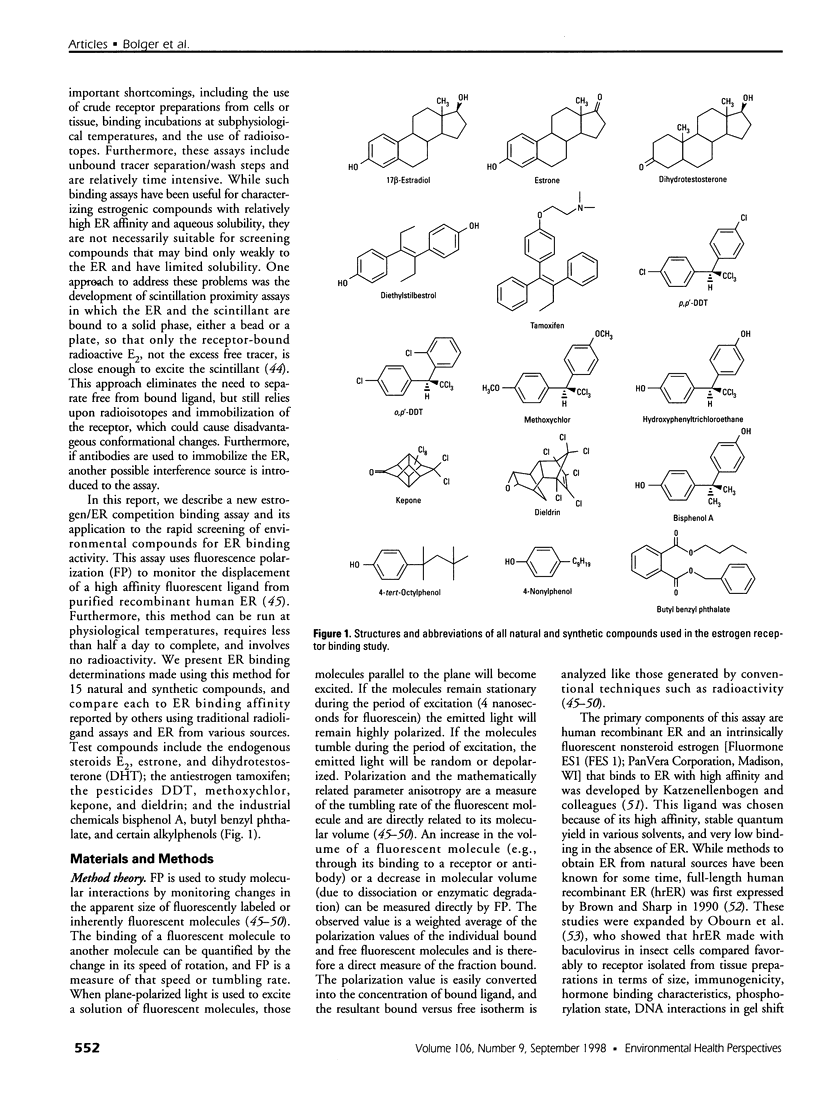
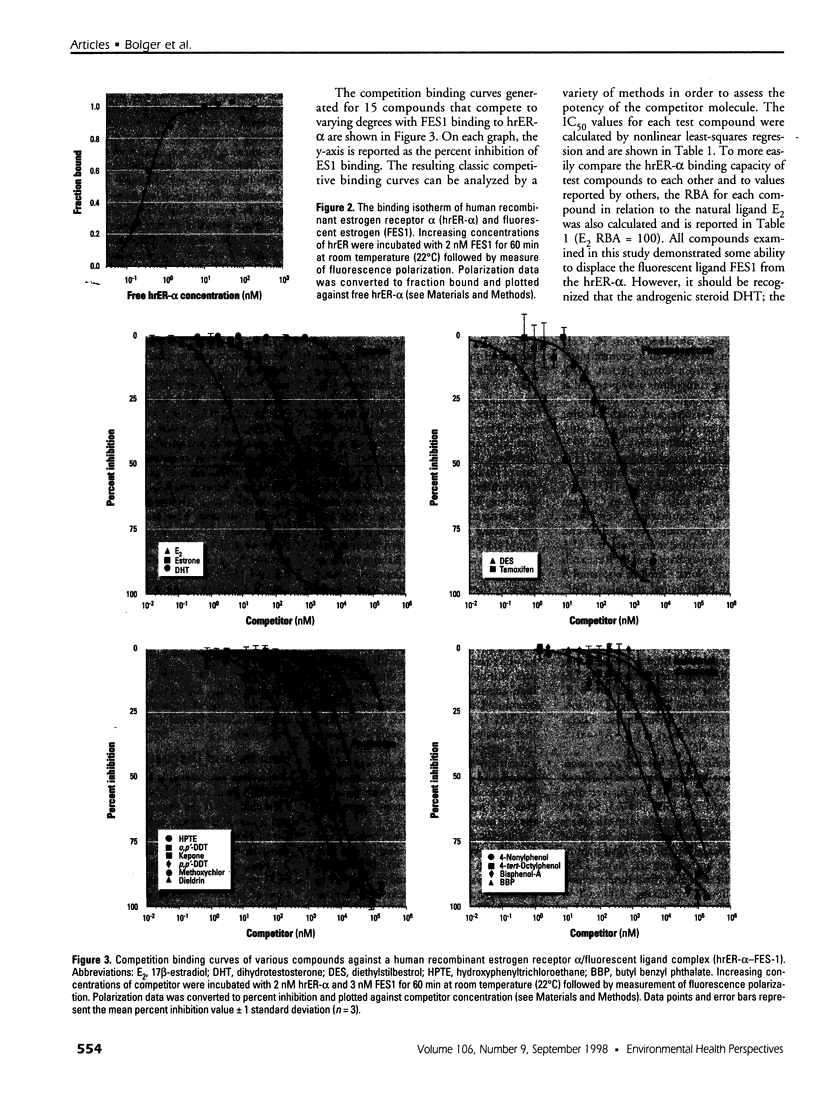
Abstract
Over the last few years, an increased awareness of endocrine disrupting chemicals (EDCs) and their potential to affect wildlife and humans has produced a demand for practical screening methods to identify endocrine activity in a wide range of environmental and industrial chemicals. While it is clear that in vivo methods will be required to identify adverse effects produced by these chemicals, in vitro assays can define particular mechanisms of action and have the potential to be employed as rapid and low-cost screens for use in large scale EDC screening programs. Traditional estrogen receptor (ER) binding assays are useful for characterizing a chemical's potential to be an estrogen-acting EDC, but they involve displacement of a radioactive ligand from crude receptor preparations at low temperatures. The usefulness of these assays for realistically determining the ER binding interactions of weakly estrogenic environmental and industrial compounds that have low aqueous solubility is unclear. In this report, we present a novel fluorescence polarization (FP) method that measures the capacity of a competitor chemical to displace a high affinity fluorescent ligand from purified, recombinant human ER-[alpha] at room temperature. The ER-[alpha] binding interactions generated for 15 natural and synthetic compounds were found to be similar to those determined with traditional receptor binding assays. We also discuss the potential to employ this FP technology to binding studies involving ER-ss and other receptors. Thus, the assay introduced in this study is a nonradioactive receptor binding method that shows promise as a high throughput screening method for large-scale testing of environmental and industrial chemicals for ER binding interactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. F., Klotz D. M., Collins B. M., Vonier P. M., Guillette L. J., Jr, McLachlan J. A. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996 Jun 7;272(5267):1489–1492. doi: 10.1126/science.272.5267.1489. [DOI] [PubMed] [Google Scholar]
- Beekman J. M., Allan G. F., Tsai S. Y., Tsai M. J., O'Malley B. W. Transcriptional activation by the estrogen receptor requires a conformational change in the ligand binding domain. Mol Endocrinol. 1993 Oct;7(10):1266–1274. doi: 10.1210/mend.7.10.8264659. [DOI] [PubMed] [Google Scholar]
- Brown M., Sharp P. A. Human estrogen receptor forms multiple protein-DNA complexes. J Biol Chem. 1990 Jul 5;265(19):11238–11243. [PubMed] [Google Scholar]
- Checovich W. J., Bolger R. E., Burke T. Fluorescence polarization--a new tool for cell and molecular biology. Nature. 1995 May 18;375(6528):254–256. doi: 10.1038/375254a0. [DOI] [PubMed] [Google Scholar]
- Cheskis B. J., Karathanasis S., Lyttle C. R. Estrogen receptor ligands modulate its interaction with DNA. J Biol Chem. 1997 Apr 25;272(17):11384–11391. doi: 10.1074/jbc.272.17.11384. [DOI] [PubMed] [Google Scholar]
- Citron M. Perplexing peroxisome proliferators. Environ Health Perspect. 1995 Mar;103(3):232–235. doi: 10.1289/ehp.95103232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T. Environmental estrogens: health implications for humans and wildlife. Environ Health Perspect. 1995 Oct;103 (Suppl 7):135–136. doi: 10.1289/ehp.95103s7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandliker W. B., Hsu M. L., Levin J., Rao B. R. Equilibrium and kinetic inhibition assays based upon fluorescence polarization. Methods Enzymol. 1981;74(Pt 100):3–28. doi: 10.1016/0076-6879(81)74003-5. [DOI] [PubMed] [Google Scholar]
- Davis D. L., Bradlow H. L., Wolff M., Woodruff T., Hoel D. G., Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect. 1993 Oct;101(5):372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. M., Toone C. K. DDT-induced feminization of gull embryos. Science. 1981 Aug 21;213(4510):922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Leonard L. S., Lovell S., Gould J. C., Babaï D., Portier C. J., McDonnell D. P. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997 Mar;143(1):205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- Gellert R. J. Kepone, mirex, dieldrin, and aldrin: estrogenic activity and the induction of persistent vaginal estrus and anovulation in rats following neonatal treatment. Environ Res. 1978 Jul;16(1-3):131–138. doi: 10.1016/0013-9351(78)90150-0. [DOI] [PubMed] [Google Scholar]
- Gellert R. J. Uterotrophic activity of polychlorinated biphenyls (PCB) and induction of precocious reproductive aging in neonatally treated female rats. Environ Res. 1978 Jul;16(1-3):123–130. doi: 10.1016/0013-9351(78)90149-4. [DOI] [PubMed] [Google Scholar]
- Grainger D. J., Metcalfe J. C. Tamoxifen: teaching an old drug new tricks? Nat Med. 1996 Apr;2(4):381–385. doi: 10.1038/nm0496-381. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Kelce W. R., Wiese T., Tyl R., Gaido K., Cook J., Klinefelter G., Desaulniers D., Wilson E., Zacharewski T. Endocrine Screening Methods Workshop report: detection of estrogenic and androgenic hormonal and antihormonal activity for chemicals that act via receptor or steroidogenic enzyme mechanisms. Reprod Toxicol. 1997 Sep-Oct;11(5):719–750. doi: 10.1016/s0890-6238(97)00025-7. [DOI] [PubMed] [Google Scholar]
- Guzelian P. S. Comparative toxicology of chlordecone (Kepone) in humans and experimental animals. Annu Rev Pharmacol Toxicol. 1982;22:89–113. doi: 10.1146/annurev.pa.22.040182.000513. [DOI] [PubMed] [Google Scholar]
- Hammond B., Katzenellenbogen B. S., Krauthammer N., McConnell J. Estrogenic activity of the insecticide chlordecone (Kepone) and interaction with uterine estrogen receptors. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6641–6645. doi: 10.1073/pnas.76.12.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. A., Henttu P., Parker M. G., Sumpter J. P. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997 Aug;105(8):802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. T., Holmes P., Humfrey C. D. Reproductive health in humans and wildlife: are adverse trends associated with environmental chemical exposure? Sci Total Environ. 1997 Oct 20;205(2-3):97–106. doi: 10.1016/s0048-9697(97)00212-x. [DOI] [PubMed] [Google Scholar]
- Hwang K. J., Carlson K. E., Anstead G. M., Katzenellenbogen J. A. Donor-acceptor tetrahydrochrysenes, inherently fluorescent, high-affinity ligands for the estrogen receptor: binding and fluorescence characteristics and fluorometric assay of receptor. Biochemistry. 1992 Nov 24;31(46):11536–11545. doi: 10.1021/bi00161a035. [DOI] [PubMed] [Google Scholar]
- Jameson D. M., Sawyer W. H. Fluorescence anisotropy applied to biomolecular interactions. Methods Enzymol. 1995;246:283–300. doi: 10.1016/0076-6879(95)46014-4. [DOI] [PubMed] [Google Scholar]
- Jordan V. C. Molecular mechanisms of antiestrogen action in breast cancer. Breast Cancer Res Treat. 1994;31(1):41–52. doi: 10.1007/BF00689675. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen J. A. The structural pervasiveness of estrogenic activity. Environ Health Perspect. 1995 Oct;103 (Suppl 7):99–101. doi: 10.1289/ehp.95103s799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R. J., Ankley G. T. A perspective on the risk assessment process for endocrine-disruptive effects on wildlife and human health. Risk Anal. 1996 Dec;16(6):731–739. doi: 10.1111/j.1539-6924.1996.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Kavlock R. J., Daston G. P., DeRosa C., Fenner-Crisp P., Gray L. E., Kaattari S., Lucier G., Luster M., Mac M. J., Maczka C. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996 Aug;104 (Suppl 4):715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelce W. R., Wilson E. M. Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J Mol Med (Berl) 1997 Mar;75(3):198–207. doi: 10.1007/s001090050104. [DOI] [PubMed] [Google Scholar]
- Korach K. S., McLachlan J. A. Techniques for detection of estrogenicity. Environ Health Perspect. 1995 Oct;103 (Suppl 7):5–8. doi: 10.1289/ehp.95103s75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993 Jun;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Carlsson B., Grandien K., Enmark E., Häggblad J., Nilsson S., Gustafsson J. A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997 Mar;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kupfer D., Bulger W. H. Metabolic activation of pesticides with proestrogenic activity. Fed Proc. 1987 Apr;46(5):1864–1869. [PubMed] [Google Scholar]
- Le Dréan Y., Kern L., Pakdel F., Valotaire Y. Rainbow trout estrogen receptor presents an equal specificity but a differential sensitivity for estrogens than human estrogen receptor. Mol Cell Endocrinol. 1995 Mar;109(1):27–35. doi: 10.1016/0303-7207(95)03482-m. [DOI] [PubMed] [Google Scholar]
- Lundblad J. R., Laurance M., Goodman R. H. Fluorescence polarization analysis of protein-DNA and protein-protein interactions. Mol Endocrinol. 1996 Jun;10(6):607–612. doi: 10.1210/mend.10.6.8776720. [DOI] [PubMed] [Google Scholar]
- Marselos M., Tomatis L. Diethylstilboestrol: I, Pharmacology, Toxicology and carcinogenicity in humans. Eur J Cancer. 1992;28A(6-7):1182–1189. doi: 10.1016/0959-8049(92)90482-h. [DOI] [PubMed] [Google Scholar]
- McBlain W. A. The levo enantiomer of o,p'-DDT inhibits the binding of 17 beta-estradiol to the estrogen receptor. Life Sci. 1987 Jan 12;40(2):215–221. doi: 10.1016/0024-3205(87)90361-4. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993 Oct;101(5):386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan J. A., Wong A., Degen G. H., Barrett J. C. Morphologic and neoplastic transformation of Syrian hamster embryo fibroblasts by diethylstilbestrol and its analogs. Cancer Res. 1982 Aug;42(8):3040–3045. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. An exact correction to the "Cheng-Prusoff" correction. J Recept Res. 1988;8(1-4):533–546. doi: 10.3109/10799898809049010. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Struck R. F., James R. Estrogenic activities of chlorinated hydrocarbons. J Toxicol Environ Health. 1978 Mar-May;4(2-3):325–339. doi: 10.1080/15287397809529664. [DOI] [PubMed] [Google Scholar]
- Newbold R. Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens. Environ Health Perspect. 1995 Oct;103 (Suppl 7):83–87. doi: 10.1289/ehp.95103s783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimrod A. C., Benson W. H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996 May;26(3):335–364. doi: 10.3109/10408449609012527. [DOI] [PubMed] [Google Scholar]
- Obourn J. D., Koszewski N. J., Notides A. C. Hormone- and DNA-binding mechanisms of the recombinant human estrogen receptor. Biochemistry. 1993 Jun 22;32(24):6229–6236. doi: 10.1021/bi00075a016. [DOI] [PubMed] [Google Scholar]
- Ozers M. S., Hill J. J., Ervin K., Wood J. R., Nardulli A. M., Royer C. A., Gorski J. Equilibrium binding of estrogen receptor with DNA using fluorescence anisotropy. J Biol Chem. 1997 Nov 28;272(48):30405–30411. doi: 10.1074/jbc.272.48.30405. [DOI] [PubMed] [Google Scholar]
- Paech K., Webb P., Kuiper G. G., Nilsson S., Gustafsson J., Kushner P. J., Scanlan T. S. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997 Sep 5;277(5331):1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Parker M. G., Arbuckle N., Dauvois S., Danielian P., White R. Structure and function of the estrogen receptor. Ann N Y Acad Sci. 1993 Jun 11;684:119–126. doi: 10.1111/j.1749-6632.1993.tb32276.x. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy K., Wang F., Chen I. C., Norris J. D., McDonnell D. P., Leonard L. S., Gaido K. W., Bocchinfuso W. P., Korach K. S., Safe S. Estrogenic activity of a dieldrin/toxaphene mixture in the mouse uterus, MCF-7 human breast cancer cells, and yeast-based estrogen receptor assays: no apparent synergism. Endocrinology. 1997 Apr;138(4):1520–1527. doi: 10.1210/endo.138.4.5056. [DOI] [PubMed] [Google Scholar]
- Reel J. R., Lamb IV J. C., Neal B. H. Survey and assessment of mammalian estrogen biological assays for hazard characterization. Fundam Appl Toxicol. 1996 Dec;34(2):288–305. doi: 10.1006/faat.1996.0198. [DOI] [PubMed] [Google Scholar]
- Shelby M. D., Newbold R. R., Tully D. B., Chae K., Davis V. L. Assessing environmental chemicals for estrogenicity using a combination of in vitro and in vivo assays. Environ Health Perspect. 1996 Dec;104(12):1296–1300. doi: 10.1289/ehp.961041296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Toft D. O. Steroid receptors and their associated proteins. Mol Endocrinol. 1993 Jan;7(1):4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994 Apr;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995 Oct;103 (Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancel G. M., Boettger-Tong H. L., Chiappetta C., Hyder S. M., Kirkland J. L., Murthy L., Loose-Mitchell D. S. Toxicity of endogenous and environmental estrogens: what is the role of elemental interactions? Environ Health Perspect. 1995 Oct;103 (Suppl 7):29–33. doi: 10.1289/ehp.95103s729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz R., Brown N. G., Allen D. L., Bigsby R. M., Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997 May;138(5):1780–1786. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- Swillens S. Interpretation of binding curves obtained with high receptor concentrations: practical aid for computer analysis. Mol Pharmacol. 1995 Jun;47(6):1197–1203. [PubMed] [Google Scholar]
- Toppari J., Larsen J. C., Christiansen P., Giwercman A., Grandjean P., Guillette L. J., Jr, Jégou B., Jensen T. K., Jouannet P., Keiding N. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996 Aug;104 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Van Aswegen C. H., Purdy R. H., Wittliff J. L. Binding of 2-hydroxyestradiol and 4-hydroxyestradiol to estrogen receptors from human breast cancers. J Steroid Biochem. 1989 Apr;32(4):485–492. doi: 10.1016/0022-4731(89)90380-4. [DOI] [PubMed] [Google Scholar]
- Wade M. G., Desaulniers D., Leingartner K., Foster W. G. Interactions between endosulfan and dieldrin on estrogen-mediated processes in vitro and in vivo. Reprod Toxicol. 1997 Nov-Dec;11(6):791–798. doi: 10.1016/s0890-6238(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Waller C. L., Juma B. W., Gray L. E., Jr, Kelce W. R. Three-dimensional quantitative structure--activity relationships for androgen receptor ligands. Toxicol Appl Pharmacol. 1996 Apr;137(2):219–227. doi: 10.1006/taap.1996.0075. [DOI] [PubMed] [Google Scholar]
- Waller C. L., Oprea T. I., Chae K., Park H. K., Korach K. S., Laws S. C., Wiese T. E., Kelce W. R., Gray L. E., Jr Ligand-based identification of environmental estrogens. Chem Res Toxicol. 1996 Dec;9(8):1240–1248. doi: 10.1021/tx960054f. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Wiese T. E., Polin L. A., Palomino E., Brooks S. C. Induction of the estrogen specific mitogenic response of MCF-7 cells by selected analogues of estradiol-17 beta: a 3D QSAR study. J Med Chem. 1997 Oct 24;40(22):3659–3669. doi: 10.1021/jm9703294. [DOI] [PubMed] [Google Scholar]