Abstract
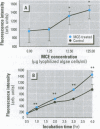
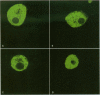
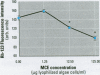
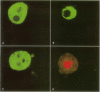
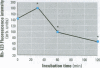
Cyanobacteria contamination of water has become a growing public health problem worldwide. Microcystis aeruginosa is one of the most common toxic cyanobacteria. It is capable of producing microcystins, a group of cyclic heptapeptide compounds with potent hepatotoxicity and tumor promotion activity. The present study investigated the effect of microcystic cyanobacteria on primary cultured rat hepatocytes by examining mitochondrial membrane potential (MMP) changes and intracellular reactive oxygen species (ROS) formation in cells treated with lyophilized freshwater microcystic cyanobacteria extract (MCE). Rhodamine 123 (Rh-123) was used as a fluorescent probe for changes in mitochondrial fluorescence intensity. The mitochondrial Rh-123 fluorescence intensity in MCE-treated hepatocytes, examined using a laser confocal microscope, responded in a dose- and time-dependent manner. The results thus indicate that the alteration of MMP might be an important event in the hepatotoxicity caused by cyanobacteria. Moreover, the parallel increase of ROS formation detected using another fluorescent probe, 2',7'-dichlorofluorescin diacetate also suggests the involvement of oxidative stress in the hepatotoxicity caused by cyanobacteria. The fact that MMP changes precede other cytotoxic parameters such as nuclear staining by propidium iodide and cell morphological changes suggests that mitochondrial damage is closely associated with MCE-induced cell injury in cultured rat hepatocytes.
Full text
PDF
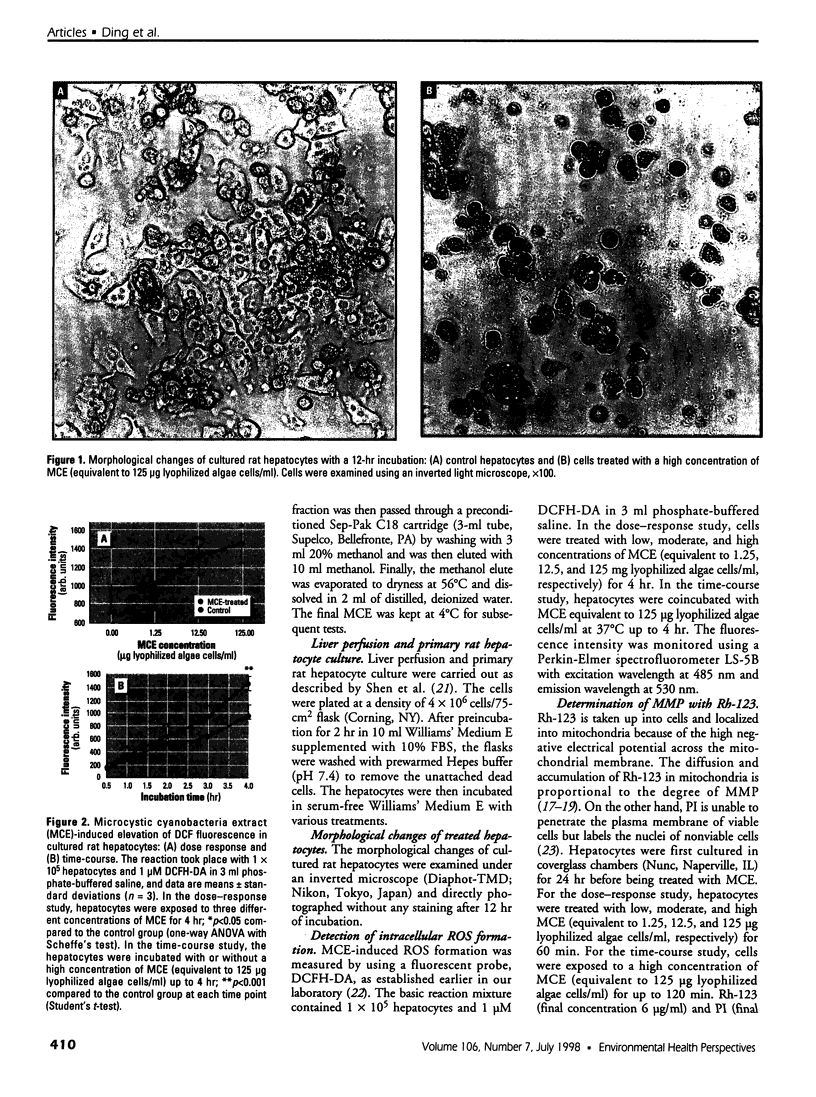
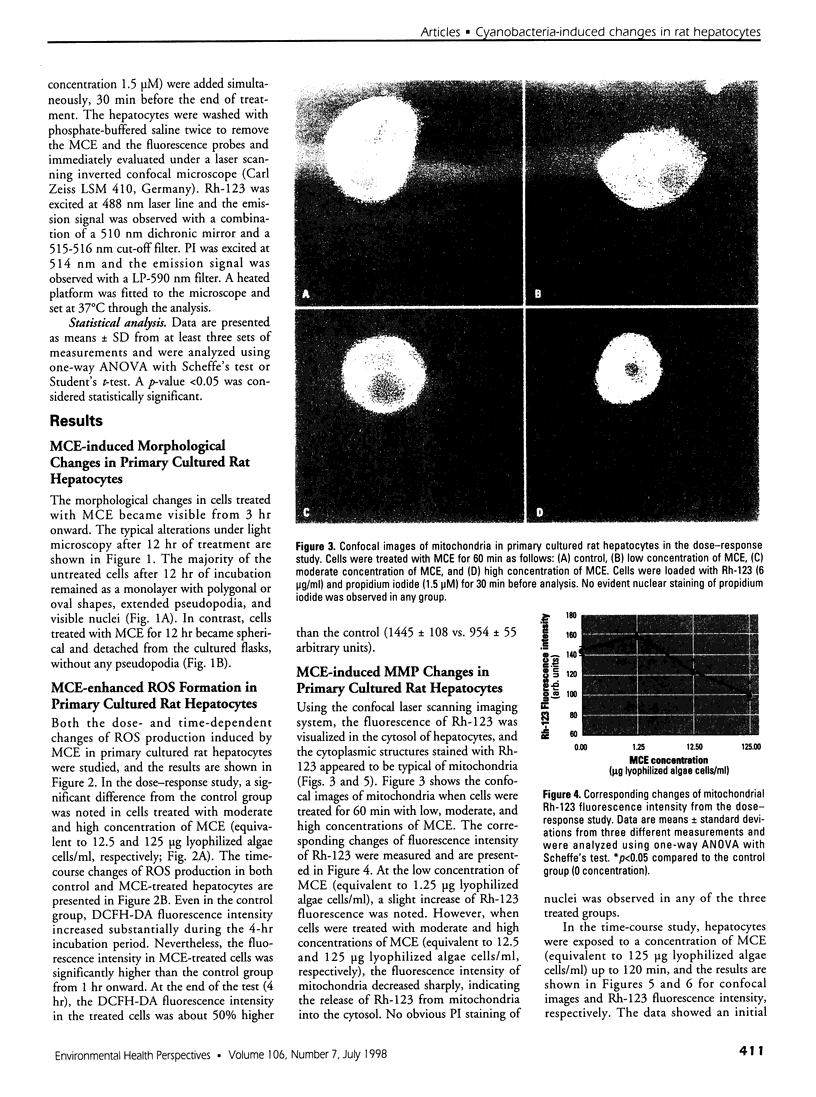
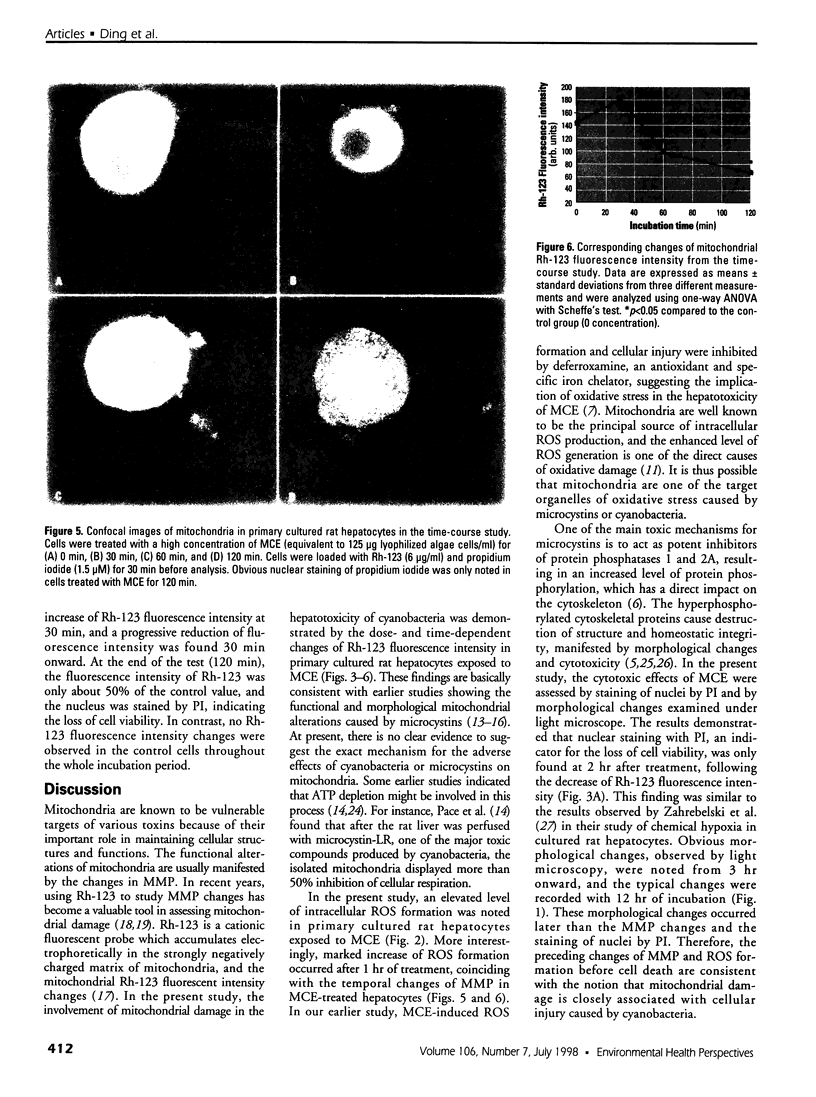

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R., Rao P. V., Bhaskar A. S., Pant S. C., Dube S. N. Liver slice culture for assessing hepatotoxicity of freshwater cyanobacteria. Hum Exp Toxicol. 1996 Feb;15(2):105–110. doi: 10.1177/096032719601500202. [DOI] [PubMed] [Google Scholar]
- Carmichael W. W. Cyanobacteria secondary metabolites--the cyanotoxins. J Appl Bacteriol. 1992 Jun;72(6):445–459. doi: 10.1111/j.1365-2672.1992.tb01858.x. [DOI] [PubMed] [Google Scholar]
- Carmichael W. W. The toxins of cyanobacteria. Sci Am. 1994 Jan;270(1):78–86. doi: 10.1038/scientificamerican0194-78. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- Eriksson J. E., Toivola D., Meriluoto J. A., Karaki H., Han Y. G., Hartshorne D. Hepatocyte deformation induced by cyanobacterial toxins reflects inhibition of protein phosphatases. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1347–1353. doi: 10.1016/s0006-291x(05)80936-2. [DOI] [PubMed] [Google Scholar]
- Harada K., Suzuki M., Dahlem A. M., Beasley V. R., Carmichael W. W., Rinehart K. L., Jr Improved method for purification of toxic peptides produced by cyanobacteria. Toxicon. 1988;26(5):433–439. doi: 10.1016/0041-0101(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Hermansky S. J., Stohs S. J., Eldeen Z. M., Roche V. F., Mereish K. A. Evaluation of potential chemoprotectants against microcystin-LR hepatotoxicity in mice. J Appl Toxicol. 1991 Feb;11(1):65–73. doi: 10.1002/jat.2550110112. [DOI] [PubMed] [Google Scholar]
- Hooser S. B., Beasley V. R., Lovell R. A., Carmichael W. W., Haschek W. M. Toxicity of microcystin LR, a cyclic heptapeptide hepatotoxin from Microcystis aeruginosa, to rats and mice. Vet Pathol. 1989 May;26(3):246–252. doi: 10.1177/030098588902600309. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Ghosh S., Wickstrom M., Miller L. A., Hess R., Haschek W. M., Beasley V. R. Comparative pathology of microcystin-LR in cultured hepatocytes, fibroblasts, and renal epithelial cells. Nat Toxins. 1995;3(3):119–128. doi: 10.1002/nt.2620030302. [DOI] [PubMed] [Google Scholar]
- Mereish K. A., Bunner D. L., Ragland D. R., Creasia D. A. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: biochemistry, histopathology, and lethality. Pharm Res. 1991 Feb;8(2):273–277. doi: 10.1023/a:1015868809990. [DOI] [PubMed] [Google Scholar]
- Mereish K. A., Solow R. Effect of antihepatotoxic agents against microcystin-LR toxicity in cultured rat hepatocytes. Pharm Res. 1990 Mar;7(3):256–259. doi: 10.1023/a:1015822028414. [DOI] [PubMed] [Google Scholar]
- Miura G. A., Robinson N. A., Geisbert T. W., Bostian K. A., White J. D., Pace J. G. Comparison of in vivo and in vitro toxic effects of microcystin-LR in fasted rats. Toxicon. 1989;27(11):1229–1240. doi: 10.1016/0041-0101(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Nishiwaki-Matsushima R., Ohta T., Nishiwaki S., Suganuma M., Kohyama K., Ishikawa T., Carmichael W. W., Fujiki H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J Cancer Res Clin Oncol. 1992;118(6):420–424. doi: 10.1007/BF01629424. [DOI] [PubMed] [Google Scholar]
- Pace J. G., Robinson N. A., Miura G. A., Matson C. F., Geisbert T. W., White J. D. Toxicity and kinetics of [3H]microcystin-LR in isolated perfused rat livers. Toxicol Appl Pharmacol. 1991 Mar 1;107(3):391–401. doi: 10.1016/0041-008x(91)90303-v. [DOI] [PubMed] [Google Scholar]
- Palmeira C. M., Moreno A. J., Madeira V. M., Wallace K. B. Continuous monitoring of mitochondrial membrane potential in hepatocyte cell suspensions. J Pharmacol Toxicol Methods. 1996 Feb;35(1):35–43. doi: 10.1016/1056-8719(95)00131-x. [DOI] [PubMed] [Google Scholar]
- Papa S. Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim Biophys Acta. 1996 Sep 12;1276(2):87–105. doi: 10.1016/0005-2728(96)00077-1. [DOI] [PubMed] [Google Scholar]
- Rahn C. A., Bombick D. W., Doolittle D. J. Assessment of mitochondrial membrane potential as an indicator of cytotoxicity. Fundam Appl Toxicol. 1991 Apr;16(3):435–448. doi: 10.1016/0272-0590(91)90084-h. [DOI] [PubMed] [Google Scholar]
- Rao P. V., Bhattacharya R., Pant S. C., Bhaskar A. S. Toxicity evaluation of in vitro cultures of freshwater cyanobacterium Microcystis aeruginosa: I. Hepatotoxic and histopathological effects in rats. Biomed Environ Sci. 1995 Sep;8(3):254–264. [PubMed] [Google Scholar]
- Shen H. M., Ong C. N., Shi C. Y. Involvement of reactive oxygen species in aflatoxin B1-induced cell injury in cultured rat hepatocytes. Toxicology. 1995 May 5;99(1-2):115–123. doi: 10.1016/0300-483x(94)03008-p. [DOI] [PubMed] [Google Scholar]
- Shen H. M., Shi C. Y., Shen Y., Ong C. N. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radic Biol Med. 1996;21(2):139–146. doi: 10.1016/0891-5849(96)00019-6. [DOI] [PubMed] [Google Scholar]
- Ubl J. J., Chatton J. Y., Chen S., Stucki J. W. A critical evaluation of in situ measurement of mitochondrial electrical potentials in single hepatocytes. Biochim Biophys Acta. 1996 Sep 12;1276(2):124–132. doi: 10.1016/0005-2728(96)00067-9. [DOI] [PubMed] [Google Scholar]
- Wickstrom M., Haschek W., Henningsen G., Miller L. A., Wyman J., Beasley V. Sequential ultrastructural and biochemical changes induced by microcystin-LR in isolated perfused rat livers. Nat Toxins. 1996;4(5):195–205. doi: 10.1002/(SICI)(1996)4:5<195::AID-NT1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wu E. Y., Smith M. T., Bellomo G., Di Monte D. Relationships between the mitochondrial transmembrane potential, ATP concentration, and cytotoxicity in isolated rat hepatocytes. Arch Biochem Biophys. 1990 Nov 1;282(2):358–362. doi: 10.1016/0003-9861(90)90129-m. [DOI] [PubMed] [Google Scholar]
- Zahrebelski G., Nieminen A. L., al-Ghoul K., Qian T., Herman B., Lemasters J. J. Progression of subcellular changes during chemical hypoxia to cultured rat hepatocytes: a laser scanning confocal microscopic study. Hepatology. 1995 May;21(5):1361–1372. [PubMed] [Google Scholar]