [Google Scholar]
- 3.Bryan, L. E., and A. J. Godfrey. 1991. β-Lactam antibiotics, p. 599-664. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. Williams & Wilkins, Baltimore, Md.
- 4.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush, K., and G. H. Miller. 1998. Bacterial enzymatic resistance: β-lactamases and aminoglycoside-modifying enzymes. Curr. Opin. Microbiol. 1:509-515. [DOI] [PubMed] [Google Scholar]
- 6.Bush, K. 2001. New β-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 7.Bush, K., C. Macalintal, B. A. Rasmussen, V. J. Lee, and Y. Yang. 1993. Kinetic interactions of tazobactam with β-lactamases from all structural classes. Antimicrob. Agents Chemother. 37:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, Y.-C., and W. H. Prusoff. 1973. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22:3099-3108. [DOI] [PubMed] [Google Scholar]
- 9.Cherry, P. C., C. E. Newall, and N. S. Watson. 1978. Chem. Commun. (J. Chem. Soc. Sect. D), p. 469-470.
- 10.Clark, D. P., and J. P. Beard. 1979. Altered phospholipid composition in mutants of Escherichia coli sensitive or resistant to organic solvents. J. Gen. Microbiol. 113:267-274. [DOI] [PubMed] [Google Scholar]
- 11.Jett, B. D., D. J. Ritchie, R. Reichley, T. C. Bailey, and D. F. Sahm. 1995. In vitro activity of various β-lactam antimicrobial agents against clinical isolates of Escherichia coli and Klebsiella spp. resistant to oxyimino cephalosporins. Antimicrob. Agents Chemother. 39:1187-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 13.Livermore, D. M. 1995. β-lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neu, H. C. 1992. The crisis in antibiotic resistance. Science 257:1064-1073. [DOI] [PubMed] [Google Scholar]
- 15.Nicolas-Chanoine, M. H. 1997. Inhibitor-resistant β-lactamases. J. Antimicrob. Chemother. 40:1-3. [DOI] [PubMed] [Google Scholar]
- 16.Nishida, K., C. Kunugita, T. Uji, F. Higashitani, A. Hyodo, N. Unemi, S. N. Maiti, O. A. Phillips, P. Spevak, K. P. Atchison, S. Salama, H. Atwal, and R. G. Micetich. 1999. In vitro and in vivo activities of Syn2190, a novel β-lactamase inhibitor. Antimicrob. Agents Chemother. 43:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Callaghan, C., A. Morris, S. M. Kirby, and A. H. Shingler. 1972. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne, D. A., R. Cramp, D. J. Winstanley, and D. J. C. Knowles. 1994. Comparative activities of clavulanic acid, sulbactam, and tazobactam against clinically important β-lactamases. Antimicrob. Agents Chemother. 38:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaendler, H. R., F. Weisner, and K. Metzger. 1993. Synthesis and antibacterial activity of (1′R, 5R, 6R)-2-tert-butyl-6-(1′-hydroxyethyl)oxapenem-3-carboxylic acid. Bioorg. Med. Chem. Lett. 3:2211-2218. [Google Scholar]
- 20.Sirot, D. 1996. Extended-spectrum plasmid-mediated β-lactamases. J. Antimicrob. Chemother. 36(Suppl. A):19-34. [DOI] [PubMed] [Google Scholar]
- 21.Spratt, B. G. 1977. Properties of the penicillin-binding proteins of Escherichia coli K12. J. Biochem. 72:341-352. [DOI] [PubMed] [Google Scholar]
- 22.Thomson, K. S., and E. Smith Moland. 2000. Version 2000: the new β-lactamases of Gram-negative bacteria at the dawn of the new millennium. Microbes Infect. 2:1225-1235. [DOI] [PubMed] [Google Scholar]