Abstract
Ertapenem (INVANZ) is a new once-a-day parental β-lactam antimicrobial agent that has been shown to be highly effective as a single agent for treatment of various community-acquired and mixed infections. The plasma pharmacokinetics of a 1-g intramuscular (i.m.) dose was compared with those of a 1-g intravenous (i.v.) dose infused over 30 min, the recommended rate of i.v. infusion for comparison, and over 120 min, which more closely mimicked the time course for absorption of the i.m. form. In a three-period crossover study (Part A), 26 healthy subjects received single doses of ertapenem administered i.m., i.v. infused over 30 min, and i.v. infused over 120 min. Blood for ertapenem analysis was collected over 24 h postdose for each treatment. In Part B, these fasted subjects received a 1-g i.m. dose of ertapenem once daily for 7 days. Following a 1-g i.m. dose and a 1-g i.v. dose infused over 120 min, the geometric mean area under the concentration curve from hour 0 to infinity (AUC0-∞) was 541.8 μg · hr/ml following i.m. administration and 591.4 μg · hr/ml following a 120-min infusion; the geometric mean ratio was 0.92 with a 90% confidence interval of 0.88 to 0.95. The geometric mean AUC0-∞ was nearly identical when 1-g doses were infused over 30 or 120 min. Although the maximum concentration of drug in serum was somewhat lower following i.m. administration than following i.v. administration, the shape of the plasma concentration profiles was roughly comparable at later time points. Ertapenem did not accumulate after multiple 1-g i.m. daily doses over 7 days. The geometric mean ratio for AUC0-24 (day 7/day 1) was 0.98 with a 90% confidence interval of 0.94 to 1.02. Thus, the relative bioavailability of the 1-g i.m. dose was 92%. Ertapenem does not accumulate following multiple daily 1-g i.m. doses over 7 days.
Ertapenem (INVANZ [MK-0826]; Merck & Co., Inc.) is a once-a-day parenteral β-lactam antimicrobial agent with excellent in vitro activity against gram-positive and gram-negative aerobic and anaerobic bacteria generally associated with community-acquired and mixed infections (C. J. Gill, J. J. Jackson, J. G. Sundelof, H. Rosen, and H. Kropp, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F125, 1996). Moreover, ertapenem has been shown to be effective for treating several community-acquired and mixed infections, including intra-abdominal infections [J. S. Solomkin, K. A. Choe, N. V. Christou, E. Dellinger, O. Malafaia, O. Rotstein, J. Tellado, A. Yellin, B. Deyi, V. Satishchandran, H. Teppler, et al., Abstr. Clin. Microbiol. Infect. Dis. 7(suppl. 1):314, abstr. P1460, 2001], skin and skin-structure infections (2), community-acquired pneumonia (5), acute pelvic infections (S. Roy, I. Higareda, E. Angel-Muller, M. Ismail, B. Deyi, C. Hague, H. Teppler, Protocol 023 Study Group, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. L888, 2001), and urinary tract infections (K. Tomera, E. Burdmann, O. Pamo, R. Gesser, Q. Jiang, W. Wimmer, The Protocol 014 Study Group, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. L1053, 2001). The anionic side chain of ertapenem makes it a structurally unique carbapenem. It also has a 1-β-methyl group on the azabicyclic ring that provides stability against human renal dehydropeptidase I. Its major metabolite via dehydropeptidase I is the open-lactam form. Its protein binding in plasma is ≥95% in the mouse, rhesus monkey, chimpanzee, and human (6). The pharmacokinetics of ertapenem following intramuscular (i.m.) administration was evaluated in the rhesus monkey (bolus, 10 mg/kg of body weight), and comparison with intravenous (i.v.) administration indicated rapid and complete absorption from the injection site.
For healthy young subjects receiving an i.v. infusion of ertapenem over 30 min, concentrations of ertapenem in plasma increased slightly less than dose proportionally at dosages from 0.5 to 3 g (A. Majumdar, K. Birk, A. Cairns, W. Neway, D. Musson, L. Tomasko, T. Bradstreet, R. Lins, G. Mistry, S. Holland, and J. D. Rogers, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 491, 2000) because of nonlinear protein binding (6, 7). The mean values for the area under the concentration curve (AUC) for i.v. doses from 0.5 to 3 g ranged from 306 to 1,407 μg · h/ml. The mean apparent plasma clearance (CLP) of the drug ranged from 27.6 to 36.3 ml/min, respectively, and the mean apparent half-life (t[1/2]) was about 3.5 to 4 h. About 36 to 53% of drug was excreted unchanged in urine. Following multiple dosing, the mean plasma profiles on day 8 were comparable to those on day 1, indicating little accumulation in young adults (A. Majumdar, K. Birk, R. A. Blum, A. M. Cairns, J. Conroy, C. Mendel, D. Musson, and J. D. Rogers, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F130, 1996).
The intent of this study was to assess the pharmacokinetic parameters of ertapenem and its bioavailability following a single 1-g i.m. dose to healthy subjects. It was stated before the study that the AUC from time 0 to infinity (AUC0-∞) for a 1-g i.m. dose of ertapenem will not be substantially different from that for a single 1-g i.v. dose infused over 2 h. The 120-min i.v. infusion time was picked to match the anticipated i.m. time required for the maximum concentration of drug in serum (Cmax) to be reached (Tmax) and to keep plasma concentrations of ertapenem in range of linear protein binding. The clinical dose for ertapenem is a 1-g i.v. infusion over 30 min. This treatment was included in this study for comparison of the duration of plasma concentrations exceeding the susceptibility breakpoint for organisms. A second hypothesis stated that ertapenem will not accumulate to a clinically significant extent in plasma when a 1-g i.m. dose is administered once daily for 7 days.
MATERIALS AND METHODS
Study design.
Healthy young subjects (20 males and 6 females) participated in a two-part crossover study. Pharmacokinetic differences between males and females have been shown to be minor and clinically insignificant in previous studies (Majumdar et al., 36th ICAAC; Majumdar et al., 40th ICAAC). In Part A, a three-period crossover, single-dose study, fasted subjects received 1-g doses of ertapenem or placebo (n = 4) administered i.m., infused i.v. over 30 min, and infused i.v. over 120 min. Heparinized blood and urine samples were collected at specified times up to 24 h postdose. In Part B, these fasted subjects received a 1-g i.m. dose of ertapenem or placebo once daily for 7 days. Blood and urine samples were collected for 24 h postdose following the final dose on day 7. There was a 7-day washout interval between doses in Part A and between the last dose in Part A and the first dose in Part B. The 26 male and female subjects who enrolled in this study had a mean age and weight of 30 ± 6 years and 74 ± 12 kg, respectively. All of the subjects were considered healthy based upon medical history, physical examination, vital signs, 12-lead electrocardiogram, and laboratory tests (hematology, blood chemistry, urinalysis). The subjects did not take any over-the-counter or prescription medications that may interfere with pharmacokinetic assessment.
The study protocol and consent form were reviewed and approved by the local institutional review board. All subjects gave written informed consent for participation in the study.
Bioanalytical procedures.
Plasma and urine samples were analyzed for total ertapenem by reversed-phase high-performance liquid chromatography with UV absorbance detection (300 nm) (4). The method for plasma samples involved a Hypersil 5-μm C18 BDS (100 by 4.6 mm) analytical column (Keystone Scientific, Bellefonte, Pa.) and a mobile phase containing 10.5% methanol in 25 mM sodium phosphate buffer, pH 6.5. The urine method was similar but with a longer analytical column (150 by 4.6 mm). The plasma and urine assays for total drug involved online extraction by column switching of stabilized sample aliquots. The analytes were extracted online with a Maxsil 10-μm ODS (50 by 4.6 mm) column (Phenomenix, Torrance, Calif.) and an aqueous mobile phase of 25 mM sodium phosphate buffer, pH 6.5. The lower limits of quantitation for the plasma and urine assays were 0.125 and 1.25 μg/ml, respectively. The intraday precision and accuracy (n = 5) for these methods were ≤10% (except at lower limits of quantitation of ≤15%) and ≤15% over the concentration ranges of 0.125 to 50 μg/ml and 1.25 to 100 μg/ml, respectively. Interday acceptance criteria for analyzed samples was <20% of nominal values for plasma quality control samples prepared at 0.25 and 40 μg/ml and urine quality control samples prepared at 10 and 75 μg/ml.
Pharmacokinetic procedures.
The area under the plasma concentration-time curve was estimated from time 0 to the last measurable concentration (AUC0-t) by the linear log trapezoidal rule. The apparent terminal elimination rate constant (β) was determined with a weighted (1/y), monoexponential, curve-fitting program, Sigma Plot. The apparent t[1/2] was calculated as ln 2/β. AUC0-∞ was calculated as the sum of AUC0-t and the last measurable concentration divided by β. The apparent CLP was obtained by dividing the actual dose administered by the corresponding AUC0-∞. The apparent renal clearance (CLR) was estimated from the urinary recovery of drug (fe) and corresponding increments of AUC. Tmax after an i.m. dose and Cmax were determined by observation.
Statistics.
A confidence interval (CI) approach was used to assess whether the AUC0-∞ is similar following a single 1-g i.m. dose and a single 1-g i.v. dose infused over 2 h. A log transformation was applied to the AUC data prior to analysis. An analysis of variance model was fitted to the log-transformed AUC0-∞ data with the following three factors included in the model: treatment, period, and subject. A two-sided 90% CI for the arithmetic mean AUC0-∞ difference (1-g i.m. substract 1-g i.v. 2 h) on the log scale was calculated from this analysis of variance model; the confidence limits were then back transformed through exponentiation to obtain the 90% CI for the geometric mean ratio (GMR). The hypothesis that the AUC0-∞ is similar following a single 1-g i.m. dose and a single 1-g i.v. dose infused over 2 h would be supported if the 90% CI for the GMR was contained in the similarity limits (0.75 to 1.33). The same method was used to assess the accumulation of ertapenem by comparing the AUC0-24 following 1-g i.m. daily doses over 7 days (Part B) to that following a single 1-g i.m. dose (Part A). This analysis was conducted under the assumptions that there was no period effect between Part A and Part B and that there was no carryover effect from the 1-g i.v. doses in Part A. A 90% CI was determined by a paired t test for the mean AUC0-24 difference between the last dose of multiple 1-g i.m. doses on day 7 and a single 1-g i.m. dose on day 1. The confidence limits were then exponentiated to obtain the 90% CI for the GMR. The hypothesis that ertapenem, when administered as 1-g i.m. daily doses for 7 days, does not accumulate would be supported if the 90% CI for the GMR was contained in the similarity limits (0.75 to 1.33).
Assuming that the true GMR was 1.0 and taking a conservative estimate of standard deviation of 0.19 for AUC, a sample size of 18 subjects (three subjects/sequence) yielded 80% probability that the observed 90% CI for the GMR would be contained within the range of 0.87 to 1.14. Under the same assumption, this study had at least a 99% probability of getting the observed 90% CI within the range of 0.75 to 1.33.
RESULTS
The 1-g dose of ertapenem given as a single i.m. and i.v. dose and multiple daily i.m. doses (7 days) were generally well tolerated. Fifteen subjects reported 32 clinical adverse experiences which were drug related. No laboratory adverse experiences were reported. The clinical adverse experiences were generally similar between i.m. and i.v. administration. A majority of the adverse experiences were mild or moderate in intensity, and the most frequent were diarrhea and erythema at the site of administration. The latter occurred only following i.m. administration of ertapenem.
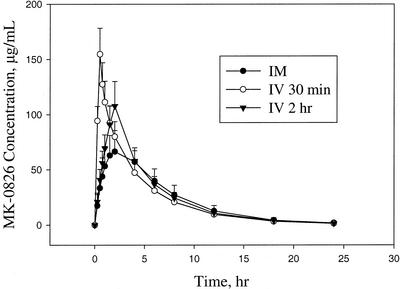
The mean plasma profiles comparing the single 1-g i.m. dose and the 1-g i.v. infusions of ertapenem over 30 and 120 min are shown in Fig. 1 (linear and semilog plot). The 1-g i.v. 120-min infusion was used to lower the total concentrations of ertapenem in plasma at the end of infusion compared to those following the 30-min infusion (see Fig. 1), thus minimizing the effect of plasma nonlinear protein binding. The pharmacokinetics of the 30-min and 120-min i.v. infusion are very similar except for a lower end-of-infusion concentration for the latter. The geometric mean AUC0-∞ values for a single 1-g i.m. dose and an i.v. dose (infused over 30 or 120 min) are comparable at 542, 597, and 591 μg · h/ml. The GMR of individual AUC0-∞ ratios (i.m./i.v., 120-min infusion) was 0.92 (i.e., a bioavailability of 92%), and the 90% CI was 0.88 to 0.95. The means and standard deviations for AUC0-∞ and other pharmacokinetic parameters following administration of ertapenem by i.m. and i.v. routes are summarized in Table 1. The Tmax of total ertapenem following i.m. administration averaged 2.2 h, with a mean Cmax of 70.6 μg/ml. The mean plasma concentration at the end of the 30-min i.v. infusion was 165 μg/ml, while that following the 120-min infusion averaged 120 μg/ml. Other pharmacokinetic parameters that describe elimination include apparent t[1/2], apparent CLR, and fe (0 to 24 h) and appear similar for i.v. and i.m. administration. The mean t[1/2] values for the i.m. dose and the i.v. 120- and 30-min infusions are 3.8, 3.7, and 3.8 h and agree with previously reported values (Majumdar et al., 40th ICAAC). The mean apparent CLR and fe values are 10.9, 11.9, and 12.7 ml/min and 33.6, 39.6, and 42.3%, respectively.
FIG. 1.
Mean concentrations of total ertapenem in plasma (± standard deviation) from healthy subjects receiving a single dose of 1 g i.m., 1 g i.v. over 30 min, and 1 g i.v. over 2 h.
TABLE 1.
Arithmetic mean values (± standard deviation) for pharmacokinetic parameters following a 1-g single i.v. and i.m. dose and the seventh daily 1-g i.m. dose of ertapenem in healthy subjects
| Parameter | 1 g i.m. | 1 g i.v., 30-min infusion | 1 g i.v., 2-h infusion | Multiple i.m. dose (day 7) |
|---|---|---|---|---|
| AUC0-24 (μg · h/ml) | 524.9 (92.8)c | 506.4a | ||
| AUC0-∞ (μg · h/ml) | 541.8a | 597.4a | 591.4a | |
| Ceoi (μg/ml)d | 164.6 (24.0) | 120.8 (40.9) | ||
| Cmax (μg/ml) | 70.6 (15.5) | 75.7 (11.2) | ||
| Tmax (hr) | 2.2 (0.9) | 1.9 (0.6) | ||
| Apparent t[1/2] (h)b | 3.8 (0.5) | 3.8 (0.5) | 3.7 (0.5) | 3.5 (0.2) |
| Apparent CLP (ml/min) | 28.4 (4.6) | 28.6 (4.8) | ||
| Apparent CLR (ml/min) | 10.9 (3.3) | 12.7 (3.2) | 11.9 (3.5) | 13.7 (3.7) |
| fe (% of dose in urine) | 33.6 (10.2) | 42.3 (10.1) | 39.6 (11.9) | 41.1 (10.0) |
| Time above susceptibility breakpoint at 4 μg/ml (h) | 18.3 (3.0) | 17.0 (2.6) | 17.0 (2.6) |
Geometric mean.
Harmonic means (jackknife standard deviations) are presented for half-life data (3).
Geometric mean, 518.1.
Ceoi, concentration at end of infusion.
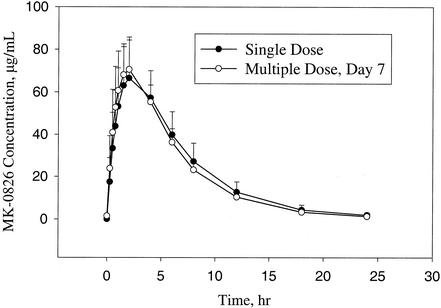
The pharmacokinetic profiles of ertapenem following a single 1-g i.m. dose and multiple once daily 1-g i.m. doses are similar, as shown in Fig. 2. The observed geometric mean AUC0-24 following a single 1-g i.m. dose was 518.1 μg · h/ml, and after the seventh multiple dose, it was 506.4 μg · h/ml. The GMR of individual AUC ratios (multiple dose/single dose) was close to 1.0 at 0.98 with a 90% CI of 0.94 to 1.02. The pharmacokinetic similarities of the single i.m. dose and multiple doses are demonstrated by the mean values and standard deviations for other parameters, as summarized in Table 1.
FIG. 2.
Mean concentrations of ertapenem in plasma (± standard deviation) from healthy subjects on day 1 following a single 1-g i.m. dose (Part A) and on the seventh day following once-daily 1-g i.m. doses for 7 days (Part B).
The time that ertapenem concentration in plasma remained above the susceptibility breakpoint for organisms (4 μg/ml for anaerobes, 2 μg/ml for Enterobacteriaceae and staphylococcus, and 1 μg/ml for streptococcus, according to the final printed labeling for approved NDA 21337 [1]) was similar for subjects receiving a single i.m. dose and those receiving the i.v. dose infused over 30 min (Table 1). The comparison was made with the 30-min infusion because ertapenem will be administered i.v. over 30 min in clinical practice. The geometric mean for time above the 4 μg/ml breakpoint was 18.1 h following a 1-g i.m. dose and 16.9 h following the i.v. dose. The GMR of individual breakpoint ratios (i.m./i.v., 30-min infusion) was 1.07 with a 95% CI of 1.02 to 1.13, and the difference was statistically significant (P = 0.016) but not of clinical importance. Similar results were observed following multiple i.m. dosing at 1 g once daily for 7 days (Table 1).
DISCUSSION
The mean AUC0-∞ values for the 1-g i.m. administration and the 1-g i.v. 120-min infusion were not significantly different, and the geometric mean bioavailability of the 1-g i.m. dose of ertapenem was 92% with a 90% CI of 88 to 95%. The maximum concentration of ertapenem in plasma following an i.m. administration occurred at 2.2 h, with a mean Cmax of 70.6 μg/ml. The mean plasma concentration of ertapenem averaged 165 μg/ml at the end of a 30-min infusion and 120 μg/ml at the end of the 120-min infusion. Other pharmacokinetic parameters, such as apparent t[1/2], apparent CLR, and urinary excretion of unchanged drug, were generally similar for the i.m. and i.v. routes of administration.
AUC0-24 values of ertapenem determined on days 1 and 7 following multiple 1-g i.m. doses of ertapenem in young adults did not indicate accumulation of ertapenem, similar to what was observed with multiple intravenous doses in young men (Majumdar et al., 36th ICAAC). The GMR of individual AUC0-24 ratios (day 7/day 1) was 0.98 with a 90% CI of 0.94 to 1.02. In addition, multiple i.m. dosing did not appear to affect the apparent t[1/2], apparent CLR, and urinary excretion.
The mean duration over which concentrations of ertapenem in plasma exceeded the susceptibility breakpoint at 4 μg/ml was 18.1 h following i.m. administration compared with 16.9 h following i.v. infusion over 30 min. The comparable times suggest that either route of administration would be efficacious for once-a-day dosing.
Ertapenem, at the clinical dose of 1 g, was generally well tolerated when administered as a single i.m. or i.v. dose and as a once-daily i.m. dose for 7 days. The most frequently occurring clinical adverse experiences were diarrhea after both i.v. and i.m. drug administration, which was usually mild, and occasionally moderate, in intensity, and mild erythema at the site of i.m. drug injection. No laboratory adverse experiences were reported.
In summary, the comparable AUC0-∞ values and times over which plasma levels of ertapenem exceed susceptibility breakpoint suggest that the clinical dose of 1 g, administered by i.m. or i.v., may be used interchangeably in the treatment of bacterial infections.
REFERENCES
- 1.Gill, C. J., J. J. Jackson, L. S. Gerckens, B. A. Pelak, R. K. Thompson, J. G. Sundelof, H. Kropp, and H. Rosen. 1998. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-826 (L-749345). Antimicrob. Agents Chemother. 42:1996-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham, D. R., C. Lucasti, O. Malafaia, R. L. Nichols, P. Holtom, N. Q. Perez, A. McAdams, G. L. Woods, T. P. Ceesay, and R. Gesser. 2002. Once a day ertapenem versus four daily doses of piperacillin-tazobactam for treatment of complicated skin and skin structure infections in adults: results of a prospective, randomized, double-blind multicenter study. Clin. Infect. Dis. 34:1460-1468. [DOI] [PubMed] [Google Scholar]
- 3.Lam, F. C., C. T. Hung, and D. G. Perrier. 1985. Estimation of variance for harmonic mean half-lives. J. Pharm. Sci. 74:229-231. [DOI] [PubMed] [Google Scholar]
- 4.Musson, D. G., K. L. Birk, A. M. Cairns, A. K. Majumdar, and J. D. Rogers. 1998. High-performance liquid chromatographic methods for the determination of new carbapenem antibiotic, L-749345, in human plasma and urine. J. Chromatogr. B 720:99-106. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz-Ruiz, G., J. Caballero-Lopez, I. R. Friedland, G. L. Woods, and A. Carides. 2002. A prospective, multicenter, double-blind, randomized, comparative study to evaluate the safety, tolerability, and efficacy of ertapenem versus ceftriaxone in the treatment of community-acquired pneumonia in adults. Clin. Infect. Dis. 34:1076-1083. [DOI] [PubMed] [Google Scholar]
- 6.Sundelof, J. G., R. Hajdu, C. J. Gill, R. Thompson, H. Rosen, and H. Kropp. 1997. Pharmacokinetics of L-749345, a long-acting carbapenem antibiotic, in primates. Antimicrob. Agents Chemother. 41:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong, B. K., P. J. Bruhin, and J. H. Lin. 1999. Dose-dependent plasma clearance of MK-0826, a carbapenem antibiotic, arising from concentration dependent plasma protein binding in rats and monkeys. J. Pharm. Sci. 88:277-280. [DOI] [PubMed] [Google Scholar]