Abstract
We recently noted that immature rats failed to exhibit a normal uterine response to exogenously administered estradiol as assessed by both biochemical (induction of gene expression) and morphological (altered uterine and vaginal histology and size) end points. An initial analysis suggested that this was due to a high degree of estrogenization from a dietary source which was producing a near maximal uterotrophic response prior to hormone treatment. Subsequent chemical analysis indicated that the feed in question contained high amounts of two well-known phytoestrogens, genistein (210 mg/kg) and daidzen (14 mg/kg), and the lot of feed in question produced a large uterotrophic effect when fed to immature ovariectomized rats. These findings illustrate that, despite increased awareness of phytoestrogens, some batches of animal feed contain very high amounts of estrogenic components which have marked effects on in vivo end points of hormone action. These observations have important implications for both basic research and screening methods that utilize in vivo approaches.
Full text
PDF
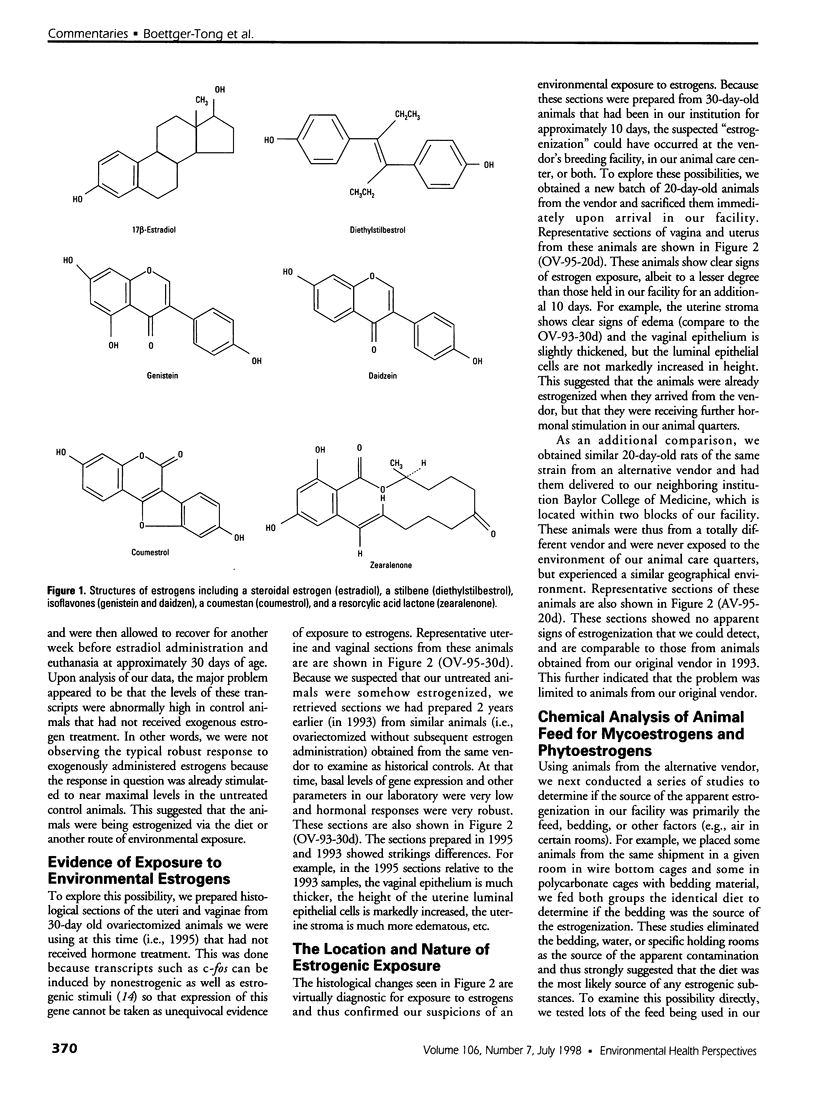
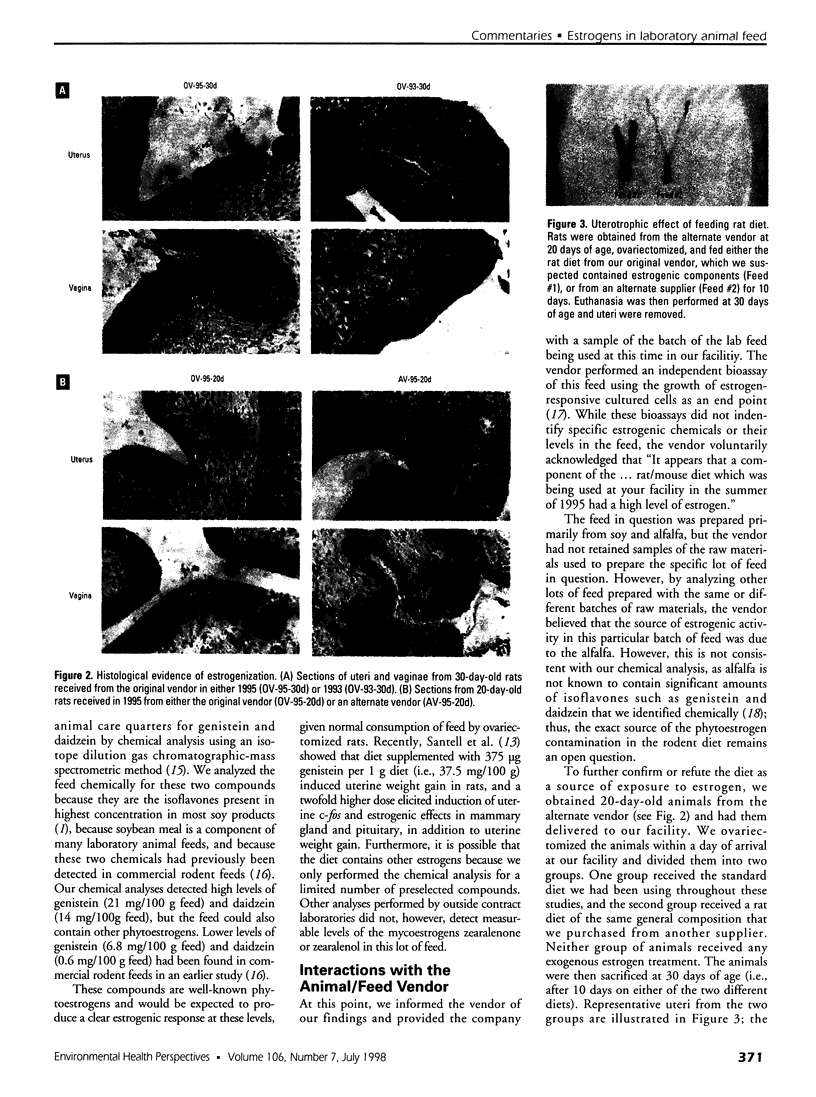


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H., Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997 Apr;29(2):95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- Franke A. A., Custer L. J., Cerna C. M., Narala K. Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Proc Soc Exp Biol Med. 1995 Jan;208(1):18–26. doi: 10.3181/00379727-208-43826. [DOI] [PubMed] [Google Scholar]
- Hyder S. M., Stancel G. M., Loose-Mitchell D. S. Steroid hormone-induced expression of oncogene encoded nuclear proteins. Crit Rev Eukaryot Gene Expr. 1994;4(1):55–116. doi: 10.1615/critreveukargeneexpr.v4.i1.30. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Katzenellenbogen J. A., Mordecai D. Zearalenones: characterization of the estrogenic potencies and receptor interactions of a series of fungal beta-resorcylic acid lactones. Endocrinology. 1979 Jul;105(1):33–40. doi: 10.1210/endo-105-1-33. [DOI] [PubMed] [Google Scholar]
- Kurzer M. S., Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- Loose-Mitchell D. S., Chiappetta C., Stancel G. M. Estrogen regulation of c-fos messenger ribonucleic acid. Mol Endocrinol. 1988 Oct;2(10):946–951. doi: 10.1210/mend-2-10-946. [DOI] [PubMed] [Google Scholar]
- Mazur W., Fotsis T., Wähälä K., Ojala S., Salakka A., Adlercreutz H. Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal Biochem. 1996 Jan 15;233(2):169–180. doi: 10.1006/abio.1996.0025. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A., Newbold R. R., Bullock B. C. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980 Nov;40(11):3988–3999. [PubMed] [Google Scholar]
- Murphy P. A., Farmakalidis E., Johnson L. D. Isoflavone content of soya-based laboratory animal diets. Food Chem Toxicol. 1982 Jun;20(3):315–317. doi: 10.1016/s0278-6915(82)80299-8. [DOI] [PubMed] [Google Scholar]
- Mäkelä S., Santti R., Salo L., McLachlan J. A. Phytoestrogens are partial estrogen agonists in the adult male mouse. Environ Health Perspect. 1995 Oct;103 (Suppl 7):123–127. doi: 10.1289/ehp.103-1518873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimrod A. C., Benson W. H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996 May;26(3):335–364. doi: 10.3109/10408449609012527. [DOI] [PubMed] [Google Scholar]
- Saloniemi H., Wähälä K., Nykänen-Kurki P., Kallela K., Saastamoinen I. Phytoestrogen content and estrogenic effect of legume fodder. Proc Soc Exp Biol Med. 1995 Jan;208(1):13–17. doi: 10.3181/00379727-208-43825. [DOI] [PubMed] [Google Scholar]
- Santell R. C., Chang Y. C., Nair M. G., Helferich W. G. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr. 1997 Feb;127(2):263–269. doi: 10.1093/jn/127.2.263. [DOI] [PubMed] [Google Scholar]
- Sheehan D. M., Branham W. S., Medlock K. L., Shanmugasundaram E. R. Estrogenic activity of zearalenone and zearalanol in the neonatal rat uterus. Teratology. 1984 Jun;29(3):383–392. doi: 10.1002/tera.1420290309. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995 Oct;103 (Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N. Cytological basis for permanent vaginal changes in mice treated neonatally with steroid hormones. Int Rev Cytol. 1976;44:193–224. doi: 10.1016/s0074-7696(08)61650-2. [DOI] [PubMed] [Google Scholar]
- Thigpen J. E., Lebetkin E. H., Dawes M. L., Richter C. B., Crawford D. The mouse bioassay for the detection of estrogenic activity in rodent diets: III. Stimulation of uterine weight by dextrose, sucrose and corn starch. Lab Anim Sci. 1987 Oct;37(5):606–609. [PubMed] [Google Scholar]
- Thigpen J. E., Li L. A., Richter C. B., Lebetkin E. H., Jameson C. W. The mouse bioassay for the detection of estrogenic activity in rodent diets: II. Comparative estrogenic activity of purified, certified and standard open and closed formula rodent diets. Lab Anim Sci. 1987 Oct;37(5):602–605. [PubMed] [Google Scholar]
- Weisz A., Bresciani F. Estrogen induces expression of c-fos and c-myc protooncogenes in rat uterus. Mol Endocrinol. 1988 Sep;2(9):816–824. doi: 10.1210/mend-2-9-816. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Rottinghaus G. E., Nonneman D. J., Dolan-Timpe M., Ross P. F. A sensitive bioassay for detection of dietary estrogens in animal feeds. J Vet Diagn Invest. 1990 Oct;2(4):268–273. doi: 10.1177/104063879000200403. [DOI] [PubMed] [Google Scholar]